Introduction
Percutaneous coronary intervention (PCI), also
termed angioplasty, is a safe and effective way to unblock coronary
arteries. During this procedure, a catheter is inserted into the
groin or arm of the patient and guided forward through the aorta
and into the coronary arteries of the heart where stenosed or
blocked arteries can be opened with a balloon positioned at the tip
of the catheter. Initially, angioplasty was performed only with
balloon catheters, but technical advances have been made and an
improved patient outcome has been achieved with the placement of
small metallic spring-like devices called ‘stents’ at the site of
the lesion. The implanted stent serves as a scaffold that keeps the
artery open. There are, however, limitations associated with
angioplasty and stenting, one of which is known as ‘restenosis’,
which is defined as a recurrence of significant narrowing in the
treated vessel (1).
Asymmetric dimethylarginine (ADMA), an endogenous
competitive inhibitor of nitric oxide synthase, increases the risk
of cardiovascular disease. ADMA inhibits vascular nitric oxide (NO)
production at concentrations found in pathophysiological
conditions. It also causes local vasoconstriction when infused
intra-arterially. ADMA is increased in the plasma of humans with
hypercholesterolemia, atherosclerosis, hypertension, chronic renal
failure, chronic heart failure and several other disorders. In
several prospective and cross-sectional studies, ADMA has evolved
as a marker of cardiovascular risk (2). It remains uncertain whether
elevations of ADMA levels may play an important pathophysiological
role in restenosis that may occur following stent implantation.
The fibrinolytic system is closely correlated with
several processes that are involved in restenosis.
Thrombin-activatable fibrinolysis inhibitor (TAFI) is a type of
fibrinolysis inhibitor that circulates as procarboxypeptidase B2
zymogen, which is converted to active form TAFIa during
coagulation. TAFI activation is catalyzed by plasmin, trypsin and
the thrombin-thrombomodulin complex. Activated TAFI suppresses
fibrinolysis through cleavage of carboxy terminal lysine residues
that are expressed during proteolysis of the fibrin polymers, which
are the binding sites of plasminogen (3).
The aim of this study was to assess the contribution
of ADMA, a novel marker of vascular endothelial dysfunction and
atherosclerosis, and TAFI, a risk factor for venous thrombosis, to
the predisposition of coronary restenosis following stent
implantation in cardiovascular patients.
Materials and methods
Patients
In total, 37 patients with coronary artery disease
(CAD) were recruited from the Department of Cardiology at Kobry El
Obba Military Hospital, Cairo, Egypt. The patients were
hospitalized for elective coronary angiography and PCI, if
necessary. Coronary angiography was performed as a consequence of
suggested or proven coronary disease by non-invasive techniques.
The study protocol was approved by the Local Ethics Committee of
Kobry El-Obba Military Hospital in Cairo. Full informed consent was
obtained from all patients prior to participation in the study.
Exclusion criteria included any concomitant acute or chronic severe
diseases such as kidney failure, hepatic insufficiency, acute
inflammatory conditions or autoimmune diseases. The routine blood
chemistry parameters for risk assessment of ischemic heart disease
were performed for each patient on the day of hospitalization.
During PCI, bare metal stents (BMS) and/or
drug-eluting stents (DES) were implanted into the coronary vessels
depending on the type of culprit coronary lesion, and the
co-morbidity of the patients. In total, 15 patients were treated
with 20 BMS, and 13 patients were treated with 18 DES, and 9
patients were treated with both 13 BMS and 14 DES. ADMA readings of
95 healthy subjects were obtained from the results of a previous
study conducted in our laboratory (4).
Sampling and storage
Prior to PCI or elective coronary angiography,
overnight fasting blood samples were collected from the patients in
order to determine ADMA and TAFI plasma levels. All the patients
were undergoing Plavix (clopidogrel) treatment and samples were
obtained prior to anticoagulant (heparin) injection. Samples were
collected in vacutainer tubes containing Na2EDTA. Plasma
samples were separated by centrifugation at 3000 rpm for 15 min.
Aliquots of collected plasma were stored at −70°C until analysis of
ADMA and TAFI was undertaken.
Prior to PCI, combined antithrombotic treatment was
administered to each patient, i.e., 300–600 mg clopidogrel and 60
IU/kg sodium-heparin. Invasive examinations (coronary and
angiography) were performed using a Philips BH 5000 monoplane or
biplane system. Recordings were subsequently analyzed by two
well-trained invasive cardiologists using Philips QCA software.
