Introduction
In recent years, the human disease spectrum has
changed with rapid economic development, and a number of infectious
diseases are now controlled effectively. However, cancer remains a
major threat to human health. Cancer is the leading cause of
mortality in economically developed countries and the second
leading cause of mortality in developing countries (1). Cervical carcinoma and gastric, colon
and lung cancer are common malignant tumors (2–5) and
the incidence and mortality of these types of cancer have increased
year by year. Therefore, cancer has become the most serious public
health issue worldwide.
Among therapeutic strategies for cancer, radical
surgery is the most effective method, however, chemotherapy plays
an integral role in patients with advanced tumors by reducing the
mortality of cancer. Cisplatin (DDP) is a commonly used
chemotherapy agent that has a broad spectrum of antitumor effects,
high antitumor activity and a curative effect on cancer. However,
the severe side-effects associated with DDP, including bone marrow
suppression, neurotoxicity and gastrointestinal reactions (6–9),
limit its therapeutic application. Therefore, the identification of
anticancer agents with high efficacy and low toxicity, as well as
combination therapies, is an important area of study.
Previous studies have reported that overexpression
of Ras-GTPase activating protein SH3 domain-binding proteins
(G3BPs) in the cytoplasm is closely associated with tumorigenesis
and metastasis in colorectal carcinoma and gastric, lung and breast
cancer (10,11). Cui et al(12) designed and screened a series of
peptides which specifically bind G3BPs. These peptides have been
demonstrated to increase the sensitivity of tumor cells to DDP and
other anticancer drugs, thus inducing apoptosis in tumor cells.
However, this sensitizing effect does not occur in normal cells.
P110 (RasGAP301–316) is one of the peptides identified
in the study. In the present study, the effects of P110 + DDP
against various types of cancer cells were examined in vitro
and in vivo.
Materials and methods
Peptide synthesis
Peptide P110 (13)
(amino acid sequence, FLKGDMFIVHNELEDG) was synthesized at Wuhan
KatyGen Pharmaceuticals, Inc. (Wuhan, China). The molecular weight
was 4,660.41 Da. It was synthesized by FMOC technology, purified by
HPLC, tested by mass spectrometry and stored at −20°C.
Cell lines and cell culture
Gastric cancer cell line, SGC-7901; colon cancer
cell line, HCT-116; cervical carcinoma cell line, HeLa; lung cancer
cell line, A-549; and human umbilical vein endothelial cells
(HUVECs) were obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). Cells were maintained in RPMI-1640 medium
(Gibco-BRL, Carlsbad, CA, USA) containing 10% fetal calf serum
(Hangzhou, China) at 37°C and 5% CO2. According to the
pre-test (14), the
IC50 of P110 in HeLa cells was 20 μM. In this study, the
concentration of P110 when combined with DDP was 20 μM. The cells
were divided into 4 groups: DDP, P110, P110 + DDP and control. The
ultimate concentration of DDP in the cancer cell analyses was 1.1,
3.3, 10, 30 and 90 μM, while in the HUVEC analyses, it was 10, 20,
50, 100 and 150 μM.
Cell cytotoxicity assay
Cell viability following treatment with various
concentrations of P110 and DDP was evaluated using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays performed in triplicate. Briefly, logarithmic growth phase
cells of SGC-7901, HCT-116, HeLa, A-549 and HUVEC were selected and
digested with 0.25% trypsin into a single cell suspension,
adjusting the cell density to 1.8×104 cells/ml. Cell
suspension (200 μl) was added to 96-well plates and cultured for 24
h. DDP and P110 + DDP at various indicated concentrations were
added. RPMI-1640 was added as a control. Following 24-h incubation,
the MTT solution was removed and 200 μl MTT was added to each well
and incubated for 4 h. After removal of the MTT solution, 200 μl
DMSO was added to each well. Next, the optical density at 570 nm
was determined using an iMark Microplate Absorbance reader
(Bio-Rad, Hercules, CA, USA). Cell proliferation inhibitory rates
were calculated as follows: Inhibition rate (%) = (1 −
A570 experimental well/A570 control well) ×
100.
