Introduction
Preeclampsia is a common pregnancy-specific syndrome
that is characterized by hypertension and proteinuria. It is a
disorder that affects at least 5% of all pregnancies worldwide
(1) and is a leading cause of
maternal and perinatal morbidity and mortality. Although the cause
of preeclampsia remains unclear, it is generally agreed that
preeclampsia results from the presence of a placenta (2) since the only treatment for
preeclampsia is the delivery of the placenta, subsequent to which
the symptoms regress rapidly. Previous studies in our laboratory
have demonstrated that miR-18a was downregulated in preeclamptic
placentas (3). miR-18a is a
component of the miR-17-92 gene cluster which is located on
chromosome 13q31.3. Evidence has shown that the ESR1 gene, which
encodes estrogen receptor α (ESRα), is a target of miR-18a
(4).
Estrogen receptors (ESRs) are members of the nuclear
receptor superfamily that mediate the pleiotropic effects of the
steroid hormone estrogen in a diverse range of developmental and
physiological processes (5). There
are two forms of the ESR, ESRα and ESRβ, each encoded by a separate
gene (ESR1 and ESR2, respectively). ESRα is expressed mainly in the
ovaries, uterus and placenta (6),
while ESRβ is widely expressed in a number of tissues (7). The receptors are activated by the
hormone 17β-estradiol (6,7). Liu et al identified that
miR-18a prevents translation of ESRα by binding to its mRNA at the
3′ untranslated region, potentially blocking the protective effects
of estrogen (4). These previous
study findings suggest that miR-18a may be involved in the
pathogenesis of preeclampsia through regulation of ESRα, which in
turn suggests the involvement of ESRα and 17β-estradiol in the
pathogenesis of preeclampsia.
A certain degree of attention has been given to
ESRα, 17β-estradiol and preeclampsia. However, studies measuring
ESRα and 17β-estradiol from preeclamptic females have demonstrated
inconsistent results, with certain studies revealing differential
ESRα and estradiol expression in preeclampsia (8,9)
while others have shown no differences (10–12).
For these reasons, we investigated the expression of estradiol and
ESRα in severe preclamptic (sPE) pregnancies compared with normal
pregnancies, with the hope that such associations may provide
insights into the causal mechanisms of preeclampsia.
Materials and methods
Sample collection and hormone assay
Sera and placental tissues were obtained with
informed consent from nulliparous females who were admitted to the
Department of Obstetrics and Gynecology, Tangdu Hospital in Xi’an,
China. The samples were obtained from patients with normal
pregnancies (control group; n=25) and from patients with sPE (sPE
group; n=25). All females underwent an elective Cesarean delivery
in the absence of labor; the clinical characteristics of the study
groups are shown in Table I.
Preeclampsia was defined according to the criteria of the
International Society for the Study of Hypertension in Pregnancy
(13,14). sPE was defined as either severe
hypertension (systolic blood pressure of ≥160 mmHg and/or diastolic
blood pressure of ≥110 mmHg on at least 2 occasions 6 h apart) plus
mild proteinuria (≥300 mg/24 h or >1+ by dipstick), or as mild
hypertension (systolic blood pressure of ≥140 mmHg and/or diastolic
blood pressure of ≥90 mmHg on at least 2 occasions 6 h apart) plus
severe proteinuria (>2 g/24 h or >2+ by dipstick) (13,14).
No other maternal complications arose in any of the preeclamptic
pregnancies, and none of our subjects had a birthweight of <10%
of average birthweight.
 | Table IClinical characteristics of normal and
preeclamptic pregnancies. |
Table I
Clinical characteristics of normal and
preeclamptic pregnancies.
| Variable | Control (n=25) | sPE (n=25) | P-valuea control vs. sPE |
|---|
| Maternal age
(years) | 30.8±2.1 | 31±1.9 | 0.912 |
| Gestational age
(weeks) | 37.5±2.0 | 36±2.7 | 0.094 |
| Birth weight (g) | 3151±386 | 3009±497 | 0.314 |
| BMI
(kg/m2) | 24.9±2.3 | 24.1±2.4 | 0.081 |
| Systolic blood
pressure (mmHg) | 110.1±9.2 | 169.6±26.2 | <0.01 |
| Diastolic blood
pressure (mmHg) | 69.1±8.2 | 112.9±17.5 | <0.01 |
| Proteinuria (g/24
h) | 0 | 2.9±1.5 | <0.01 |
Tissue blocks (~1 cm3 each) were sampled
randomly from varying lobules (10 sites) of each placenta to
achieve uniformity and adequate sampling. Villous portions were
dissected from the decidual side of the placentas (avoiding
macroscopic areas of necrosis and infarction), snap-frozen in
liquid nitrogen overnight and stored at −80°C until use. Blood was
obtained from preeclamptic patients and normal control subjects and
placed into a serum separation vacutainer tube before treatment.