Semi-qualitative analyses were performed
independently by two experienced interventional cardiologists. The
location of the restenotic lesions with respect to the stented
segment was classified as being within the stent body or at the
proximal or distal margin of the stent. Stenosis within the stent
or at the margins was further classified as being either diffuse or
focal.
As part of a routine follow-up program to detect
restenosis 4 months following successful CS, patients underwent
follow-up diagnostic coronary angiography. Fasting blood samples
were collected from the patients on EDTA prior to the angiographic
follow up and centrifuged at 3000 rpm for 20 min. The plasma
aliquots were then stored at −70°C until analysis of ADMA and
TAFI.
Determination of ADMA and TAFI in plasma
samples
Quantitative determination of ADMA and TAFI in
plasma was performed by the ELISA technique using an
ADMA® - ELISA kit supplied by DLD Diagnostika GmbH
(Hamburg, Germany) and IMUBIND® TAFIa/ai Antigen ELISA
kit supplied by American Diagnostica Inc. (ADI) (Stamford, CT,
USA).
Statistical analysis
Statistical analysis was performed using the
statistical package for social sciences (SPSS, Chicago, IL, USA)
version 19 and GraphPad Prism version 5.5. Data were presented as
the mean values ± SEM. P<0.05 was considered to indicate a
statistically significant difference. Categorical variables were
analyzed using the Pearson’s Chi-square test, whereas continuous
variables were evaluated using the Student’s t-test to compare
coronary risk parameters of the stent group prior to CS and during
follow-up coronary angiography. One-way ANOVA tests were performed
for comparison among the different groups.
Results
Patient characteristics
Table I shows the
clinical, angiographic and procedural characteristics of the
studied patients. It also shows that 37.8% of patients had no
in-stent restenosis four months following stent placement. However,
