Introduction
Cancer is a leading cause of mortality in humans.
According to the International Agency for Research on Cancer
approximately 18.7% of individuals worldwide are likely to develop
some form of cancer in their lifetime, and 11.2% of individuals
worldwide may succumb to the disease (1). In the last 20 years, advances in
cancer research and the consequent development of more effective
therapies have significantly increased the survival times
associated with certain types of cancer (1). However, there have been few
breakthroughs that are useful in all patients, particularly those
with advanced stage cancer. One of the most favorable new
approaches to cancer treatment currently being developed is tumor
biotherapy. In particular, targeting cytokines has been shown to
induce the proliferation of immunocompetent cells that have
antitumor activity (2–4).
One of the strongest and most broad-ranging
immunocyte-stimulating cytokines is interleukin-12 (IL-12), which
is known to have powerful antivirus and antitumor activity, and is
also a core regulating factor in natural immunity (4–6).
However, the potential therapeutic effects of administering
recombinant IL-12 are limited due to the toxicity and side-effects.
In addition, the expression and purification of recombinant IL-12
is expensive, the half-life of IL-12 in vivo is short and
certain individuals generate antibodies against IL-12 (7,8).
Despite these shortcomings, progress has been made towards
developing novel methods of IL-12 delivery by combining recombinant
IL-12 with an appropriate carrier. The most significant attempts
have involved a gene therapy approach to delivery of IL-12 for
which several positive results in animal models of tumors and in
phase I clinical trials have been produced (9–11).
The construction of a plasmid containing human
recombinant IL-12, IL-12 pcDNA6-V5-His-p70 (pcDNA6-p70), has been
described and it has been demonstrated that this plasmid has
biological activity in vitro and in vivo (12). The aim of this study was to
investigate the therapeutic effects of administering the
IL-12-encoding plasmid pcDNA6-p70 to mice bearing transplanted
tumors and to determine the safety profile of the plasmid in
vivo in tumor-bearing mice. Results from this study may form
the basis for further investigation of the potential of gene
therapy using pcDNA6-p70 in humans.
Materials and methods
Reagents
The recombinant plasmid pcDNA-p70, which codes for
human IL-12, was constructed as previously described (12). Methyl thiazolyl tetrazolium (MTT)
and lactate dehydrogenase (LDH) were obtained from Sigma (St.
Louis, MO, USA), cyclophosphamide from the 12th Pharmaceutical
Factory (Shanghai, China), RPMI-1640 complete medium from Gibco
(Carlsbad, CA, USA), and the ELISA detection kits for mouse IFN-γ
from the Jingmei Bioengineering Co., Ltd (Shenzhen, China).
Laboratory animals and S-180 cells
Kunming mice were provided by the Center for
Laboratory Animals in Qingdao Drug Identification Office (Shandong,
China). The S-180 cells were obtained from the Institute of Materia
Medica of the Shandong Academy of Medical Science (Shandong,
China). The study was approved by the ethics committee of Shandong
Medical College.
Cell modification
The S-180 cell line was transfected with pcDNA6-p70
by polyethyleneimine (PEI; Sigma). The modified cell line
(S-180/IL-12) was selected in 10% FBS RPMI-1640 with Blasticidin S
HCl (final concentration 10 mg/ml; Invitrogen, Carlsbad, CA, USA)
for 14 days and cultured in maintenance solution (Blasticidin S
HCl, 2 mg/ml). The modified cell line (S-180/IL-12;
2×105) was cultured in complete medium for 48 h. The
expression level of pcDNA6-p70 in the supernatant was detected
using a human IL-12 ELISA kit.
S-180 tumor-bearing mouse model and the
administration of pcDNA6-p70
S-180 cells were injected into the peritoneal cavity
and the resulting ascites were extracted, washed with physiological
saline and the density of the cells within the fluid was adjusted
to between 2×107 and 6×107 cells/ml before
0.2 ml was injected into the right armpit of the mice (n=30). The
majority of the tumors were formed by the 4th day following S-180
cell transplantation. The mice were randomly divided into three
groups (n=10 in each). The first group received pcDNA6-p70
(dissolved in purified water; 100 μg/mouse), the second
cyclophosphamide (dissolved in 0.9% saline; 40 mg/kg), and the
third 0.9% saline (100 μl/mouse). The compounds were directly
injected into the tumor on the 4th, 7th, 10th, 14th and 17th days
following transplantation of the S-180 cells. On the 20th day, the
mice were weighed and blood samples were collected from the tail
vein. The mice were sacrificed on the 21st day and the tumor,
spleen and thymus of each mouse was removed and weighed. The ratio
of tumor suppression was calculated by dividing the weight of the
tumors obtained from the mice administered pcDNA6-p70 or
cyclophosphamide by the weight of the tumors removed from the mice
administered physiological saline. The spleen and thymus indices
were calculated by dividing the respective organ weight by body
weight × 1000.