Serum was collected following centrifugation for 5 min at 3,000 rpm
and was stored at −80°C until the estimation of estradiol, which
was performed by commercially available kits according to the
manufacturer’s instructions (Estradiol assay, Cobase601, Roche,
Mannheim, Germany). The study protocol was approved by the review
board of the Fourth Military Medical University (Xi’an, China).
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
RT-PCR analysis was performed on 50 tissue samples,
one obtained from each subject. The total RNA was isolated from the
placentas using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. The purity and
concentration of the total RNA were measured on a spectrophotometer
(Jenway Ltd., Bibby Scientific Limited, Staffordshire, UK) using
their absorbance values (260/280 nm). RNA integrity was confirmed
by electrophoresis in a 1.5% agarose denaturing gel and 1 μg total
RNA was subsequently reverse transcribed into cDNA using a
RevertAid™ First Strand cDNA Synthesis kit (MBI, Fermentas,
Vilnius, Lithuania).
The primers for the RT-PCR were designed according
to specific cDNA sequences in the NCBI database (ESRα, accession
no: NM_000125; β-actin, accession no: NM_001101). The primer
sequences and the reaction conditions are shown in Table II. A 25-μl PCR master mix was
prepared as follows: 1 μl RT products, 200 μmol/l dNTPs, 2 mmol/l
MgCl2, 1 IU Taq DNA polymerase and 10 pmol of
each primer.
 | Table IIPrimer sequences and reaction
conditions for RT-PCR. |
Table II
Primer sequences and reaction
conditions for RT-PCR.
| Gene | Primer sequences | Annealing temperature
(°C) | Cycle (n) |
|---|
| ESRα | F:
CCTGGCTAGAGATCCTGAT
R: CCCTGGTTCCTGTCCAAGA | 56 | 31 |
| β-actin | F:
TCATCACTATTGGCAACGAGC
R: AACAGTCCGCCTAGAAGCAC | 55 | 25 |
Amplification was ensured to occur within the
exponential phase of PCR using preliminary experiments. PCR
products were subjected to electrophoresis on agarose gels and the
relative densities of ESRα genes normalized to β-actin were
analyzed using the Image-Pro plus (software version 6.0; Media
Cybernetics, Silver Spring, MD, USA).
Western blot analysis
Tissues were homogenized and incubated on ice in
PRO-PREP™ Protein Extraction Solution (SBS, Beijing, China). The
supernatant was collected and protein estimation was carried out
using the Bradford method. A total of 50 μg of protein per lane was
used for western blot analysis. All proteins were heated at 100°C
and separated by sodium dodecy1 sulfate-polyacry1amide gel
electrophoresis (SDS-PAGE) on a 10% gel. The proteins were next
transferred to a polyvinylidene difluoride membrane (Hybond,
Amersham Biosciences, Little Chalfont, UK) by semi-dry
electroblotting. Nonspecific reactivity was blocked by placing the
membrane in 5% skimmed milk in PBST. The membrane was then
incubated with rabbit antibodies against human ESRα (1:2,500,
Epitomic, Burlingame, CA, USA). The membrane was subsequently
washed in PBST and incubated with horseradish peroxidase secondary
antibody (1:5,000, Kangwei, Beijing, China). Chemiluminescent
detection was carried out using the Enhanced Chemiluminescent
Substrate (Pierce, Rockford, IL, USA). Subsequent to stripping, the
same membrane was used to reprobe with the rabbit-anti-human
β-actin antibody (1:3,000, Abcam, Cambridge, UK) to detect β-actin
as the internal loading control.
Statistical analysis
All values are presented as the mean ± SD of three
individual experiments performed in triplicate. Comparison of the
values between groups was performed using one-way ANOVA by SPSS
11.0 software. P<0.05 was considered to indicate a statistically
significant difference.