Annexin V/propidium iodide (PI)
staining
To quantify the percentage of cells undergoing
apoptosis, the Annexin V-FITC kit (Multi Sciences (Lianke) Biotech
Co., Ltd., Hangzhou, China) was used according to the
manufacturer’s instructions. Briefly, HCT-116 cells were incubated
for 24 and 48 h with P110 (20 μM) and DDP (10 μM) alone or in
combination. Next, the cells were washed twice with cold PBS and
resuspended in binding buffer at a concentration of
1×106 cells/ml. Following incubation, 100 μl solution
was transferred to a 5-ml culture tube and 5 μl Annexin V-FITC and
10 μl PI were added. The tube was gently centrifuged and incubated
for 15 min at room temperature in the dark. At the end of
incubation, 400 μl binding buffer was added and the cells were
analyzed immediately by flow cytometry (Beckman Coulter, Inc.,
Fullerton, CA, USA). Flow cytometry was performed using CellQuest
software.
Acute toxicity test in mice
Male BALB/c mice, 18–22 g, were obtained from
Beijing Vital River Laboratories (Beijing, China). All mice were
bred in a specific pathogen-free environment. Four groups of male
mice (n=5/group) were treated with P110 in graded doses (125, 250,
500, 1,000 mg/kg body weight) by intraperitoneal injection.
Mortality and other physical signs of toxicity, including skin
changes, respiratory movements and weight, were observed over 30
days following drug administration and the LD50 was
determined.
Xenograft tumors in mice
C26 colon cancer tumor-bearing mice were purchased
from the Institute of Biotechnology Research, Chinese Academy of
Medical Sciences (Beijing, China). Tissues derived from the C26
colon cancer tumor-bearing mice were processed into single cell
suspensions using 0.85% normal saline (1:10). Male BALB/c mice were
injected with 0.2 ml tumor cells subcutaneously in the right armpit
region and were randomly divided into 8 groups (n=10/group): saline
control; DDP 1 mg/kg/every other day; P110 25 mg/kg/day; P110 50
mg/kg/day; P110 100 mg/kg/day; P110 (25 mg/kg/day) + DDP; P110 (50
mg/kg/day) + DDP; and P110 (100 mg/kg/day) + DDP. DDP and P110 were
dissolved in 0.85% normal saline. The following day, mice were
injected intraperitoneally with the specified doses. On day 11, all
mice were sacrificed and the tumor xenografts were removed and
weighed. Tumor growth inhibitory rates were calculated using the
following formula: Inhibition rate (%) = (1 − mean test tumor
weight/mean control tumor weight) × 100. The study was approved by
the Institutional Review Board of Renmin Hospital of Wuhan
University.
Detection of tumor apoptosis genes
Rabbit anti-Bax and anti-Bcl-2 polyclonal
antibodies, anti-cytochrome c (cyt c) and
anti-caspase-3 were purchased from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China). The streptavidin
peroxidase (SP) anti-rabbit/mouse Universal Immunohistochemistry
kit was purchased from Dako (Carpinteria, CA, USA). The removed
tumors were paraffinized and cut into sections of 4–5 μm.
Expression of Bax, Bcl-2, cyt c) and caspase-3 was detected
by SP immunohistochemistry. Bax-, Bcl-2-, cyt c- and
caspase-3-positive protein expression in the cytoplasm appeared
brown. Negative samples revealed no brown staining in the cytoplasm
and nucleus and light blue staining of the nucleus.
Combined effect of the two drugs
The median effect method of Chou and Talalay
(15,16) was used to analyze the interaction
between DDP and P110. The drug combination index (CI) was
calculated. CI<1 indicated synergy, CI=1 indicated additivity
and CI>1 indicated antagonism.
Statistical analysis
Results were analyzed by SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA) and one-way ANOVA (multiple comparisons)
and t-tests (two groups comparisons) were performed accordingly.