MMT assay used to measure the
proliferation of spleen cells
To measure the proliferation of the spleen cells,
cell suspension was constructed from each mouse spleen where the
number of viable cells was >95% (staining by trypan blue). The
cell suspension was adjusted to 2×106 cells/ml with
RPMI-1640 nutrient medium containing 10% solcoseryl. The cells were
transferred to a 96-well plate (100 μl/well; n=3 wells for each
mouse spleen sample) and 5 μl Concanavalin A (ConA) was added to
each well. Control wells for the assay contained 5 μl RPMI-1640
nutrient medium alone. The cells were incubated at 37°C in 5%
CO2 and 95% humidity for 72 h. Shortly before the
incubation time was complete, 20 μl MTT (5 mg/ml) was added to each
well. Following completion of the incubation period, 150 μl
dimethyl sulfoxide (DMSO) was added to each well, the contents were
thoroughly mixed and the absorbance was read at 570 nm using ELISA
(Jingmei Bioengineering Co., Ltd.). The amount of lymphocyte
proliferation was calculated by subtracting the absorbance reading
of the control wells from that of the sample wells.
LDH assay used to measure the cytotoxic
activity of natural killer (NK) cells
In order to measure the activity of NK cells, an
effector cell suspension containing mouse spleen cells diluted to a
density of 1×107 cells/ml with RPMI-1640 culture medium
and a target cell suspension of S-180 cells with a density of
2×105 cells/ml were prepared. The two cell suspensions
were added together in a 96-well plate with a ratio of effector to
target cells of 50:1 in each well (sample mixture, n=3 wells for
each mouse spleen sample). At the same time, two control mixtures
were set up; one to indicate the maximum release of LDH possible
(100 μl S-180 cells + 100 μl 2% Triton X-100), and the other to
indicate the level of spontaneous release of LDH from the target
cells (100 μl S-180 cells + 100 μl RPMI-1640 culture media). The
sample and control mixtures were incubated at 37°C in 5%
CO2 and 90% humidity for 2 h before 100 μl of the
supernatant from each well was transferred into another 96-well
plate and heated at 37°C for 10 min. Next, 100 μl fresh LDH
detection mixture [0.032 mg NBT, 0.08 mg NAD+, 0.008 mg PMS and 4
μl sodium lactate (1 mol/l)] was added to each well, incubated for
10 to 15 min at room temperature in the dark, and then the reaction
was terminated with 30 μl citric acid (1 mol/l). The absorbance of
each reaction mixture was measured using ELISA (ELISA Equipment,
Source). Cytotoxic activity of NK cells was calculated using the
formula: (absorbance of sample mixture - absorbance of
spontaneously released LDH)/(absorbance of maximum LDH - absorbance
of spontaneous released LDH) × 100%.
Detection of IFN-γ in tumor-bearing
mice
IFN-γ was detected using ELISA. A 40 μl sample of
blood collected from the caudal vein of each mouse’s tail was mixed
with 100 μl 0.9% saline and centrifuged for 10 min (800 rpm). The
resulting supernatant was then subjected to ELISA using an ELISA
kit. The absorbance of the reaction mixture was measured at 490 nm
and the IFN-γ content of the mouse serum was calculated
(pg/ml).
Immunohistochemisty
Immunohistochemistry was performed on 4 μm sections.
The primary antibody (anti-human IL-12 monoclonal antibody; R&D
Systems, Minneapolis, MN, USA) was incubated overnight. Sections
were visualized with 3,3′-diaminobenzidine (DAB)-chromogen and
lightly counterstained with haematoxylin. Diluted goat serum was
used as the negative control for the primary antibody.
