Introduction
Pancreatic cancer is one of the malignancies with
very poor prognosis and the 5-year survival rate is approximately
3% (1). It is regarded as the 8th
most common cause of death from cancer worldwide (2), and the 4th leading cause of
cancer-related death in the United States (3). The survival rate has not improved, as
shown by studies on gemcitabine-based combination therapy (4). A breakthrough in the treatment of
this disease may be required to improve the survival
conditions.
B7H1 [CD274, programmed death 1 ligand 1 (PD-L1)] is
a B7-related protein with an immunoglobulin (Ig)-like molecule
first identified in 1999 (5).
Ample evidence confirms that B7H1 is widely expressed in various
human gastrointestinal cancers, including pancreatic (6), gastric (7), esophageal (8) and colon (5) cancers, and its expression is
constitutive or inducible. B7H1 delivers an inhibitory signal to
its receptor, programmed death-1 (PD-1), in T cells, causing the
suppression of immune responses (9). The binding of B7H1 and PD-1 occurs
through the formation of the PD-1/PD-L1 complex, which makes the 2
proteins interact through the conserved front and side of their Ig
variable (IgV) domains containing some conserved residues (10). Blocking of the B7H1 and PD-1
interaction by neutralizing antibodies restores the cytotoxic T
lymphocyte (CTL)-mediated lysis of tumor cells in vitro
(11).
Most studies have focused on the mechanisms of the
B7H1 suppressive effect on T cells mediated by PD-1/PD-L1. They
have found that mechanisms of action are involved, such as the
induction of apoptosis (12) and
the exhaustion of T cells (13).
However, in a certain study, mixed B7H1+
and B7H1- cells were cultured together with
antigen-specific CD8+ CTL in vitro. The
researchers did not detect the suppression of the cytolytic
function of CD8+ CTL after short-term culture, while
they found that B7H1- cells presented preferential lysis
(11). This conclusion suggests
that B7H1 transfers a reverse signal to tumor cells themselves,
apart from its role as a ligand to the PD-1 receptor, followed by
certain mechanisms that induce the death of B7H1+ cells.
To demonstrate this hypothesis, we induced apoptosis in a number of
human pancreatic cancer cells with different expression levels of
B7H1 and designed small peptides to interfere with the function of
B7H1. Our results indicate that B7H1 expression in pancreatic
cancer cells reduces drug-induced apoptosis and that synthetic
small peptides interrupt this inhibition.
Materials and methods
Cell lines and cultures
The Panc-1 and BxPC-3 human pancreatic cancer cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium
(Gibco, Carslbad, CA, USA) supplemented with 10% fetal bovine serum
(Gibco), 100 U/ml of penicillin and 100 μg/ml of streptomycin at
37°C in a humidified incubator containing 5% CO2.
Treatments were performed after the cells adhered.
Peptide synthesis and treatment
Two small peptides with 6 hydrophilic amino acid
residues were synthesized by solid phase synthesis (Hangzhou Angtai
Biotech. Co., Ltd., Hangzhou, China). Peptide 1 (P1) was designed
according to the amino acid sequence of the connecting site of the
PD-1/PD-L1 complex; Peptide 2 (P2) was synthesized using the
similar amino acid residues, but with an uncorrelated sequence as
the control. Peptides were lysed in phosphate-buffered saline (PBS)
and incubated with the cells at the concentration of 50 μg/ml.
Fluorescein isothiocyanate (FITC; 5 μl)-conjugated P1 and P2
(Angtai Biotechnology) at the concentration of 1 mg/ml were
incubated with BxPC-3 cell samples for flow cytometry (FCM)
assay.
FCM assay
BxPC-3 and Panc-1 cells treated with or without
interferon (IFN)-γ (500 U/ml) for 24 h in 6-well plates were
harvested and washed twice with cold PBS. Each sample was incubated
with 5 μl anti-human B7H1 phycoerythrin (PE), mouse IgG1 isotype
control PE (eBioscience, San Diego, CA, USA), FITC-conjugated P1 or
control P2 for 30 min at 4°C in the dark. After being washed twice
with PBS again, the cells were resuspended in 500 μl PBS at the
concentration of 1×105/ml and analyzed by FCM (FACScan;
BD Biosciences, Franklin Lakes, NJ, USA). In brief, for apoptosis
analysis, cells grown in 6-well plates were pre-treated with P1,
P2, recombinant human PD-1 Ig or human IgG (Sino Biological,
Beijing, China) at the concentration of 50 μg/ml. After 24 h, the
cells were treated with staurosporine (STS) at 0.5 μM for another
24 h at 37°C and were then collected. After being washed with PBS,
the cells were incubated with Alexa Fluor 488 annexin V and
propidium iodide (PI) (Invitrogen, Renfrew, UK) for 15 min at room
temperature in the dark, and then the samples were measured by
FCM.