P<0.05 was considered to indicate a statistically significant
difference. All data are presented as the mean ± SD.
Results
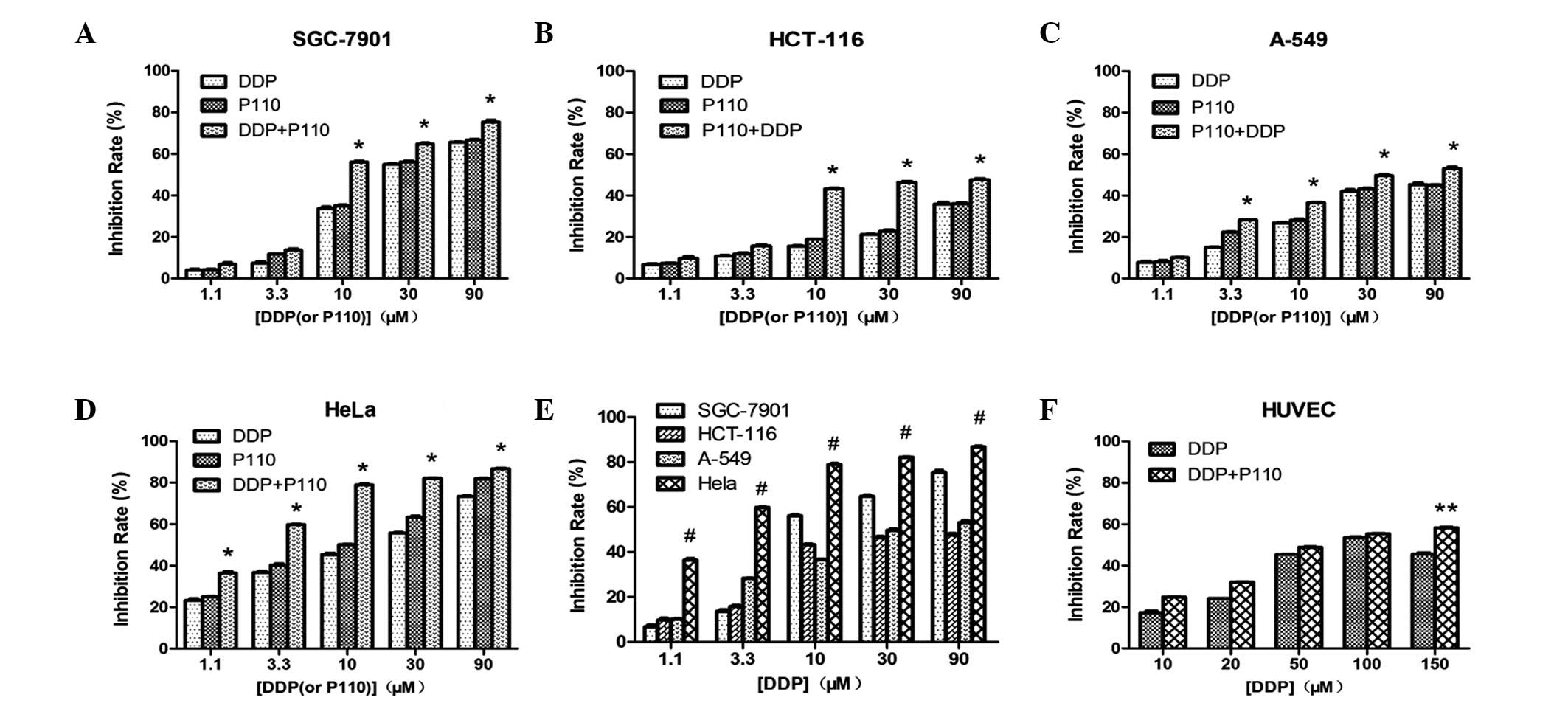
Effect of single drug exposure on the
growth of cancer cells
Cancer cells were treated with various
concentrations of P110 or DDP for 24 h and cell viability was
determined using the MTT assay (Fig.