The samples were immunohistochemically stained for
IL-12 and were assessed without knowledge of the
clinicopathological features. At least 500 carcinoma cells were
examined in 10 randomly selected fields (x200) within the same
section under light microscopy to determine the staining status of
IL-12. Samples were considered positive when the unequivocal
staining of the cytoplasm and/or nuclear compartment was observed
in >10% of the tumor cells.
Safety evaluation for pcDNA6-p70 in
normal mice
In addition to the main study groups, two further
groups of normal mice were administered pcDNA6-p70 (n=10) or 0.9%
saline (n=10) and were monitored for adverse effects relating to
the drug treatment. pcDNA6-p70 (100 μl/time, 5 μg/g) or
physiological saline (100 μl/time) were injected hypodermically
into the right armpit of each respective group of mice, and
temperature, weight and general state of health (including
appetite, fur color, response to stimulation and locomotor
activity) were recorded on the 1st, 2nd, 3rd, 7th and 14th day
following the first injection.
Statistical analysis
Statistical differences between the outcomes of each
measurement of the therapeutic activity of pcDNA6-p70 were analyzed
using the one-way ANOVA test and SPSS 14.0 statistical software.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
IL-12 expression determined by ELISA
The expression of S-180/IL-12 in the modified cell
line was 0.8–1.4 ng/ml (assessed in the supernatant from
2×105 cells grown for 48 h in 2 ml of medium) measured
by ELISA (data not shown), indicating that pcDNA6-p70 was
successfully expressed in S-180/IL-12.
Expression of IL-12 in the tumor
Tumor sections were scanned under low-power
magnification (x200) to select the most intense areas; >40% of
the tumor cells was considered positive. A strong positive
expression was considered in interstitial substance mononuclear
cells.
Inhibitory effect of pcDNA6-p70 on the
growth of S-180 tumors in mice
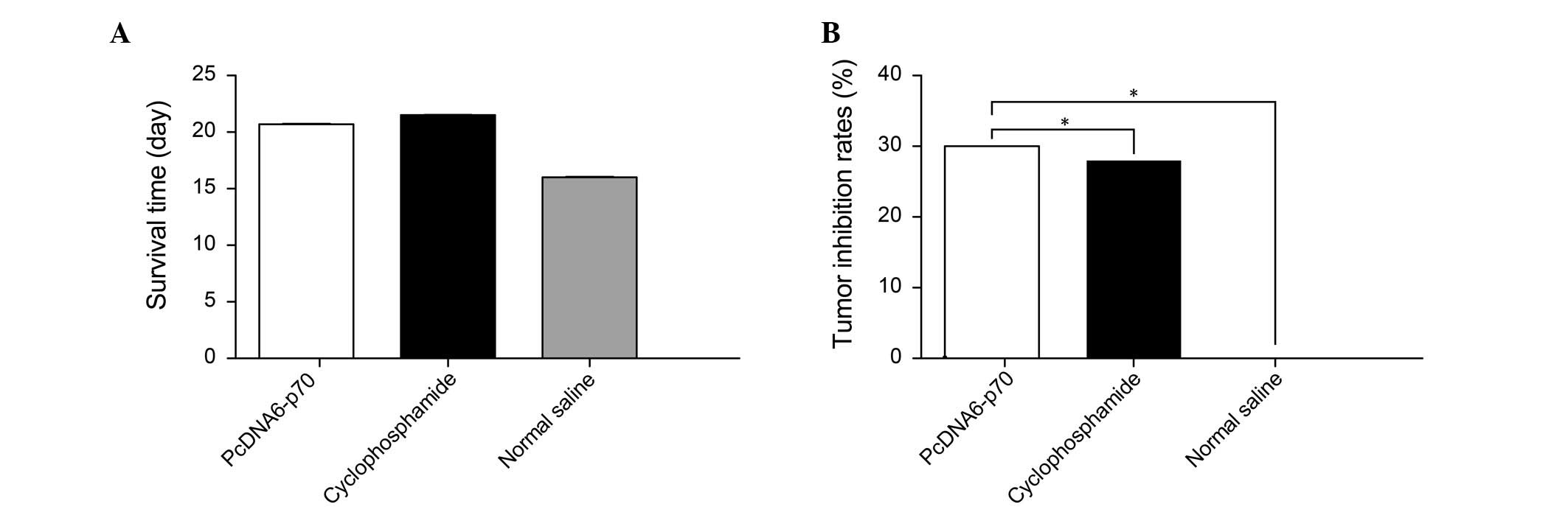
Compared with the tumor-bearing mice administered
0.9% saline, the mice treated with pcDNA6-p70 demonstrated 30%
inhibition of the growth of the transplanted tumor cells
(P<0.01) (Fig. 1). The tumors
on the mice administered pcDNA6-p70 were visibly smaller (Fig. 2), and the mice survival time was
also prolonged compared to the saline control group (Fig. 1). Notably, the inhibition of tumor
growth and prolongation of survival time observed with pcDNA6-p70
was similar to that observed with administration of the clinical
chemotherapeutic agent cyclophosphamide.