Results
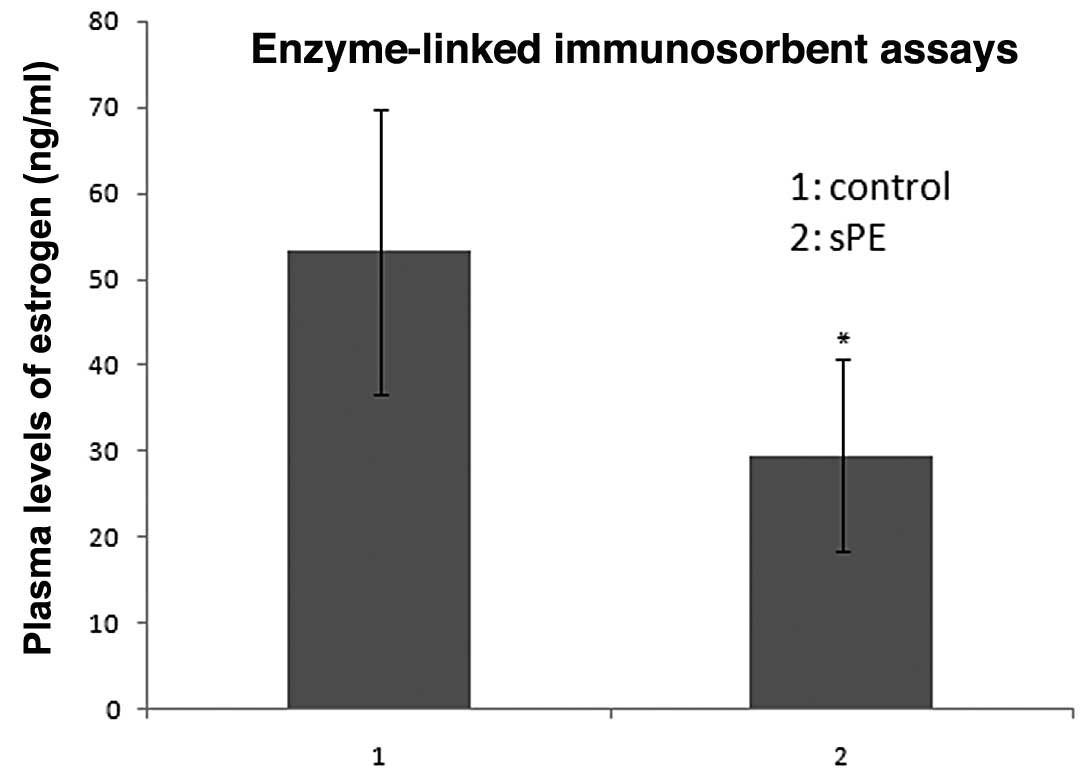
Estradiol expression in normal and
preeclamptic serum samples
Estradiol serum concentrations that were measured in
the 25 normal pregnant females ranged between 23.06 and 85.90
ng/ml, with a median value of 53.220 ng/ml. By contrast, estradiol
serum concentrations that were measured in the 25 patients with
preeclampsia ranged between 9.54 and 59.15 ng/ml, with a median
value of 29.550 ng/ml. The estradiol serum concentrations were
significantly lower in the preeclamptic pregnant females than in
the normal pregnant females (29.550±11.172 vs. 53.220±16.560 ng/ml,
respectively; P<0.05; Fig.
1).