1A-D). The growth of SGC-7901, HCT-116, HeLa and A-549 cells
was found to be significantly inhibited in a
concentration-dependent manner in vitro (P<0.05). The
growth inhibitory effect of P110 on the tumor cells was slightly
higher than that of DDP, but the difference was not determined to
be statistically significant (P>0.05).
Combined effect of P110 and DDP on the
growth of cancer cells
When the combined effects were studied, the cells
were exposed for 24 h to the two drugs concurrently. At DDP
concentrations of 10, 30 and 90 μM, the inhibitory rate of P110 +
DDP was higher than that of DDP or P110 alone and the difference
was identified to be statistically significant (P<0.05; Fig. 1A-D). Of note, the inhibitory effect
of the combination treatment on the proliferation of HCT-116 and
HeLa cells plateaued at DDP concentrations of 10, 30 and 90 μM and
the differences between the effects at these concentrations were
not observed to be statistically significant (P>0.05; Fig. 1B and D). At the same concentration,
the inhibitory effect of the combination treatment in HeLa cells
was higher than that in the other cell types (P<0.05; Fig. 1E).
Growth inhibitory effect on HUVECs
When the DPP concentration was 10, 20, 50, 100 and
150 μM, the inhibitory rate was 17.2±0.8, 24.0±0.1, 45.4±0.1,
53.5±0.3 and 45.7±0.5%, respectively and the inhibitory rate of