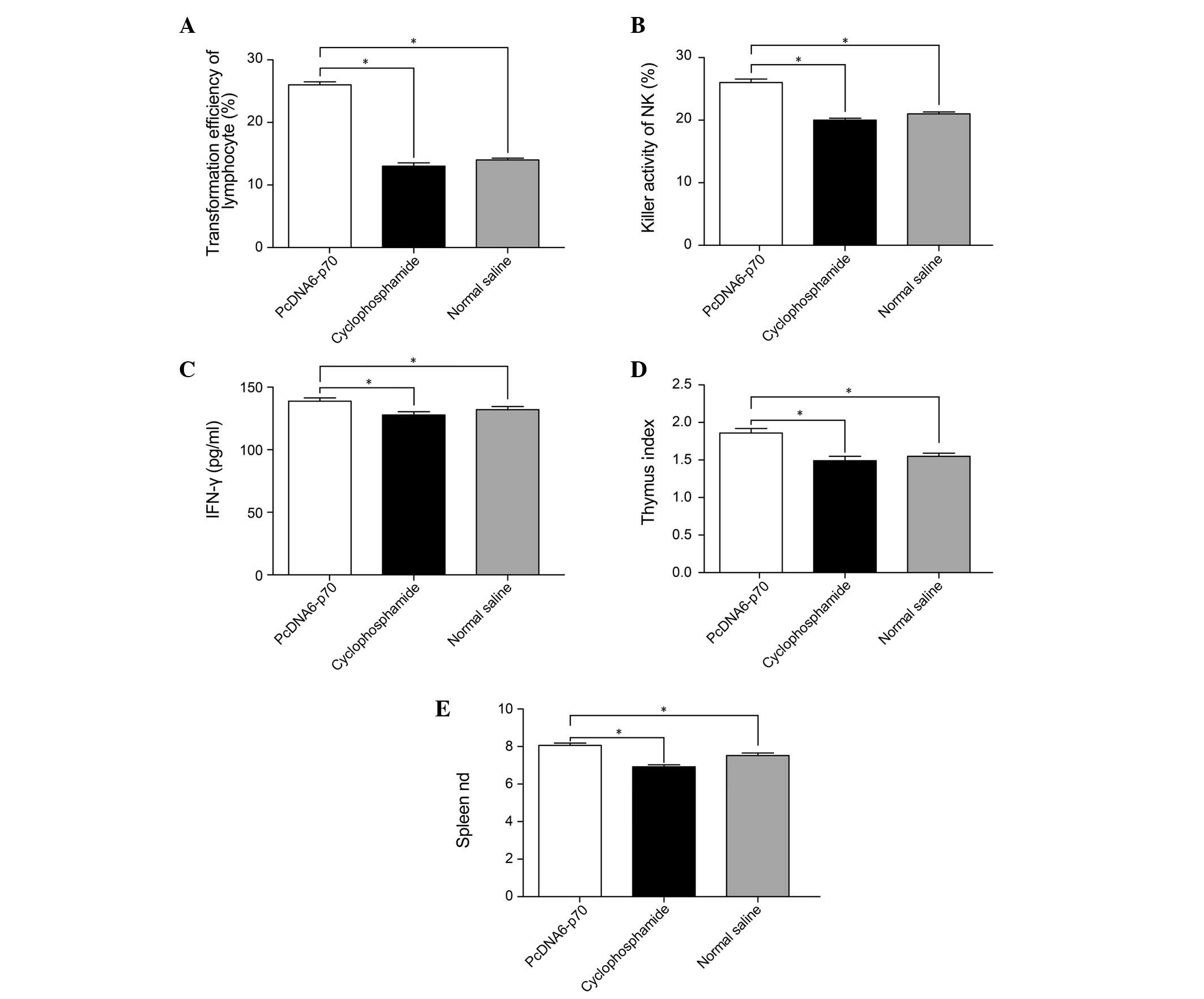
Effects of pcDNA6-p70 on the immune
function of tumor-bearing mice
Measurements of the relative size of the spleen and
thymus, the number of lymphocytes, the activity of NK cells and the
IFN-γ content of the serum in tumor-bearing mice indicated that
pcDNA6-p70 had a significant effect on immune function. Compared
with the tumor-bearing mice administered 0.9% saline, the mice
administered pcDNA6-p70 had higher spleen and thymus indices, more
proliferative lymphocytes, higher NK cell activity and increased
IFN-γ serum content (P<0.01) (Fig.