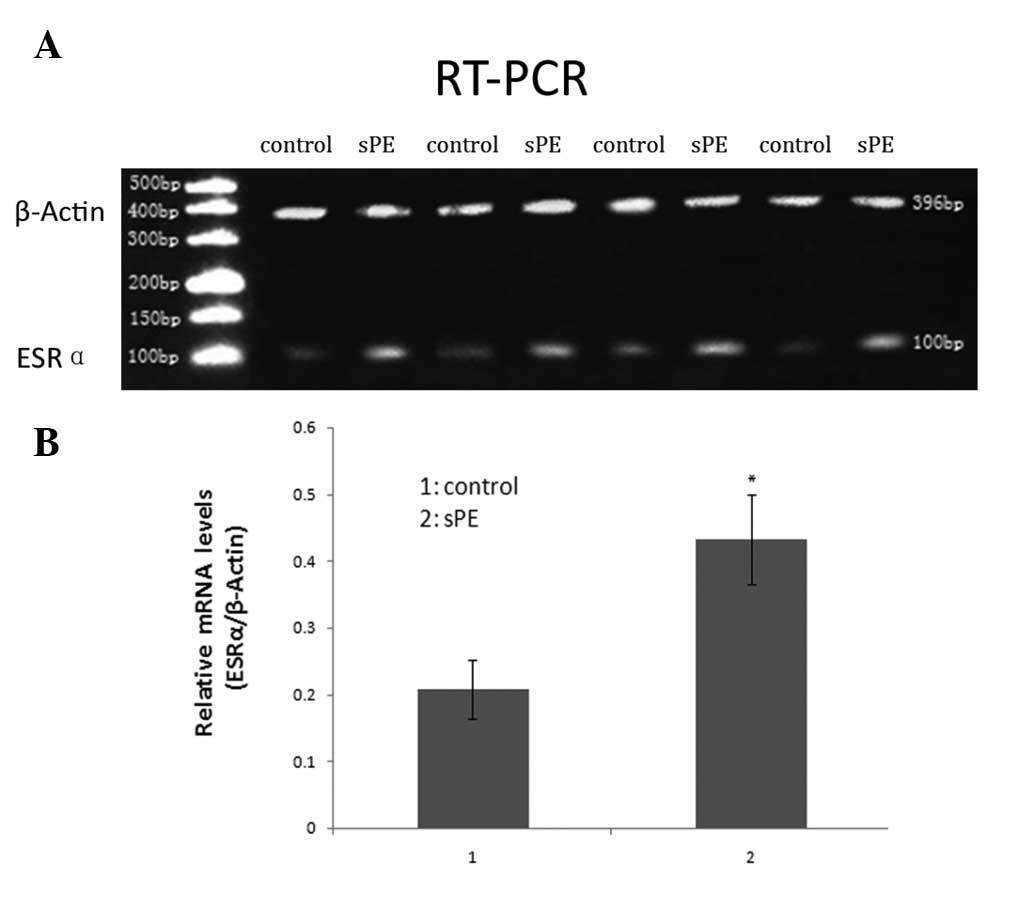
ESRα expression in normal and
preeclamptic chorionic villi
We compared the expression levels of ESRα mRNA in
chorionic villi from normal pregnancies with those from patients
with sPE using RT-PCR analysis. Relative expression of ESRα was
calculated by normalizing to β-actin expression. The mean level of
ESRα mRNA in the preeclamptic chorionic villi was significantly
higher than that of normal controls (P<0.05): 0.432±0.067 (sPE)
and 0.207±0.044 (control) by densitometric quantitation (Fig. 2).
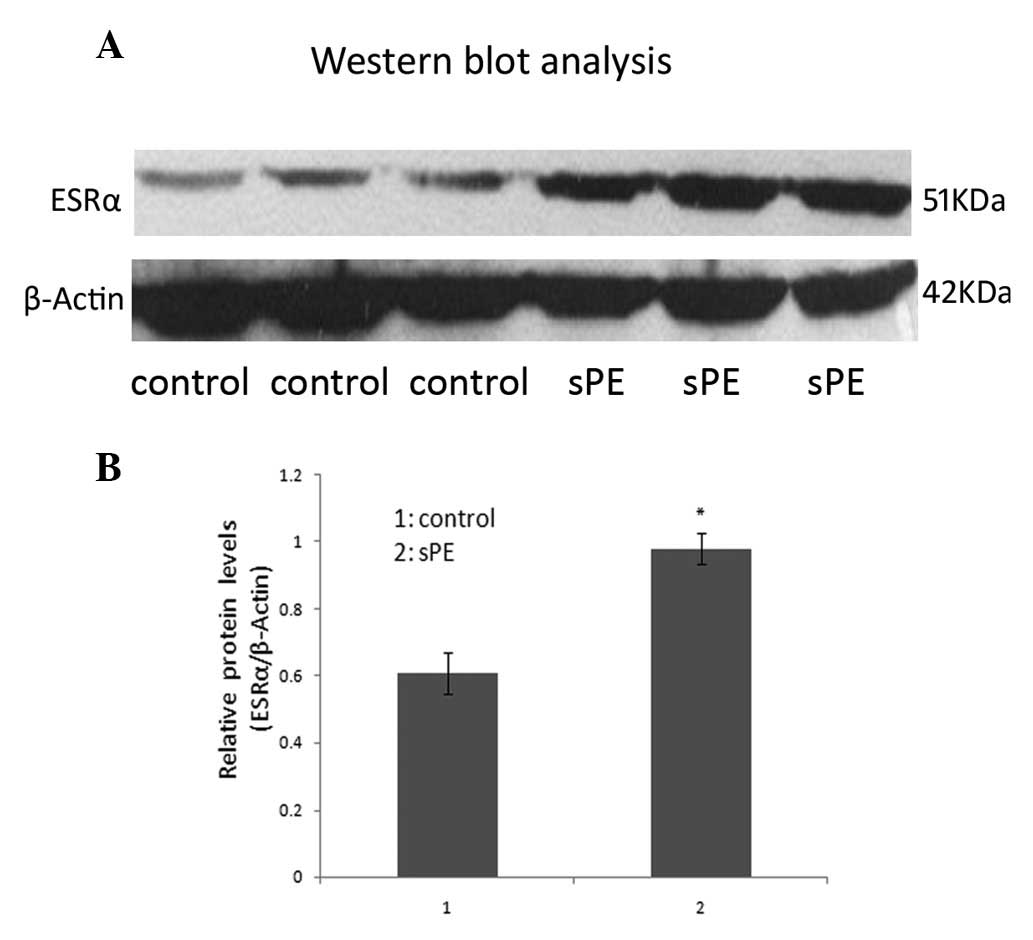
To determine whether the mRNA levels correlated with
the amount of ESRα protein, we measured the ESRα protein expression
by western blot analysis. Similar results were obtained as shown in
Fig. 3; the mean level of ESRα
protein corrected by β-actin protein was significantly higher in
the preeclamptic villi than that in normal controls (P<0.05):
0.98±0.047 (sPE) and 0.61±0.061 (control) by densitometric
quantitation.
Discussion
The present study demonstrated a significant
decrease in the expression of estradiol in patients with sPE, and a
significant increase of ESRα expression in preeclamptic pregnancies
compared with normal pregnancies.
The estrogens are a group of sex hormones secreted
primarily by the ovaries, however, during pregnancy estrogen is
secreted by the placenta. Estrogen is involved in the development
and maintenance of the female phenotype, germ cell maturation and
pregnancy. There are several types of estrogen but the three main
types are estrone (E1), estradiol (E2) and estriol (E3). At equal
concentrations, E2 has a stronger biological effect than E1 which
in turn is more powerful than E3 (15). We therefore investigated the E2
level (but not E1 or E3) by enzyme-linked immunosorbent assay
(ELISA) analysis, and detected that E2 was underexpressed in
preeclamptic pregnancies when compared with normal pregnancies.