P110 + DDP was 24.9±0.1, 32.0±0.2, 48.9±0.2, 55.4±0.2 and
58.3±0.3%, respectively, indicating that the inhibitory effect was
concentration-dependent. The differences between the rates for P110
+ DDP and DDP were not identified to be statistically significant,
except at 150 μM DDP (P<0.05; Fig.
1F).
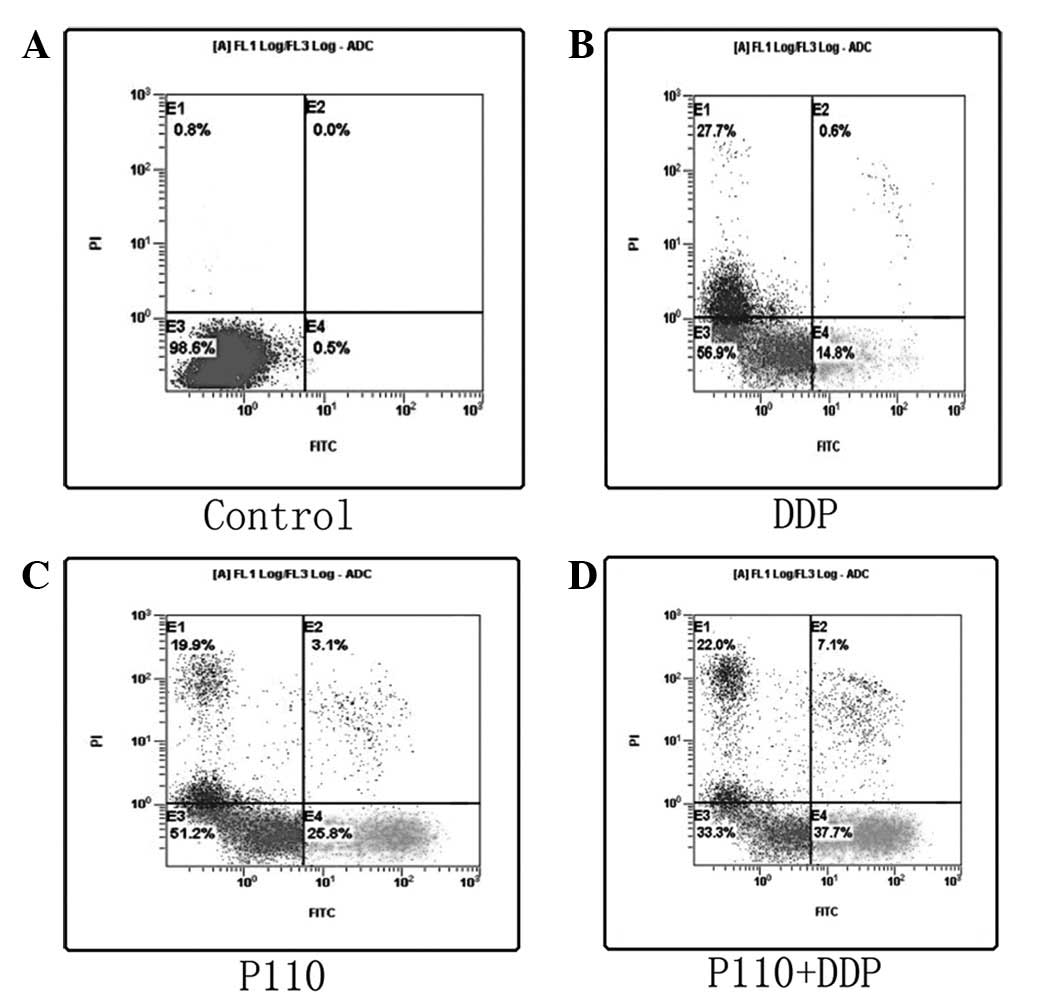
Apoptosis induced by P110 and DDP
Apoptosis induced by P110 and DDP was confirmed
using Annexin V/PI staining to detect the externalization of
phosphatidylserine on the cell membrane. As revealed in Fig. 2, the proportion of Annexin
V+/PI− cells increased progressively in
HCT-116 cells incubated at low concentrations of P110 (20 μM) and
DDP (10 μM) for 48 h. P110 and DDP alone were found to
significantly increase apoptosis compared with the control
(P<0.05; Fig. 2B and C) and the
combined effects were stronger than the effects of P110 and DDP
alone (P<0.05; Fig. 2D).
Consistent results were obtained in other cancer cells (data not
shown).
Acute toxicity of P110 in vivo
To evaluate the toxicity of P110 in BALB/c mice,
P110 in graded doses (125, 250, 500 and 1,000 mg/kg) was
administered to four groups of male mice by intraperitoneal
injection. At day 30, all mice were alive. Skin changes,
respiratory movements and the weights of the mice in each group
were not observed to be significantly different. The