2). These results varied from the effects of cyclophosphamide
where lymphocyte proliferation was significantly lower compared
with the mice treated with pcDNA6-p70 as in the case of IFN-γ serum
content and the mouse spleen and thymus indices (P<0.05)
(Fig. 3).
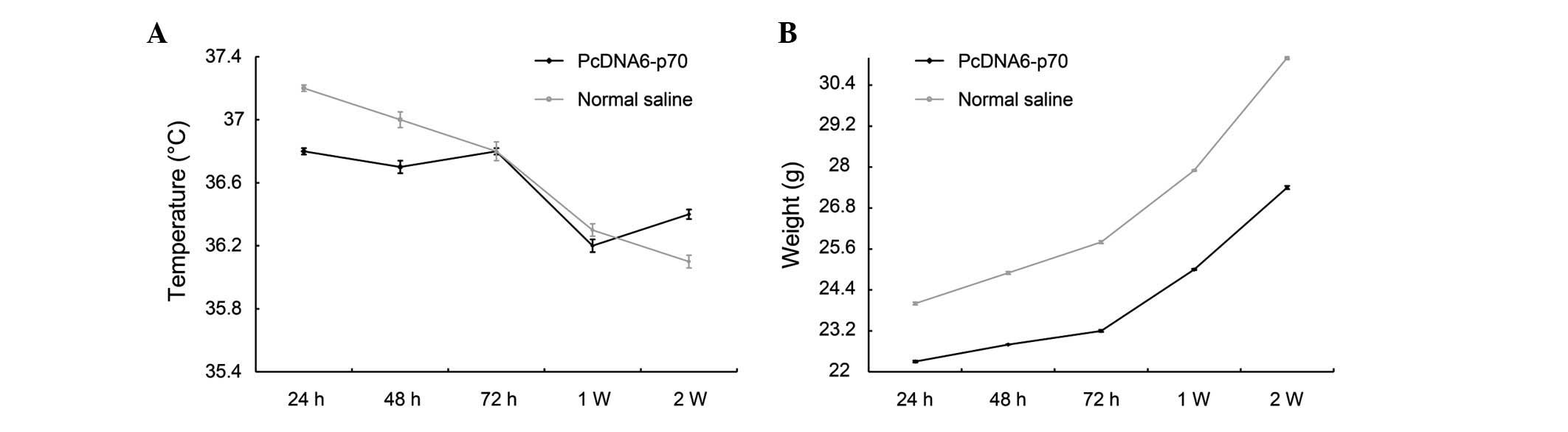
Safety of pcDNA6-p70 in normal mice
The only adverse reaction observed following the
administration of pcDNA6-p70 was the development of anorexia in a
few mice during the first 3–5 days following injection. This effect
quickly disappeared and the mean body temperature and weight
demonstrated no significant deviation or differences between the
mice administered pcDNA6-p70 and the mice administered 0.9% saline
(Fig. 4).
Discussion
Although the mechanisms of tumor development are
complicated, malfunction of immune surveillance is considered to be
one of the most significant factors in the process (13,14).
This suggests that a key approach in antitumor therapy should
reinforce the ability of the immune system to recognize tumor
antigens as ‘foreign’ and the enhancement of the activity of
specific cellular immunity. A number of immune cells types play a
role in the correlation between tumor development and immune
function. The first line of antitumor defense is the non-specific
NK cells, which are followed by more specific T cells (13,14).