These results are similar to the findings recorded by Zeisler et
al and Hertig et al(9,16).
Normal pregnancy itself is a state of systemic inflammation with an
elevation in the white blood cell count. However, the immune system
is suppressed during normal pregnancy in order to protect the fetus
against immune cell lysis. It is generally agreed that there is an
activated systemic maternal inflammatory response in preeclampsia
(17) that affects the circulating
leukocytes, and that circulating IL-6 and IL-8 are increased in
preeclampsia. Evidence indicates that estradiol has an
anti-inflammatory effect (18).
Schaefer et al observed that estradiol has an inhibitory
effect on IL-1β-mediated inflammatory responses in uterine
epithelial cells, which suggests a link between the endocrine and
immune systems, and indicates that estradiol may be crucial for
protecting the fetus against immune cells lysis during pregnancy
(19). Therefore, the significant
decrease in the expression of estradiol in patients with sPE may
impair this ability to protect the fetus against immune cells
lysis.
Estrogens mediate their action on a target tissue by
binding to their receptors, which are nuclear transcription
factors. To date, two ESRs (ESRα and ESRβ), encoded by different
genes, have been described (20).
ESRα is expressed predominantly in the ovaries, uterus, testes and
placenta, while ESRβ is expressed in numerous systems and tissues,
including the central nervous, cardiovascular and immune systems
and the urogenital and gastrointestinal tracts (21). In the uterus, there is a greater
quantity of ESRα present than ESRβ. Also, it is now evident that
ESRα is mainly involved in reproductive events (22). We therefore investigated the ESRα
level (but not ESRβ) by RT-PCR and western blot analysis, and
identified that ESRα mRNA and protein levels were increased
significantly in comparison with normal pregnancies (P<0.05).