siRNA interference
Transfection-related products were all purchased
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA. BxPC-3
cells were diluted in fresh medium without antibiotics and
transferred to 6-well plates. Cells grown to a confluence of 50–60%
were transfected with 8 μl of B7H1 siRNA or control siRNA per well
according to the manufacturer’s recommendations. After transfection
for 24 h, the efficacy of the siRNA interference was determined by
western blot analysis.
MTS assay
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) assays were performed according to the
manufacturer’s instructions (CellTiter 96 AQueous Non-Radioactive
Cell Proliferation assay; Promega, Madison, WI, USA). Briefly,
cells were seeded in 96-well culture plates at an optimal density
(5×103 cells/well) in triplicate wells. After 24 h, the
medium was changed and the cells were treated with peptides (50
μg/ml) for another 24 h. Then, STS (0.5 μM) was added. After 24 or
48 h, 20 μl of MTS solution was added to each well (at the total
volume of 120 μl/well) for 2 h at 37°C. The absorbance was measured
at 570 nm using a microtitration plate spectrophotometer.
Western blot analysis
To evaluate the expression of the B7H1 protein and
the active degrees of the apoptosis-related protein, poly
(ADP-ribose) polymerase 1 (PARP-1), and caspase-3, cellular samples
were analyzed by western blot analysis. Cell extracts were prepared
with RIPA lysis buffer (Beyotime Biotechnology, Haimen, China). The
total protein concentration was measured by the bicinchoninic acid
(BCA; Beyotime Biotechnology) method using bovine serum albumin as
the standard sample. After the samples were heat-denatured, a total
of 40 μg of protein samples was subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (6 or 12%, as
required) and transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). Membranes were then blocked with
5% non-fat milk in Tris-buffered solution with 0.5% Tween-20 (TBST)
for 1 h at room temperature and incubated with primary antibodies
specific to B7H1 (R&D Systems, Minneapolis, MN, USA), PARP-1
and caspase-3 (Epitomics, Burlingame, CA, USA) overnight at 4°C.
After being washed 3 times, membranes were incubated with secondary
antibodies (Zhongshan Goldenbridge Biotechnology Co., Beijing,
China) or GAPDH (Shanghai Weike Biochemical Reagent Co., Shanghai,
China) for 1 h at room temperature. Signals were detected by
enhanced chemiluminescence detection reagents (Millipore) using
ImageQuant LAS-4000 (Fujifilm, Tokyo, Japan). The bands were
analyzed using Multi-Gauge software (Fujifilm).
Immunofluorescence
Cells were cultured on microscope slides and washed
3 times with PBS for 15 min before being fixed with 4%
paraformaldehyde at room temperature for 15 min. After being washed
3z times with PBS for 30 min, the cells were incubated for 2 h at
room temperature with FITC-conjugated P1 or P2. After a further
washing step, images were captured on a wide-field fluorescent
microscopy (Zeiss, Jena, Germany).