IL-12, secreted by antigen-presenting cells (e.g., monocytes,
macrophages and dendritic cells), is an important antitumor and
immune regulatory factor (15),
which has several antitumor effects. IL-12 stimulates the
differentiation and proliferation of T cells and NK cells,
strengthens the cytotoxicity of cytotoxic T lymphocytes, NK cells
and macrophages, and induces the secretion of IFN-γ (16). The induction of the proliferation
of type I T helper (Th1) cells by IL-12 results in the secretion of
cytokines, including IL-12 and IFN-γ, and a consequent increase in
the expression of MHC (17).
However, the anti-tumor effect of IL-12 is mostly determined by the
secondary generation of IFN-γ and administration of an antibody
against IFN-γ blocks the antitumor effects of IL-12 (16). The secretion of IFN-γ produces
antitumor effects through various mechanisms, including stimulation
of the cytotoxic cytokine TNF-β, proliferation of lymphocytes,
induction of nitrous oxide and suppression of the growth of blood
vessels within tumors (18). The
induction of NK cells and cytotoxic T cells by IL-12 also results
in the production of a large quantity of IFN-γ (18,19).
Various experiments have indicated that any one of
these mechanisms alone can have specific antitumor activity. For
example, IL-12 has been demonstrated to inhibit the growth of
tumors in a mouse model where NK cells are inactive. By contrast,
the antitumor effect of IL-12 was lower in a nude mouse model
indicating that T cells are involved in the anti-tumor mechanism
(19). In addition to
lymphocyte-mediated antitumor mechanisms, IL-12 also activates
non-lymphocyte pathways, including those regulated by IP-10, an
important chemokine that exerts an antitumor effect through
inhibition of tumor vascularization (20). Injection of adenovirus expressing
IL-12 (Adcmv-IL-12) into mice carrying RenCa tumors resulted in the
cell infiltration of macrophages and neutrophilic granulocytes into
the tissues surrounding the tumor blood vessels (8). Similarly, in a tumor model lacking
CD4+, CD8+ or NK cells, there was
infiltration of numerous non-lymphocytes and activation of Kupffer
cells, indicating that the antitumor effect of non-lymphocytes is
correlated with the activation of IP-10 (20). Thus, the antitumor effect of IL-12
is exerted by direct and indirect actions of lymphocyte and
non-lymphocyte components of the host immune system.
In this study, it was demonstrated that a number of
the antitumor effects of endogenous IL-12 can be replicated with
the administration of pcDNA6-p70 in tumor-bearing mice. pcDNA6-p70
increases the weight of the spleen and thymus, and also increases
the level of IFN-γ. It also promotes the activation of cytotoxic
lymphocytes, stimulates the secretion of silent or activated
peripheral T cells, promotes mouse spleen cell proliferation and
activity of NK cells. From these findings, it can be concluded that
pcDNA6-p70 controls the growth of tumors and kills tumor cells by
regulating the immune system and stimulating T and NK cells. In the
cyclophosphamide control group, although cyclophosphamide extended
the survival time of the mice, the immune function was slightly
decreased, as expected. Cyclophosphamide is a widely used antitumor
drug. Its antitumor effects are mediated by its activated form,
phosphamide chlormethine, which is produced through hydrolysis by
excess phosphatase in the liver or tumor in vivo.
Cyclophosphamide is able to inhibit various types of tumor, but is
particularly effective in malignant lymphoma, acute or chronic
lymphocytic leukemia and multiple myeloma. However, the toxic
side-effects of cyclophosphamide are evident in clinical treatment
and include moderate to severe immunosuppression. Other common
side-effects include gastrointestinal reaction, inhibition of bone
marrow, alopecia and sterile cystitis (21). The findings from this study suggest
that recombinant IL-12 is able to exert a greater antitumor effect
by activating endogenous antitumor pathways compared with the
exogenous effects of chemotherapeutics, such as cyclophophamide,
and that the use of pcDNA6-p70 is capable of avoiding the
side-effects of chemotherapeutics. Numerous studies in animals have
demonstrated that recombinant IL-12 has a greater therapeutic
effect in over 20 types of tumors, with the greatest effect in lung
neoplasms and lymph neoplasms (8–12).