ESRα is known to play a significant role in proliferation in the
maturation of estrogen-dependent cells (23). Eissa et al stated that
significantly enhanced ESR expression is exhibited in term deciduas
from females with sPE, which indicates a unique role for ESR in
pregnancy (8). Since the
trophoblast is a major source of placental hormones, ESRα
expression by trophoblast cells may be involved in the stimulation
of placental hormonal estrogen production. We observed a
significant decrease in the expression of estradiol in patients
with sPE, whereas the increased ESRα expression may be a
compensatory mechanism in these cases.
It should be noted that there are two existing forms
of preeclampsia: early-onset (symptoms at <34 weeks, type I) and
late-onset (symptoms at >34 weeks, type II). Early-onset
preeclampsia is associated with placental risk factors, while
late-onset preeclampsia is associated with the female etiology
connected with disturbances in factors regulating inflammatory
process, implantation and placentation (24). As placental tissues of <34 weeks
of gestation were not easily obtainable, we used placental tissues
of 36±2.7 weeks gestation (Table
I) in the present study. Further investigation is required to
clarify the differences in estradiol and ESRα expression between
early-onset and late-onset preeclampsia.
Further studies have been carried out by our study
group in order to unravel the mechanisms of upregulating ESRα
expression in preeclamptic placentas. We have been focused on
miRNAs, noncoding RNA molecules of 21 to 24 nt that regulate the
expression of target genes in a post-transcriptional manner
(25). We have carried out a
comparison between the miRNA expression profiles of the PE
placentas and the controls. Through microarray analysis and real
time RT-PCR confirmation, we have identified certain miRNAs that
are differently expressed in PE placentas, and from using
computational target predictions we have also identified that the
targets of these miRNAs included ESRα (3), suggesting that miRNAs were
potentially involved in the regulation of ESRα expression. Further
studies using miRNAs and ESRα are currently ongoing in our
group.
In summary, estradiol expression was demonstrated to
be significantly lowered in preeclamptic pregnancies, while we
observed a significant increase in the expression of ESRα for
patients with sPE. Our findings suggest that estradiol and ESRα may
be involved in placentation and may be factors in the etiology of
PE.
Acknowledgements
This study was supported in part by the Chinese
Natural Science Foundation, Grants No. 31000660 (X.-M.Z.) and No.
30973208 (G.-W.Y.) and the Tangdu Hospital Elite Talent Fund
(X.-M.Z.).
References
|
1
|
Driul L, Damante G, D’Elia A, Springolo F,
Ianni A, Di Leonardo C, Angelini M and Marchesoni D: Screening for
pre-eclampsia in a low-risk population at 24 weeks: uterine artery
Doppler flow velocimetry and genetic variants of factor V,
prothrombin and methylenetetrahydrofolate reductase. Minerva
Ginecol. 56:385–390. 2004.(In Italian).
|
|
2
|
Redman CW: Current topic: pre-eclampsia
and the placenta. Placenta. 12:301–308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–e7. 2009.PubMed/NCBI
|
|
4
|
Liu WH, Yeh SH, Lu CC, Yu SL, Chen HY, Lin
CY, Chen DS and Chen PJ: MicroRNA-18a prevents estrogen
receptor-alpha expression, promoting proliferation of
hepatocellular carcinoma cells. Gastroenterology. 136:683–693.
2009. View Article : Google Scholar
|
|
5
|
Shao W and Brown M: Advances in estrogen
receptor biology: prospects for improvements in targeted breast
cancer therapy. Breast Cancer Res. 6:39–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuiper GG, Carlsson B, Grandien K, Enmark
E, Häggblad J, Nilsson S and Gustafsson JA: Comparison of the
ligand binding specificity and transcript tissue distribution of
estrogen receptors alpha and beta. Endocrinology. 138:863–870.
1997.PubMed/NCBI
|
|
7
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:5925–5930.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eissa S, Mostafa MM, El-Gendy AA and Senna
IA: Quantitative immunological detection of total estrogen receptor
(cytosolic and nuclear) in term decidua of preeclampsia: a
preliminary study. Clin Chem. 43:405–406. 1997.PubMed/NCBI
|
|
9
|
Zeisler H, Jirecek S, Hohlagschwandtner M,
Knöfler M, Tempfer C and Livingston JC: Concentrations of estrogens
in patients with preeclampsia. Wien Klin Wochenschr. 114:458–461.