Statistical analysis
All statistical analyses were carried out using the
SPSS 19.0 statistical software package. All data were obtained from
at least 3 individual experiments. Values are expressed as the
means ± SD. Statistical analysis between groups was performed by
one-way ANOVA. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
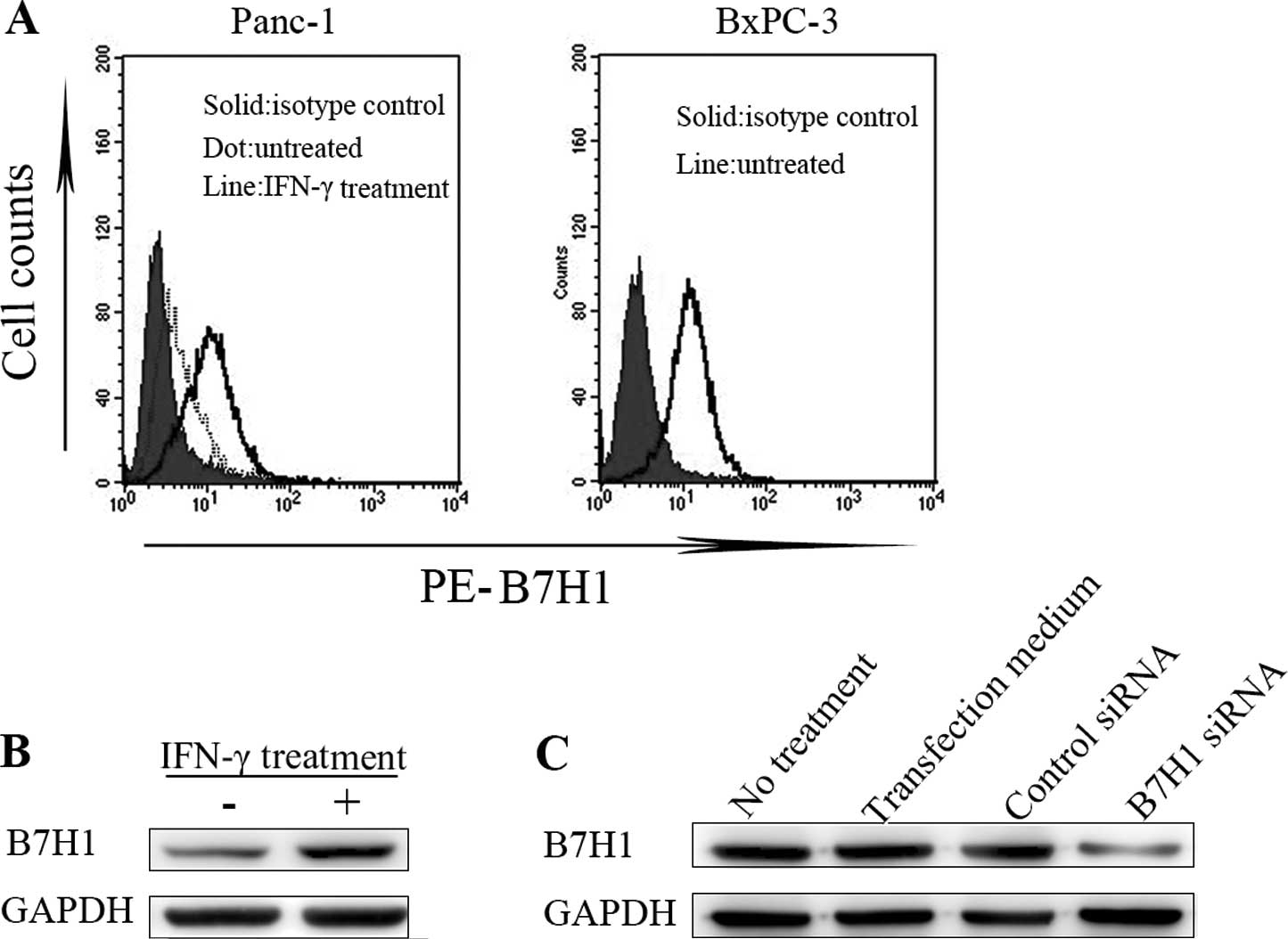
B7H1 is constitutively expressed in
Panc-1 cells and highly expressed in BxPC-3 cells
We used FCM to determine the levels of B7H1 protein
expression on the cell membrane in BxPC-3 and Panc-1 cells. There
was a low-level constitutive expression of B7H1 in Panc-1 cells.
This expression was upregulated 24 h after treatment with IFN-γ
(500 U/ml), while B7H1 expression was high in BxPC-3 cells
untreated with IFN-γ (Fig. 1A). We
then extracted the total protein from cells treated as mentioned
above to detect the B7H1 expression levels by western blot
analysis. The results indicated a similar tendency. Panc-1 cells
presented significantly higher levels of B7H1 expression after
treatment with IFN-γ (Fig. 1B).
The expression levels in BxPC-3 cells were effectively
downregulated by siRNA silencing (Fig.
1C). In our study, following B7H1 siRNA transfection, Panc-1
and BxPC-3 cells had a low expression of B7H1, while Panc-1 cells
treated with IFN-γ and BxPC-3 cells had a high B7H1 expression.