Translation of this antitumor activity into patients, however, is
limited by the side-effects of the direct application of
recombinant IL-12.
In this study, we also demonstrated that the direct
injection of pcDNA6-p70 produced a high and continuous local
cytokine density in the tumor, compared to a low density in the
blood. This type of action corresponds with the physiological
functions of autocrine and paracrine cytokines. The high density of
cytokines in the local environment can increase antigen expression
(including MHC) in the tumor cells and recruit immunocytes
(including T cells, B cells and NK cells). Furthermore, it can
induce other cytokines. All these factors reinforce the immune
functions in different ways, thereby effectively enhancing the
antitumor immunity.
Due to the side-effects caused by the direct use of
IL-12, research into gene therapy using a carrier system combined
with recombinant IL-12 is a promising treatment modality. In this
study, normal mice treated with pcDNA6-p70 demonstrated no
significant adverse effects compared to control mice treated with
0.9% saline. This included no significant differences in
temperature, weight or general state of health (including appetite,
fur color, response to stimulation and locomotor activity).
Similarly, the safety of plasmids bearing recombinant IL-12 has
also been revealed by other studies. Wolff et al
demonstrated that there was no integration of recombinant plasmids
into the host genome by screening more than 1800 plasmids after
Escherichia coli containing recombinant plasmid DNA were
injected into mice. The methylating pattern of the plasmid DNA in
the injected Escherichia coli remained the same for 19
months in the muscles of mice, indicating that there was no plasmid
replication (22). Similarly, a
study by Jiao et al in primates found that there was no
anti-DNA detected even after reinjection (23). Imboden et al also reported
that there were no abnormalities in organ histology and serum
biochemical markers in mice administered the recombinant plasmid
(pNGVL-3-mIL12) at a dose of 0.5 to 5 μg. Levels of serum IFN-γ
were also normal, demonstrating that recombinant plasmids
containing IL-12 are safe in vivo and could be used in gene
therapy (24).
In conclusion, results of this study have shown that
the in vivo delivery of recombinant IL-12 using a plasmid
vector (pcDNA6-p70) has a significant antitumor effect, and with
further development and testing may be useful in the clinic.
Abbreviations:
|
MTT
|
methyl thiazolyl tetrazolium
|
|
NBT
|
nitroblue tetrazolium
|
|
LDH
|
lactate dehydrogenase
|
|
DMSO
|
dimethyl sulfoxide
|
|
NK
|
natural killer
|
|
NAD+
|
nicotinamide adenine dinucleotide
|
|
DAB
|
diaminobenzidine
|
|
MHC
|
major histocompatibility complex
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tepper RI, Pattengale PK and Leder P:
Murine interleukin-4 displays potent anti-tumor activity in
vivo. Cell. 57:503–512. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iinuma H, Okinaga K, Fukushima R, et al:
Superior protective and therapeutic effects of IL-12 and IL-18
gene-transduced dendritic neuroblastoma fusion cells on liver
metastasis of murine neuroblastoma. J Immunol. 176:3461–3469. 2006.
View Article : Google Scholar
|
|
4
|
Siddiqui F, Li CY, Larue SM, et al: A
phase I trial of hyperthermia-induced interleukin-12 gene therapy
in spontaneously arising feline soft tissue sarcomas. Mol Cancer
Ther. 6:380–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coca S, Enrech S, Moreno Garcia V, et al:
Evaluation of the antitumor activity of interleukin-12 in an
experimental murine model of colorectal cancer induced by 1,2
dimethyl-hydrazine (DMH). Rev Esp Enferm Dig. 97:619–628. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hill HC, Conway TF Jr, Sabel MS, et al:
Cancer immunotherapy with interleukin 12 and granulocyte-macrophage
colony-stimulating factor-encapsulated microspheres: coinduction of
innate and adaptive antitumor immunity and cure of disseminated
disease. Cancer Res. 62:7254–7263. 2002.
|
|
7
|
Wolff JA, Malone RW, Williams P, et al:
Direct gene transfer into mouse muscle in vivo. Science.