2002.PubMed/NCBI
|
|
10
|
Schiessl B, Mylonas I, Hantschmann P, Kuhn
C, Schulze S, Kunze S, Friese K and Jeschke U: Expression of
endothelial NO synthase, inducible NO synthase, and estrogen
receptors alpha and beta in placental tissue of normal,
preeclamptic, and intrauterine growth-restricted pregnancies. J
Histochem Cytochem. 53:1441–1449. 2005. View Article : Google Scholar
|
|
11
|
Troisi R, Potischman N, Roberts JM, Ness
R, Crombleholme W, Lykins D, Siiteri P and Hoover RN: Maternal
serum oestrogen and androgen concentrations in preeclamptic and
uncomplicated pregnancies. Int J Epidemiol. 32:455–460. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acromite M, Ziotopoulou M, Orlova C and
Mantzoros C: Increased leptin levels in preeclampsia: associations
with BMI, estrogen and SHBG levels. Hormones (Athens). 3:46–52.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ACOG Committee on Practice Bulletins -
Obstetrics. ACOG practice bulletin. Diagnosis and management of
preeclampsia and eclampsia. Number 33, January 2002. Obstet
Gynecol. 99:159–167. 2002.PubMed/NCBI
|
|
14
|
National Institutes of Health; National
Heart, Lung and Blood Institute; National High Blood Pressre
Program. Working Group Report on High Blood Pressure in Pregnancy.
NIH Publication No. 00-3029. revised 2000.
|
|
15
|
Romani W, Patrie J, Curl LA and Flaws JA:
The correlations between estradiol, estrone, estriol, progesterone,
and sex hormone-binding globulin and anterior cruciate ligament
stiffness in healthy, active females. J Womens Health (Larchmt).
12:287–298. 2003. View Article : Google Scholar
|
|
16
|
Hertig A, Liere P, Chabbert-Buffet N, Fort
J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N,
Lefevre G, et al: Steroid profiling in preeclamptic women: evidence
for aromatase deficiency. Am J Obstet Gynecol. 203:477.e1–e9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Redman CW and Sargent IL: Pre-eclampsia,
the placenta and the maternal systemic inflammatory response - a
review. Placenta. 24(Suppl A): S21–S27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Molloy EJ, O’Neill AJ, Grantham JJ,
Sheridan-Pereira M, Fitzpatrick JM, Webb DW and Watson RW:
Sex-specific alterations in neutrophil apoptosis: the role of
estradiol and progesterone. Blood. 102:2653–2659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schaefer TM, Wright JA, Pioli PA and Wira
CR: IL-1beta-mediated proinflammatory responses are inhibited by
estradiol via down-regulation of IL-1 receptor type I in uterine
epithelial cells. J Immunol. 175:6509–6516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sowers MR, Jannausch ML, McConnell DS,
Kardia SR and Randolph JF Jr: Endogenous estradiol and its
association with estrogen receptor gene polymorphisms. Am J Med.
119(9 Suppl 1): S16–S22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drummond AE, Baillie AJ and Findlay JK:
Ovarian estrogen receptor alpha and beta mRNA expression: impact of
development and estrogen. Mol Cell Endocrinol. 149:153–161. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gustafsson JA: Estrogen receptor beta - a
new dimension in estrogen mechanism of action. J Endocrinol.
163:379–383. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bukovsky A, Caudle MR, Cekanova M,
Fernando RI, Wimalasena J, Foster JS, Henley DC and Elder RF:
Placental expression of estrogen receptor beta and its hormone
binding variant - comparison with estrogen receptor alpha and a
role for estrogen receptors in asymmetric division and
differentiation of estrogen-dependent cells. Reprod Biol
Endocrinol. 1:362003. View Article : Google Scholar
|
|
24
|
Cudihy D and Lee RV: The pathophysiology
of pre-eclampsia: current clinical concepts. J Obstet Gynaecol.
29:576–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pasquinelli AE, Hunter S and Bracht J:
MicroRNAs: a developing story. Curr Opin Genet Dev. 15:200–205.
2005. View Article : Google Scholar : PubMed/NCBI
|