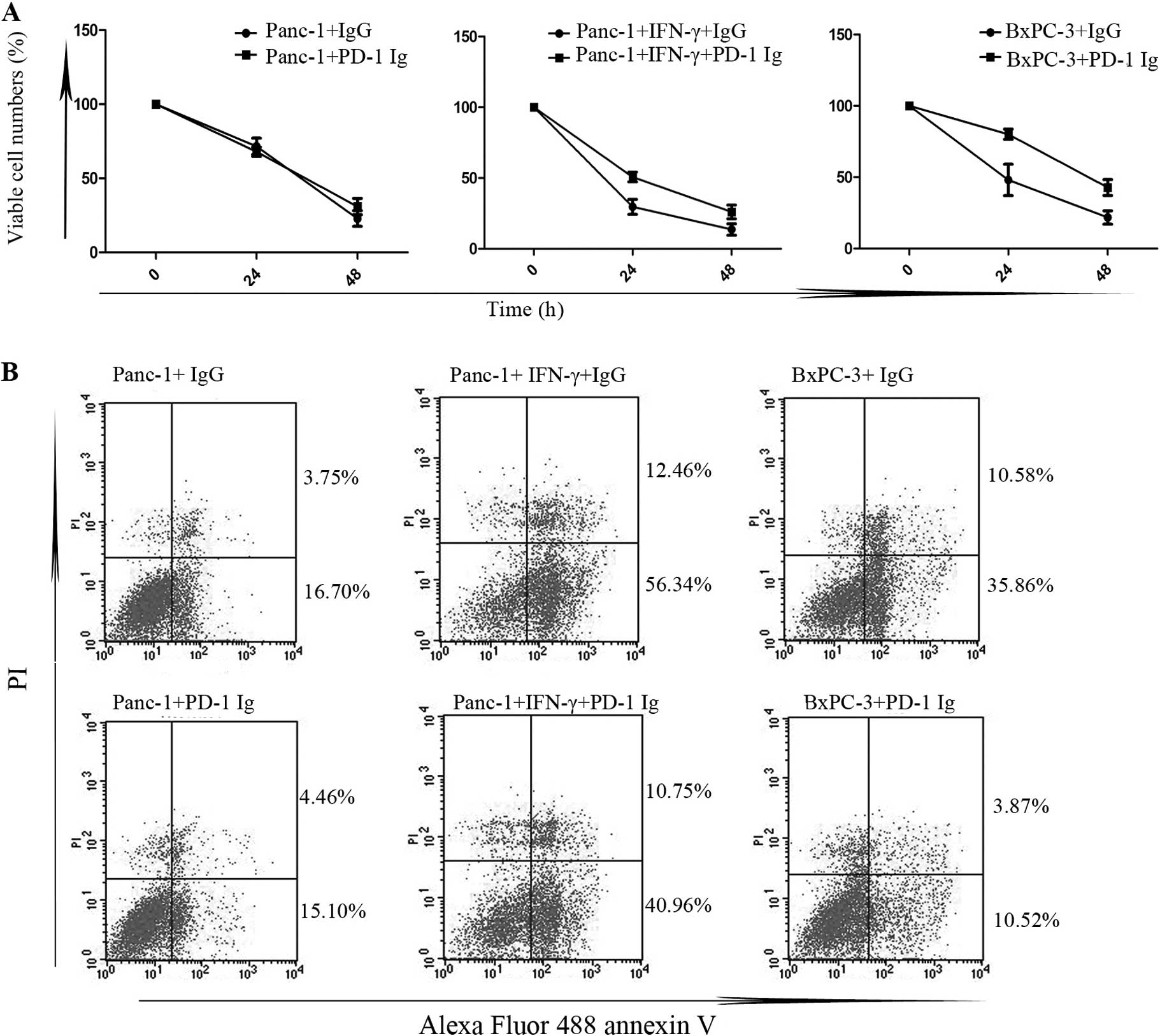
Binding to PD-1 Ig protects tumor cells
from STS-induced apoptosis
Panc-1 cells and cells with a high expression of
B7H1 were incubated with PD-1 Ig fusion protein (50 μg/ml) or
control IgG (50 μg/ml) for 24 h after they adhered to 6-well
plates. After extensive washing, the cells were treated with STS
(0.5 μM) for 24–48 h. MTS assay was used to determine the cell
proliferation in the different groups after treatment with STS
(Fig. 2A). There was a higher
percentage of cell viability in cells with a high expression of
B7H1 pre-treated with PD-1 Ig compared to the controls (P<0.05),
while the differences in Panc-1 cells with low B7H1 expression
levels were not significant (P>0.05). There was an inhibitory
action in the cell death caused by STS from the formation of the
PD-1/PD-L1 complex. To explore the mechanisms involved, we then
examined cell apoptosis by FCM assay (Fig. 2B). After treatment with STS for 24
h, the percentages of early apoptotic cells in the cells with a
high expression of B7H1 pre-treated with PD-1 Ig were significantly
lower than those in the controls (P<0.05) and, as expected,
there were no significant differences in the Panc-1 cells
(P>0.05). These results suggest that the formation of the
PD-1/PD-L1 complex suppresses STS-induced tumor cell apoptosis in
cells with a high expression of B7H1.