the remaining patients (62.2%) were subjected to in-stent
restenosis either with <50% of artery diameter (27.0% of
patients) or >50% (35.2% of patients).
 | Table IClinical, angiographic and procedural
characteristics of the studied groups. |
Table I
Clinical, angiographic and procedural
characteristics of the studied groups.
| Characteristics | |
|---|
| Age (years), mean ±
SEM | 55.3±1.45 |
| Total no. of
patients, n | 37 |
| Patients with
angiographic follow up, n (%) | 29 (78.4) |
| Patients with
multislice CT scan follow up, n (%) | 8 (21.6) |
| Diabetic patients, n
(%) | 12 (32.4) |
| Non-diabetic
patients, n (%) | 25 (67.6) |
| Hypertensive, n
(%) | 20 (54) |
| Non-hypertensive, n
(%) | 17 (45.95) |
| Patients with bare
metal stent (BMS), n | 24 |
| Patients with
drug-eluting stent (DES), n | 22 |
| Smokers, n |
| Current smokers | 8 |
| Quit around 5 months
before PCI | 19 |
| Non-smokers, n | 10 |
| Patients with patent
stents, n | 14 |
| Patients with
in-stent restenosis (<50% of artery diameter), n | 10 |
| Patients with
in-stent restenosis (>50% of artery diameter), n | 13 |
Coronary stenting and plasma ADMA
levels
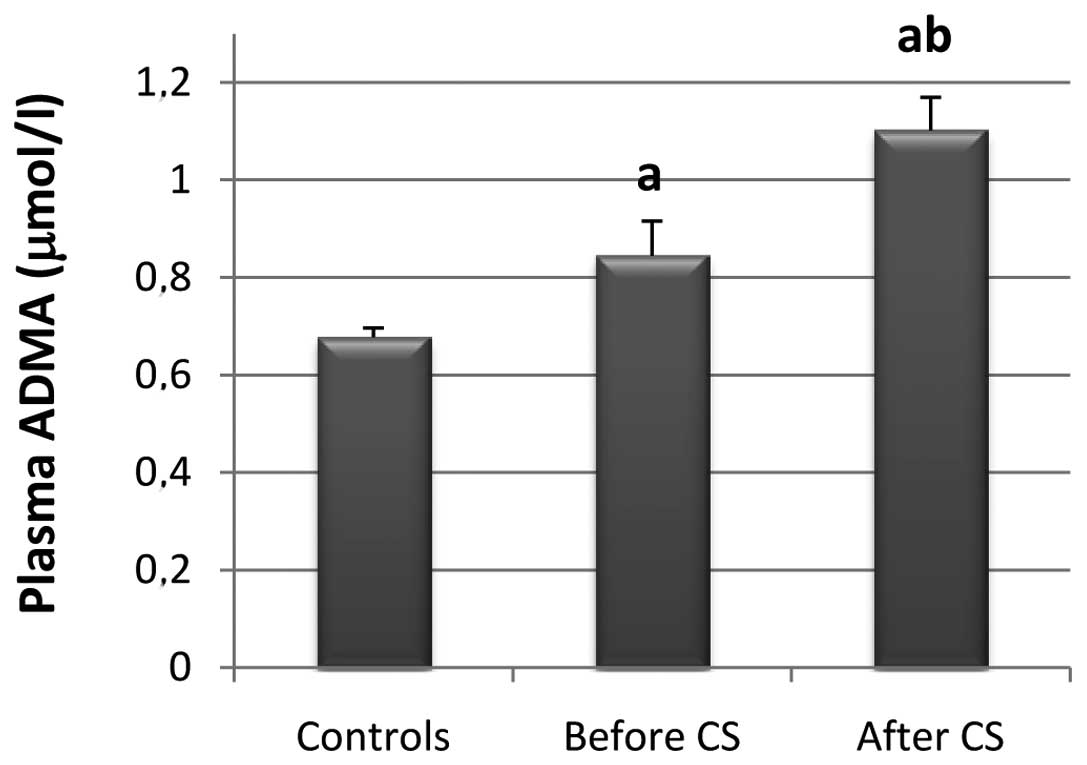
Results showed that the mean plasma ADMA level was
significantly higher by 19.73% in CAD patients (n=37) as compared
with the healthy control subjects (n=95; P=0.0021) (Fig. 1). In addition, in CAD patients the
ADMA levels were significantly higher by 23.33% following CS as
compared to those prior to CS (P=0.0123).
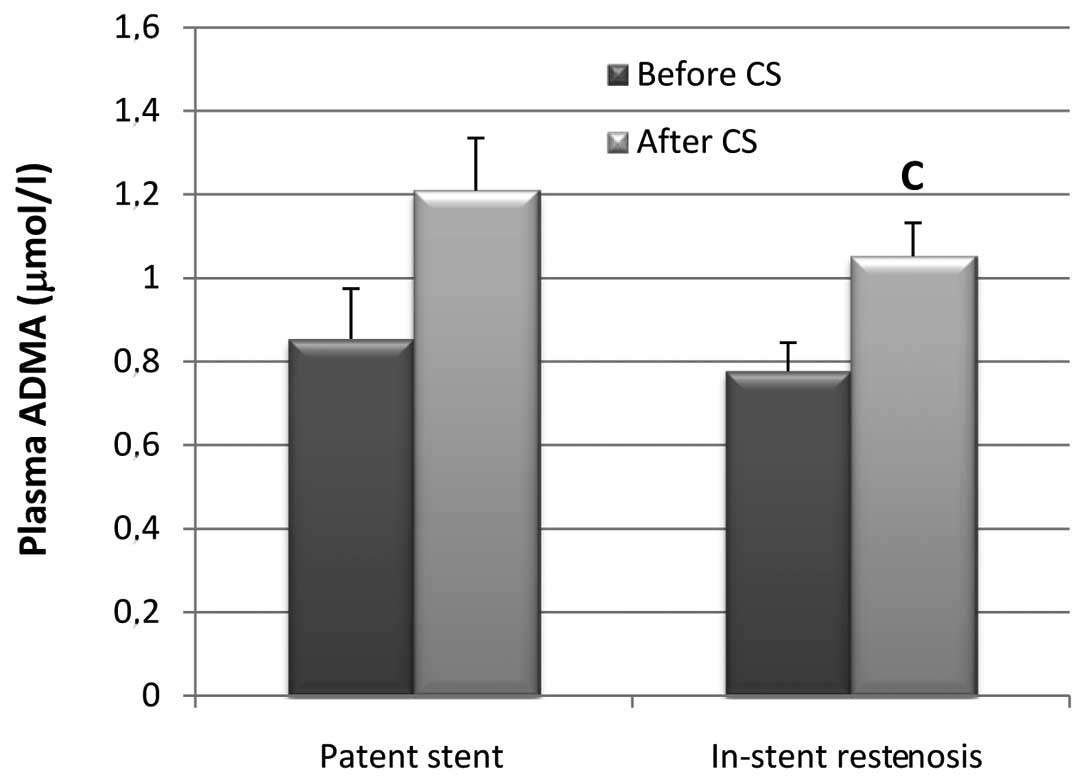
In-stent restenosis and plasma ADMA
levels
In the 14 CAD patients with patent stents, ADMA
levels prior to CS were not significantly different from those
levels after CS (P=0.0535). However, in all CAD patients with
in-stent restenosis (n=23), ADMA levels after CS were found to be
significantly higher by 26.16% than those levels before CS
(P=0.0102) (Fig. 2).