247:1465–1468. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang KS, Cho WK, Yoo J, Yun HJ, Kim S and
Im DS: Adenovirus-mediated interleukin-12 gene transfer combined
with cytosine deaminase followed by 5-fluorocytosine treatment
exerts potent antitumor activity in Renca tumor-bearing mice. BMC
Cancer. 5:512005. View Article : Google Scholar
|
|
9
|
Shi F, Rakhmilevich AL, Heise CP, et al:
Intratumoral injection of interleukin-12 plasmid DNA, either naked
or in complex with cationic lipid, results in similar tumor
regression in a murine model. Mol Cancer Ther. 1:949–957.
2002.PubMed/NCBI
|
|
10
|
Sangro B, Mazzolini G, Ruiz J, et al:
Phase I trial of intratumoral injection of an adenovirus encoding
interleukin-12 for advanced digestive tumors. J Clin Oncol.
22:1389–1397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keke F, Hongyang Z, Hui Q, Jixiao L and
Jian C: A combination of flk1-based DNA vaccine and an
immunomodulatory gene (IL-12) in the treatment of murine cancer.
Cancer Biother Radiopharm. 19:649–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang WQ, Wang LN and Liu ZJ: Functional
evaluation of recombinant human IL-12 ex vivo with cytokine follow
cytometry (CFC). Chin J Micobiol Immunol. 26:383–384. 2006.
|
|
13
|
Wagner HJ, Bollard CM, Vigouroux S, et al:
A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s
disease by targeting interleukin 12 to the tumor environment using
tumor antigen-specific T cells. Cancer Gene Ther. 11:81–91.
2004.
|
|
14
|
Wang LL and Wu JM: Clinical Laboratory
Immunology. The People’s Medical Publishing House; Beijing: pp.
369–370. 2008
|
|
15
|
Duan X, Jia SF, Koshkina N and Kleinerman
ES: Intranasal interleukin-12 gene therapy enhanced the activity of
ifosfamide against osteosarcoma lung metastases. Cancer.
106:1382–1388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaime-Ramirez AC, Mundy-Bosse BL,
Kondadasula S, et al: IL-12 enhances the antitumor actions of
trastuzumab via NK cell IFN-gamma production. J Immunol.
186:3401–3409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tatsumi T, Huang J, Gooding WE, et al:
Intratumoral delivery of dendritic cells engineered to secrete both
interleukin (IL)-12 and IL-18 effectively treats local and distant
disease in association with broadly reactive Tc1-type immunity.
Cancer Res. 63:6378–6386. 2003.
|
|
18
|
Segal JG, Lee NC, Tsung YL, Norton JA and
Tsung K: The role of IFN-gamma in rejection of established tumors
by IL-12 : source of production and target. Cancer Res.
62:4696–4703. 2002.PubMed/NCBI
|
|
19
|
Lode HN, Dreier T, Xiang R, Varki NM, Kang
AS and Reisfeld RA: Gene therapy with a single chain interleukin 12
fusion protein induces T cell-dependent protective immunity in a
syngeneic model of murine neuroblastoma. Proc Natl Acad Sci USA.
95:2475–2480. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Z, Baguley BC and Ching LM:
Interferon-inducible protein 10 induction and inhibition of
angiogenesis in vivo by the antitumor agent
5,6-dimethylxanthenone-4-acetic acid (DMXAA). Cancer Res.
61:1517–1521. 2001.PubMed/NCBI
|
|
21
|
Yang SHJ: Pharmacology. The People’s
Medical Publishing House; Beijing: pp. 5012005
|
|
22
|
Wolff JA, Dowty ME, Jiao S, et al:
Expression of naked plasmids by cultured myotubes and entry of
plasmids into T tubules and caveolae of mammalian skeletal muscle.
J Cell Sci. 103:1249–1259. 1992.PubMed/NCBI
|
|
23
|
Jiao H, Soejima Y, Ohe Y, Miura K, Tamura
T and Saijo N: Differential macrophage-mediated cytotoxicity to
P388 leukemia cells and its drug-resistant cells examined by a new
MTT assay. Leuk Res. 16:1175–1180. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imboden M, Shi F, Pugh TD, et al: Safety
of interleukin-12 gene therapy against cancer: a murine
biodistribution and toxicity study. Hum Gene Ther. 14:1037–1048.
2003. View Article : Google Scholar : PubMed/NCBI
|