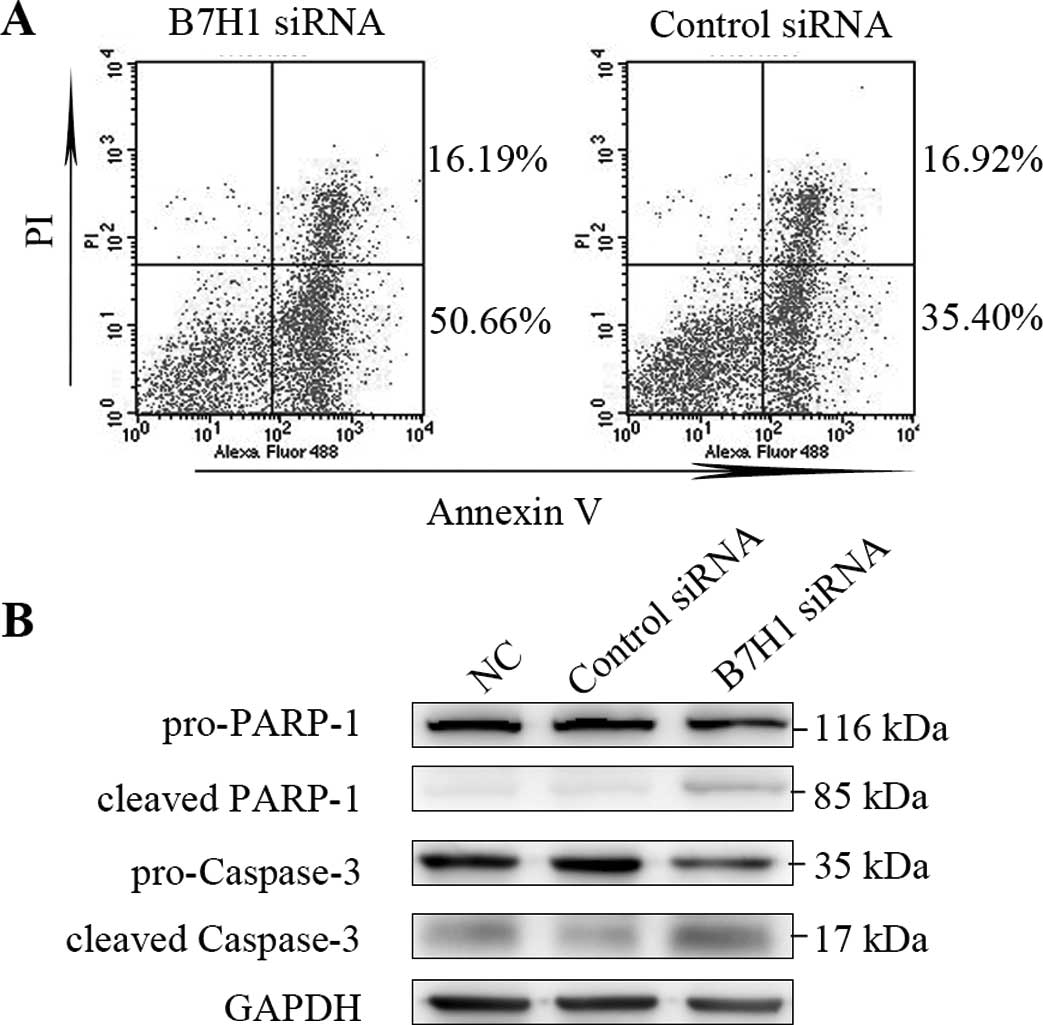
B7H1 knockdown increases apoptosis in
BxPC-3 cells
In order to examine whether the expression levels of
B7H1 are related to cell apoptosis in BxPC-3 cells, FCM assay and
western blot analysis were performed. The percentage of cells at
the early stages of apoptosis in cells transfected with B7H1 siRNA
was significantly increased compared to the control-transfected
cells (P<0.05; Fig. 3A).