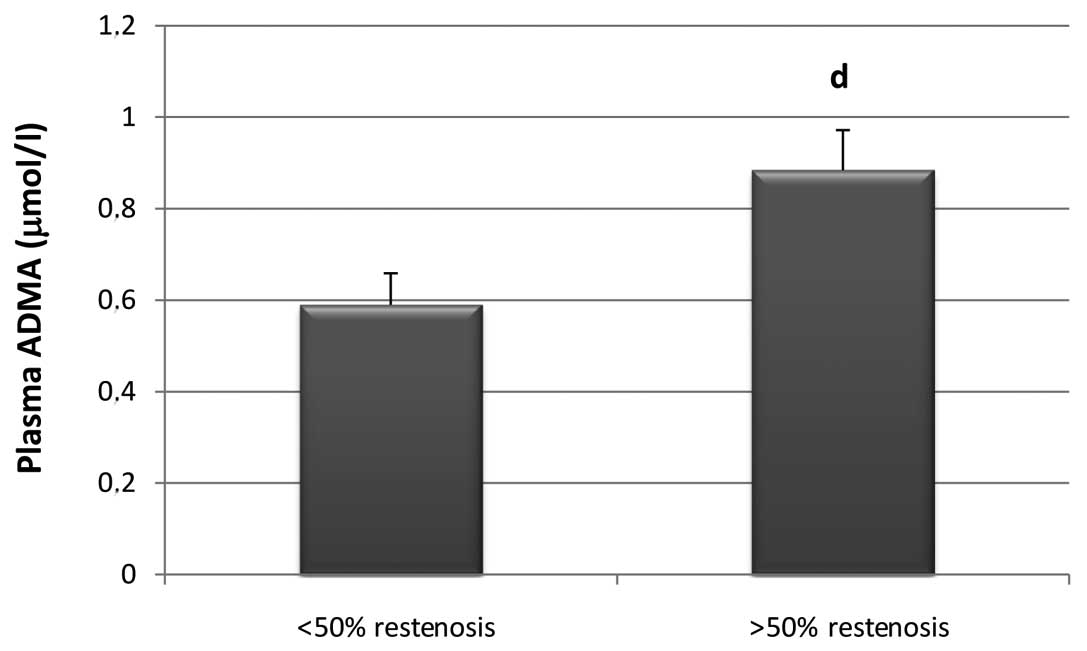
It was also revealed that plasma ADMA levels prior
to CS in patients with >50% in-stent restenosis were
significantly higher by 32.3% than ADMA levels prior to CS in
patients with <50% in-stent restenosis (P=0.0327) (Fig. 3).