Caspase-3 and PARP-1 play key roles during the process of cell
apoptosis. We observed marked increases in the cleavage activities
of caspase-3 and PARP-1 in the cells transfected with B7H1 siRNA,
which indicated the enhancement of apoptosis. This suggests that
high levels of B7H1 expression reduce STS-induced tumor cell
apoptosis. On the other hand, a successful siRNA interference may
increase apoptosis in these cells.
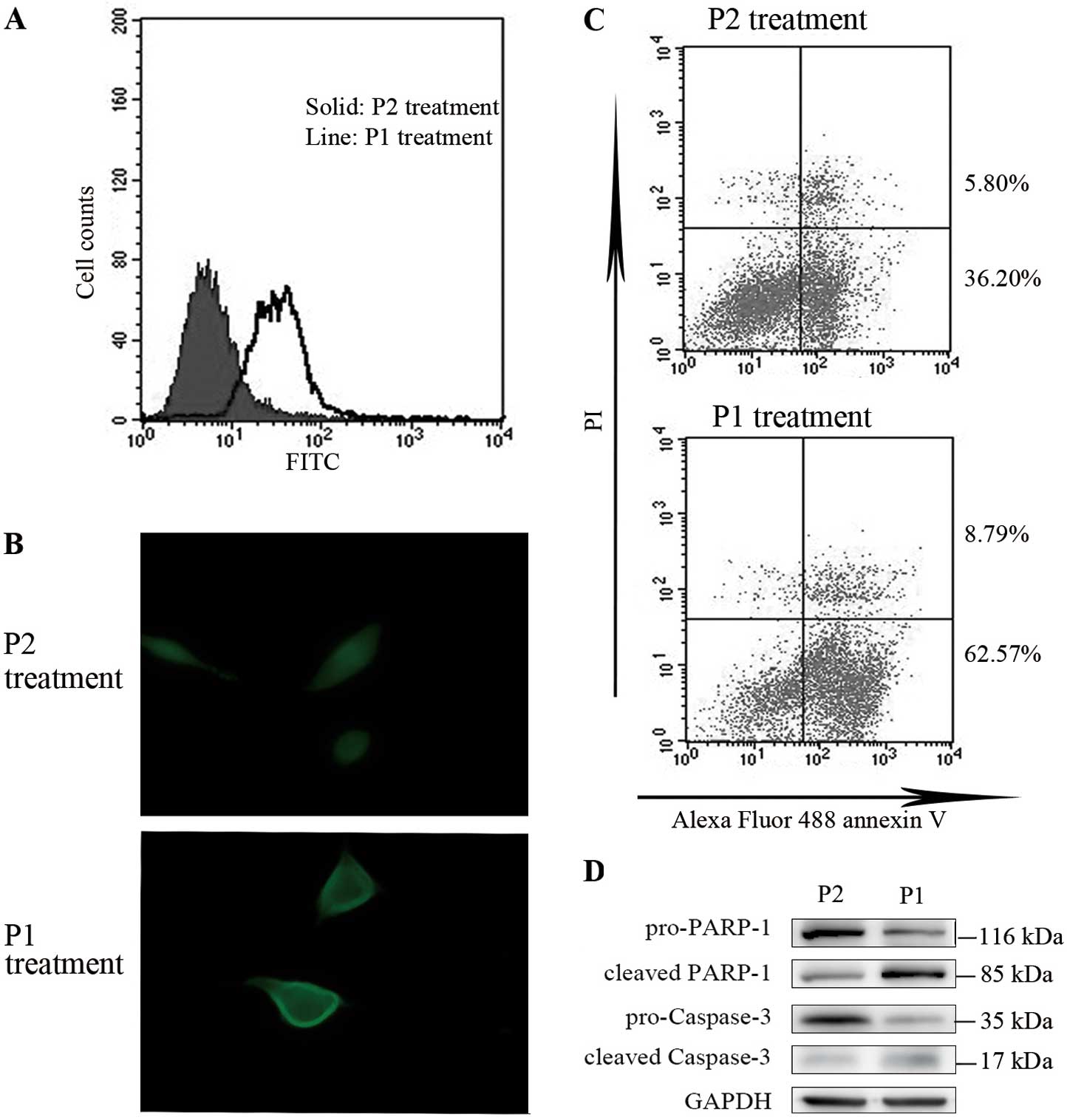
Synthetic peptides designed according to
PD-1/PD-L1 complex increase apoptosis in BxPC-3 cells
Synthetic peptide P1 was a small peptide containing
6 amino acid residues. The amino acid sequence,
Ser-Asn-Gln-Thr-Asp-Lys, was the same as human PD-1 residues,
73–78, which acted as the connecting site to PD-L1 during the
formation of the PD-1/PD-L1 complex. Peptide P2, with the residues,
Ala-Asp-Tyr-Lys-Arg-Ile, was synthesized to act as the control in
our study. We used FITC-conjugated P1 and P2 to investigate the
efficacy of these synthetic peptides binding to the cell membrane
and whether this binding is relative to the expression levels of
B7H1. BxPC-3 cell samples incubated with FITC-conjugated synthetic
peptides for 30 min were washed extensively and analyzed by FCM.
The level of P1 binding was significantly higher than the P2
control (Fig. 4A), which showed a
similar tendency in B7H1 expression. To confirm our result, we
performed immunofluorescence analysis to detect whether FITC-P1
binds to the cell membrane effectively. Cells with P1 treatment
presented significantly higher fluorescence on the membrane than
the control cells (Fig. 4B). With
these results, we pre-treated BxPC-3 cells with synthetic peptides
and then induced apoptosis by STS, as previously mentioned. In the
FCM assays, the cell percentage in the early stages of apoptosis in
P1 pre-treated cells was significantly higher than the P2 control
cells (P<0.05; Fig. 4C). The
detection of PARP-1 and caspase-3 showed markedly increasing levels
of cleaved proteins in P1 pre-treated cells (Fig. 4D). This may be explained by the
interference of the anti-apoptotic effect caused by the PD-1/PD-L1
complex formation, which occurred by the binding of P1.
Discussion
Previous studies have indicated that inhibitory
signals are transferred to T cells by B7H1 in tumor cells, which
lead to immune suppression (11,14,15).
The blockage of B7H1 and its receptor, PD-1, in T cells by
antibodies improves antitumor immunity (14). Disruption of the B7H1 gene
upregulates T cell responses (16). These studies suggest that B7H1 and
its receptor contribute to tumor cells escaping from immune
destruction.
However, in this study, we attempted to explain the
mechanism of the resistance to antitumor immunity in
B7H1+ cancer cells from another point of view. The
concept that programmed cell death by apoptosis serves as a natural
barrier to cancer development has been established by compelling
functional studies conducted over the past 2 decades. Yet, another
research has revealed how apoptosis is attenuated in tumors that
succeed in progressing to states of high-grade malignancy and
resistance to therapy (17).
In the present study, we found that B7H1 expressed
at a high level inhibits STS-induced cancer cell apoptosis and that
the successful B7H1 knockdown increases apoptosis by disrupting
this inhibition in the BxPC-3 human pancreatic cell line. A certain
study previously demonstrated that B7H1 siRNA knockdown led to an
increase in spontaneous apoptosis, as well as doxorubicin-induced
apoptosis in breast cancer cells (18), and another study reported that B7H1
expression in cancer cells plays a role in the induction of the
anti-apoptotic mechanism (19).
All these results indicate that the expression levels of B7H1 are
related to apoptosis in tumor cells. In due time, this may
contribute to the inhibition of B7H1+ tumor cell lysis
in immune responses. However, the underlying molecular mechanisms
of the impact are unknown, and further research will help in
developing tumor prognosis and therapy.
Furthermore, B7H1 is confirmed to bind its receptor,
PD-1, and form the PD-1/PD-L1 complex. It is the structural basis
that induces B7H1 to produce inhibitory effects on immune
responses. This complex transfers reverse signals to
B7H1+ tumor cells besides its forward direction to T
cells. This was also detected in our study using PD-1 Ig to mimic
the formation of the PD-1/PD-L1 complex. We found that drug-induced
apoptosis in cells with a high expression of B7H1 increased
significantly after treatment. Since the complex has several
conserved domains described previously, small molecule drugs
designed to interfere with its inhibitory signal may soon become a
reality.