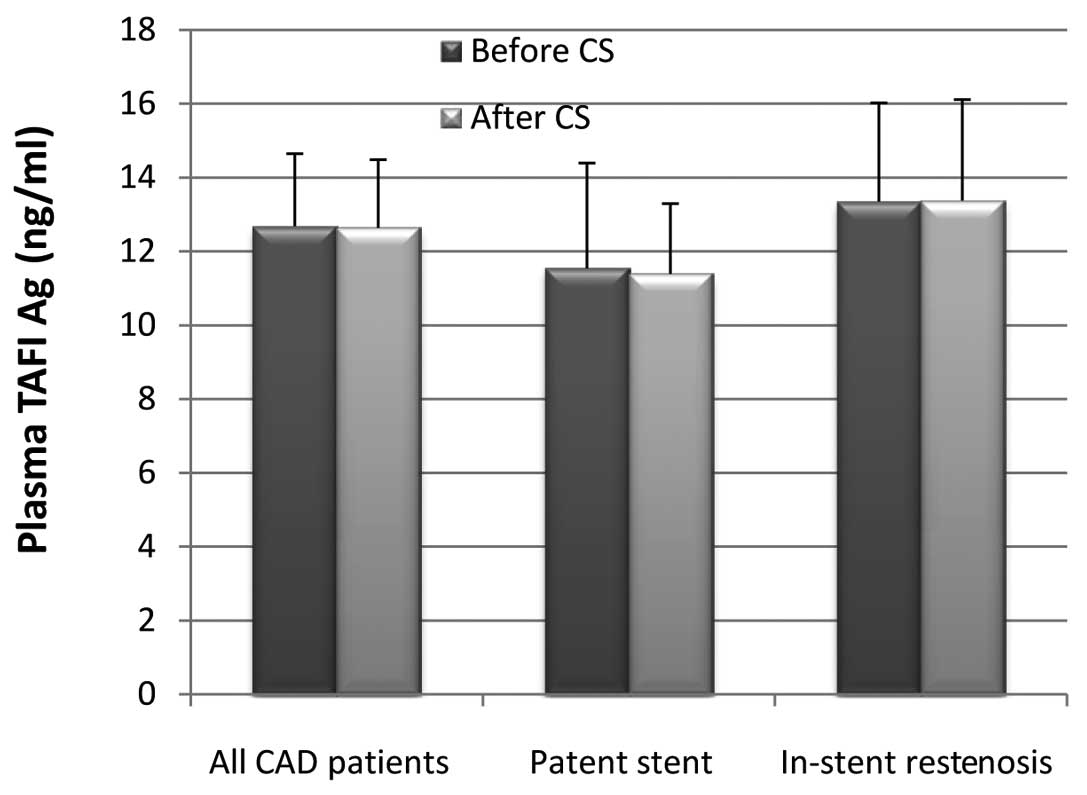
Coronary stenting and plasma TAFI
levels
It is of note that in all 37 CAD patients, whether
with patent stent or in-stent restenosis, or pre- or post-CS, the
plasma TAFI levels did not significantly change (Fig. 4).
Discussion
NO secreted by the endothelium is important in
ensuring cardiac homeostasis. NO provides basal tonus by relaxing
vascular smooth muscle cells (5).
It inhibits the adhesion of leukocytes and thrombocytes, as well as
the activation and aggregation of the latter. Additionally, NO
inhibits the formation of superoxide radicals, oxidation of
low-density lipoproteins, and the migration and proliferation of
smooth muscle cells (6–8). Decreased levels of NO initiate
atherosclerosis and lead to its exacerbation (9,10).
Decreased NO activity, also thought to be one of the factors
responsible for restenosis, develops following arterial injury
(11). Restenosis following
balloon angioplasty is a consequence of neointimal hyperplasia and
vessel remodeling. NO inhibits neointimal hyperplasia.
ADMA is a substance that inhibits NO synthesis by
blocking the NOS enzyme. High concentrations of plasma ADMA may be
used to predict the severity of coronary atherosclerosis and the
development of CAD (12).
Increased ADMA concentration produces elevation in aortic and
arterial blood pressure. Elevated blood pressure is associated with
the presence of coronary artery narrowing (13). A multicentered, case-control
CARDIAC (Coronary Artery Risk Determination investigating the
effect of ADMA Concentration) study revealed that every 1 μmol/l
increase in plasma ADMA concentration led to a 2.35-fold increase
in the risk of CAD (14). Our
study supports previous reports and demonstrates the significant
elevation of mean plasma ADMA levels (P=0.0021) in CAD patients as
compared to control healthy subjects. The observation that ADMA
levels increase early in the development of atherosclerosis
suggests that ADMA has the potential to be both a marker and a
mediator of vascular lesions (15).
Sahinarslan et al(16) investigated the correlation of
plasma ADMA concentration with lesion distribution and severity at
coronary artery angiography. Those authors enrolled patients with
stable angina and divided them into two groups. Group I included
the patients with normal coronary arteries. All other patients were
included in group II. Results of that study showed that ADMA levels
were higher in group II compared to group I, and the
L-arginine/ADMA ratio was lower in group II patients compared to
group I patients. ADMA positively correlated with the coronary
atherosclerotic score. Moreover, ADMA was an important predictor of
angiographically defined CAD. Thus, those authors (16) supported the hypothesis that plasma
ADMA concentration is a good predictor of CAD.