Small peptides less than 8–10 amino acid residues
are easily absorbed by the gastrointestinal tract with little
degradation. On the other hand, small peptides may cause less
side-effects compared to other treatments, such as chemotherapy and
radiotherapy. The amino acid residues binding to B7H1 may interrupt
the integrity of the domains and cause some interference. In our
study, we designed a small peptide containing 6 amino acid residues
to act as a drug. After demonstrating its successful binding to
cells with a high expression of B7H1, we discovered that its
binding increased apoptosis in B7H1+ tumor cells.
In conclusion, our study demonstrates that the high
expression of B7H1 and the formation of the PD-1/PD-L1 complex
inhibit drug-induced apoptosis in pancreatic cancer cells in
vitro. Synthetic small peptides enhance drug-induced apoptosis
in pancreatic cancer cells with a high expression of B7H1. We are
the first to demonstrate that synthetic small peptides can increase
apoptosis in B7H1+ cancer cells. Our results may lead to
a breakthrough in the treatment of pancreatic cancer.
Acknowledgements
This study was supported by the Key Social
Development Project of Major Science and Technology (2011C13036-1).
The study was performed at the Biomedical Research Center, Sir Run
Run Shaw Hospital, Zhejiang University School of Medicine,
Hangzhou, China.
Abbreviations:
|
Ig
|
immunoglobulin
|
|
PD-1
|
programmed death-1
|
|
CTL
|
cytotoxic T lymphocyte
|
|
IFN
|
interferon
|
|
PE
|
phycoerythrin
|
|
STS
|
staurosporine
|
|
PBS
|
phosphate-buffered saline
|
|
P1
|
peptide 1
|
|
P2
|
peptide 2
|
|
FITC
|
fluorescein isothiocyanate
|
|
FCM
|
flow cytometry
|
|
MTS
|
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
References
|
1
|
Lowenfels AB and Maisonneuve P:
Epidemiology and risk factors for pancreatic cancer. Best Pract Res
Clin Gastroenterol. 20:197–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
3
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
4
|
Merl MY, Li J and Saif MW: The first-line
treatment for advanced pancreatic cancer. In: Highlights from the
‘2010 ASCO Gastrointestinal Cancers Symposium’; Orlando, FL, USA.
January 22–24, 2010; JOP. 11. pp. 148–150. 2010, PubMed/NCBI
|
|
5
|
Dong H, Zhu G, Tamada K, et al: B7-H1, a
third member of the B7 family, costimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nomi T, Sho M, Akahori T, et al: Clinical
significance and therapeutic potential of the programmed death-1
ligand/programmed death-1 pathway in human pancreatic cancer. Clin
Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu SM, Meng Q, Zhang QX, et al:
Expression and significance of B7-H1 and its receptor PD-1 in human
gastric carcinoma. Zhonghua Zhong Liu Za Zhi. 30:192–195.
2008.PubMed/NCBI
|
|
8
|
Ohigashi Y, Sho M, Yamada Y, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L: Coinhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
David Y, Yoshimasa T, Masashi I, et al:
The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of
antibodies and T cell receptors. PNAS. 105:3011–3016. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano F, Kaneko K, Tamura H, et al:
Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates
cancer therapeutic immunity. Cancer Res. 65:1089–1096.
2005.PubMed/NCBI
|
|
12
|
Dong H, Strome SE, Salomao DR, et al:
Tumor associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwai Y, Ishida M, Tanaka Y, et al:
Involvement of PD-L1 on tumor cells in the escape from host immune
system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad
Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blank C, Brown I, Peterson AC, et al:
PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T
cell receptor (TCR) transgenic CD8+ T cells. Cancer Res.
64:1140–1145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Latchman YE, Liang SC, Wu Y, et al:
PD-L1-deficient mice show that PD-L1 on T cells, antigen presenting
cells, and host tissues negatively regulates T cells. Proc Natl
Acad Sci USA. 101:10691–10696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghebeh H, Lehe C, Barhoush E, et al:
Doxorubicin downregulates cell surface B7-H1 expression and
upregulates its nuclear expression in breast cancer cells: role of
B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 12:R482010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azuma T, Yao S, Zhu G, et al: B7-H1 is a
ubiquitous antiapoptotic receptor on cancer cells. Blood.
111:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|