Our results showed that ADMA levels were
significantly higher (P=0.0123) by 23.33% in CAD patients (n=37)
four months after CS as compared to levels prior to stenting. This
finding may be attributed to the increased ADMA levels after
myocardial ischemia-reperfusion injury (17). This study also revealed a
significant increase of 26.16% in plasma ADMA levels following CS
in all CAD patients with in-stent restenosis. This observation was
not valid for patients with patent stent, which may prove a
positive correlation between increased ADMA levels and the
incidence of cardiovascular events and in-stent restenosis. In
contrast to our findings, Ajtay et al(18) demonstrated that in patients with
CAD, PCI stent placement markedly decreased the plasma level of
ADMA. Coronary angiography alone resulted in an increase of
ADMA.
Krempl et al(19) found that ADMA is significantly
elevated in patients with unstable angina. A reduced ADMA level at
6 weeks after PCI may indicate a decreased risk of recurrent
cardiovascular events. In their study, Krempl et al(19) investigated the role of ADMA in the
clinical outcome of patients with unstable angina. Their results
showed that baseline ADMA concentration in controls was
significantly lower than that in patients with CAD. Additionally,
patients with unstable angina had significantly higher baseline
ADMA levels than patients with stable angina and there was a
significant reduction in ADMA levels at 6 weeks after PCI in
patients with unstable angina who experienced no recurrent
cardiovascular event. By contrast, those authors found that in
patients who experienced another acute event following CS, ADMA
concentrations remained elevated 6 weeks after PCI.
Results of multivariate analysis indicated that
plasma ADMA independently almost tripled the risk for recurrent
symptomatic stenosis of an arteriovenous fistula (AVF) following
percutaneous transluminal angioplasty. These results suggest the
involvement of ADMA in the progression of symptomatic restenosis of
AVFs after PCI and require preventive strategies that target ADMA
and/or endothelial dysfunction to decrease the risk for AVF
restenosis (20).
TAFI is a recently described inhibitor of
fibrinolysis which is involved in the regulation of the balance
between coagulation and fibrinolysis. High TAFI plasma levels may
therefore contribute to a hypo-fibrinolytic state and to an
increased risk of thrombotic disorders. There are contradictory
results regarding TAFI levels in CAD patients, possibly due to the
differences in the characteristics of patients and the time of
blood sampling among different studies (21).
The fibrinolytic system is closely correlated with
several processes that are involved in restenosis. In the study by
Lau et al(3), it was found
that high pre-procedural plasma levels of TAFI antigen increased
the risk of restenosis following PCI. Animal studies have shown
that inhibition of the early initiators of the coagulation pathway,
such as factor VII and tissue factor, decrease late neointimal
hyperplasia (22). Therefore, a
potential target for the inhibition of restenosis is to limit early
thrombosis. High levels of TAFI have been associated with deep
venous thrombosis (23) and CAD
(24). Increased TAFI levels were
found to be a risk factor for the development of angina pectoris
among apparently healthy males (25). A high TAFI level was associated
with a 2-fold increase in the risk of recurrence of venous
thrombosis in comparison with patients with lower TAFI levels
(26). Contradictory results have
been reported for the role of TAFI in myocardial infarction.
Previous studies have shown that patients with a recent myocardial
infarction presented lower values of TAFI antigen while elevated
TAFI levels were actually protective against myocardial infarction
(27,28).
As the list of substrates for TAFI grows, it becomes
clear that besides the regulation of fibrinolysis, TAFI is likely
to play a potentially important role in processes such as blood
pressure regulation, inflammation and wound healing. Different
studies markedly suggest that besides being important in the
regulation of fibrinolysis, TAFI may also have an important
function in the regulation of inflammation. High TAFI levels are
protective against arterial thrombosis by inactivation of
inflammatory mediators such as bradykinin and C5a (29).
Paola Cellai et al(21) measured plasma TAFI activity and
antigen levels in 44 patients admitted to the Coronary Care Unit
and in a group of 44 healthy controls, matched for age and gender,
to detect a possible association of their levels with acute CAD. No
differences were found in TAFI, either at the activity or antigen
levels, between patients and controls.
Our data revealed that the difference between TAFI
levels prior to and following CS in all CAD patients was not
significant. Moreover, our findings have shown that TAFI levels
prior to CS in CAD patients with in-stent restenosis were not
significantly different from those after CS, indicating that TAFI
does not play a role in in-stent restenosis.
In conclusion, the salient findings of our study are
as follows: i) The mean plasma level of ADMA is significantly
higher in CAD patients than that reported for healthy subjects, ii)
plasma ADMA levels are significantly higher in CAD patients four
months after CS as compared to levels prior to stenting, iii) CAD
patients who developed in-stent restenosis during angiographic
follow-up had a significant increase in ADMA levels following CS,
in contrast to patients with patent stent who did not show this
significant increase, iv) plasma ADMA levels prior to stenting in
patients with >50% in-stent restenosis were significantly higher
than those in patients who developed <50% in-stent restenosis;
v) in non-diabetic CAD patients who developed in-stent restenosis,
ADMA levels were higher by 19% after CS and vi) TAFI levels did not
significantly change following CS in CAD patients. Therefore, we
can conclude that ADMA, but not TAFI, is correlated with the
pre-disposition of in-stent restenosis following CS.
References
|
1
|
Dangas G and Kuepper F: Cardiology patient
page. Restenosis: repeat narrowing of a coronary artery: prevention
and treatment. Circulation. 105:2586–2587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boger RH: Asymmetric dimethylarginine
(ADMA) and cardiovascular disease: insights from prospective
clinical trials. Vasc Med. 10:S19–S25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau HK, Segev A, Hegele RA, Sparkes JD,
Teitel JM, et al: Thrombin-activatable fibrinolysis inhibitor
(TAFI): a novel predictor of angiographic coronary restenosis.
Thromb Haemost. 90:1187–1191. 2003.PubMed/NCBI
|
|
4
|
Gad MZ, Hassanein SI, Abdel-Maksoud SM,
Shaban GM, Abou-Aisha K, et al: Assessment of serum levels of
asymmetric dimethylarginine (ADMA), symmetric dimethylarginine
(SDMA) and L-arginine in coronary artery disease. Biomarkers.
15:746–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loscalzo J and Welch G: Nitric oxide and
its role in the cardiovascular system. Prog Cardiovasc Dis.
38:87–104. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hogg N, Kalyanaraman B, Joseph J, Struck A
and Parthasarathy S: Inhibition of low-density lipoprotein
oxidation by nitric oxide. Potential role in atherogenesis. FEBS
Lett. 334:170–174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marks DS, Vita JA, Folts JD, Keaney JF Jr,
Welch GN, et al: Inhibition of neointimal proliferation in rabbits
after vascular injury by a single treatment with a protein adduct
of nitric oxide. J Clin Invest. 96:2630–2638. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg UC and Hassid A: Nitric
oxide-generating vasodilators and 8-bromo-cyclic guanosine
monophosphate inhibit mitogenesis and proliferation of cultured rat
vascular smooth muscle cells. J Clin Invest. 83:1774–1777. 1989.
View Article : Google Scholar
|
|
9
|
Valkonen VP, Paiva H, Salonen JT, Lakka
TA, Lehtimaki T, et al: Risk of acute coronary events and serum
concentration of asymmetrical dimethylarginine. Lancet.
358:2127–2128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoccali C, Bode-Boger S, Mallamaci F,
Benedetto F, Tripepi G, et al: Plasma concentration of asymmetrical
dimethylarginine and mortality in patients with end-stage renal
disease: a prospective study. Lancet. 358:2113–2117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janero DR and Ewing JF: Nitric oxide and
postangioplasty restenosis: pathological correlates and therapeutic
potential. Free Radic Biol Med. 29:1199–1221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu TM, Ding YA, Charng MJ and Lin SJ:
Asymmetrical dimethylarginine: a novel risk factor for coronary
artery disease. Clin Cardiol. 26:458–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wykretowicz A, Metzler L, Milewska A,
Balinski M, Rutkowska A, et al: Noninvasively assessed pulsatility
of ascending aortic pressure waveform is associated with the
presence of coronary artery narrowing. Heart Vessels. 23:16–19.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schulze F, Lenzen H, Hanefeld C, Bartling
A, Osterziel KJ, et al: Asymmetric dimethylarginine is an
independent risk factor for coronary heart disease: results from
the multicenter Coronary Artery Risk Determination investigating
the Influence of ADMA Concentration (CARDIAC) study. Am Heart J.
152:493 e1–8. 2006.
|
|
15
|
Boger RH: Asymmetric dimethylarginine, an
endogenous inhibitor of nitric oxide synthase, explains the
‘L-arginine paradox’ and acts as a novel cardiovascular risk
factor. J Nutr. 134:2842S–2847S. 2004.
|
|
16
|
Sahinarslan A, Cengel A, Biberoglu G,
Hasanoglu A, Turkoglu S, et al: Plasma asymmetric dimethylarginine
level and extent of lesion at coronary angiography. Coron Artery
Dis. 17:605–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cziraki A, Ajtay Z, Nemeth A, Lenkey Z,
Sulyok E, et al: Effects of coronary revascularization with or
without cardiopulmonary bypass on plasma levels of asymmetric
dimethylarginine. Coron Artery Dis. 22:245–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ajtay Z, Scalera F, Cziraki A, Horvath I,
Papp L, et al: Stent placement in patients with coronary heart
disease decreases plasma levels of the endogenous nitric oxide
synthase inhibitor ADMA. Int J Mol Med. 23:651–657. 2009.PubMed/NCBI
|
|
19
|
Krempl TK, Maas R, Sydow K, Meinertz T,
Boger RH, et al: Elevation of asymmetric dimethylarginine in
patients with unstable angina and recurrent cardiovascular events.
Eur Heart J. 26:1846–1851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu CC, Wen SC, Yang CW, Pu SY, Tsai KC, et
al: Plasma ADMA predicts restenosis of arteriovenous fistula. J Am
Soc Nephrol. 20:213–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paola Cellai A, Antonucci E, Alessandrello
Liotta A, Fedi S, Marcucci R, et al: TAFI activity and antigen
plasma levels are not increased in acute coronary artery disease
patients admitted to a coronary care unit. Thromb Res. 118:495–500.
2006.PubMed/NCBI
|
|
22
|
Jang Y, Guzman LA, Lincoff AM,
Gottsauner-Wolf M, Forudi F, et al: Influence of blockade at
specific levels of the coagulation cascade on restenosis in a
rabbit atherosclerotic femoral artery injury model. Circulation.
92:3041–3050. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Tilburg NH, Rosendaal FR and Bertina
RM: Thrombin activatable fibrinolysis inhibitor and the risk for
deep vein thrombosis. Blood. 95:2855–2859. 2000.PubMed/NCBI
|
|
24
|
Silveira A, Schatteman K, Goossens F, Moor
E, Scharpe S, et al: Plasma procarboxypeptidase U in men with
symptomatic coronary artery disease. Thromb Headmost. 84:364–368.
2000.PubMed/NCBI
|
|
25
|
Morange PE, Juhan-Vague I, Scarabin PY,
Alessi MC, Luc G, et al: Association between TAFI antigen and
Ala147Thr polymorphism of the TAFI gene and the angina pectoris
incidence. The PRIME Study (Prospective Epidemiological Study of
MI). Thromb Haemost. 89:554–560. 2003.PubMed/NCBI
|
|
26
|
Eichinger S, Schonauer V, Weltermann A,
Minar E, Bialonczyk C, et al: Thrombin-activatable fibrinolysis
inhibitor and the risk for recurrent venous thromboembolism. Blood.
103:3773–3776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Juhan-Vague I, Morange PE, Aubert H, Henry
M, Aillaud MF, et al: Plasma thrombin-activatable fibrinolysis
inhibitor antigen concentration and genotype in relation to
myocardial infarction in the north and south of Europe.
Arterioscler Thromb Vasc Biol. 22:867–873. 2002. View Article : Google Scholar
|
|
28
|
Juhan-Vague I and Morange PE: Very high
TAFI antigen levels are associated with a lower risk of hard
coronary events: the PRIME Study. J Thromb Haemost. 1:2243–2244.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bouma BN and Mosnier LO: Thrombin
activatable fibrinolysis inhibitor (TAFI) at the interface between
coagulation and fibrinolysis. Pathophysiol Haemost Thromb.
33:375–381. 2003. View Article : Google Scholar : PubMed/NCBI
|