LD50 of P110 was calculated to be >1,000 mg/kg.
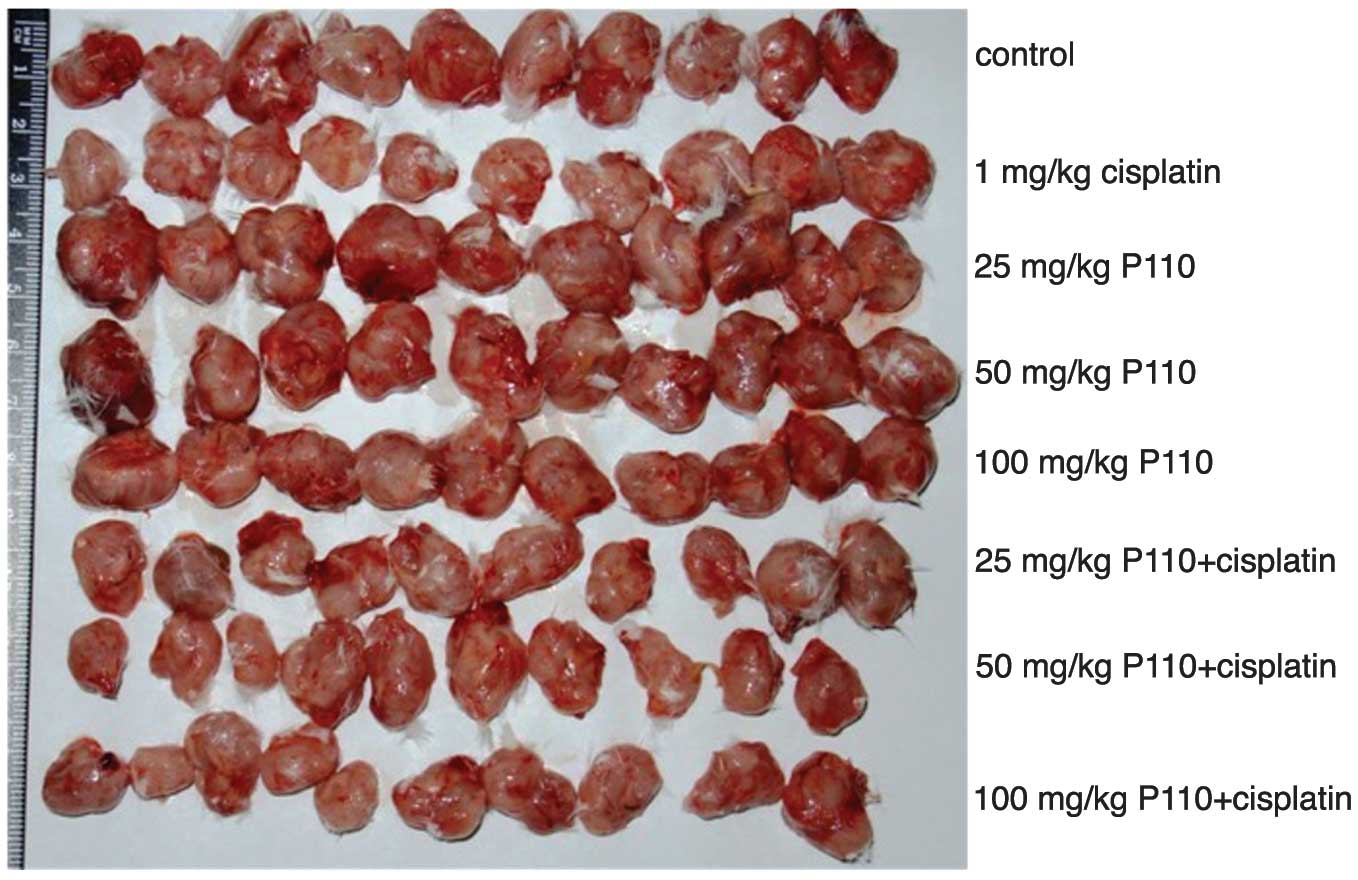
P110 + DDP inhibits colon cancer cells in
vivo
As demonstrated in Fig.
3, mice treated with P110 (25, 50 and 100 mg/kg) + DDP (1
mg/kg) developed significantly smaller tumors than mice treated
with 0.85% normal saline (P<0.05). The tumor growth inhibitory
rates in the P110 + DDP group were 23.0, 30.4 and 34.2%,
respectively and 22.7% in the group treated with DDP alone.
Compared with control, P110 alone had no effect on tumor growth
(P>0.05; Table I).
 | Table IInhibitory effect of DDP and P110 on
C26 xenotransplanted tumors in mice. |
Table I
Inhibitory effect of DDP and P110 on
C26 xenotransplanted tumors in mice.
| Group | n | Weight (g) | Inhibition rate
(%) |
|---|
| Control | 10 | 1.65±0.3 | |
| 1 mg/kg DDP | 10 | 1.28±0.3a | 22.7 |
| 25 mg/kg P110 | 10 | 2.02±0.6 | 0.0 |
| 50 mg/kg P110 | 10 | 1.97±0.4 | 0.0 |
| 100 mg/kg P110 | 10 | 1.58±0.2 | 4.1 |
| 25 mg/kg P110 + 1
mg/kg DDP | 10 | 1.27±0.4a | 23.0 |
| 50 mg/kg P110 + 1
mg/kg DDP | 10 | 1.15±0.4a,b | 30.4 |
| 100 mg/kg P110 + 1
mg/kg DDP | 10 | 1.19±0.3a,b | 34.2 |
Combined effect of the two drugs
The CIs of P110 (at doses of 25, 50 and 100 mg/kg) +
DDP (1 mg/kg) were 0.99, 0.90 and 0.88, respectively. All CIs were
<1. These results indicate that the interaction between P110 and
DDP is synergistic.
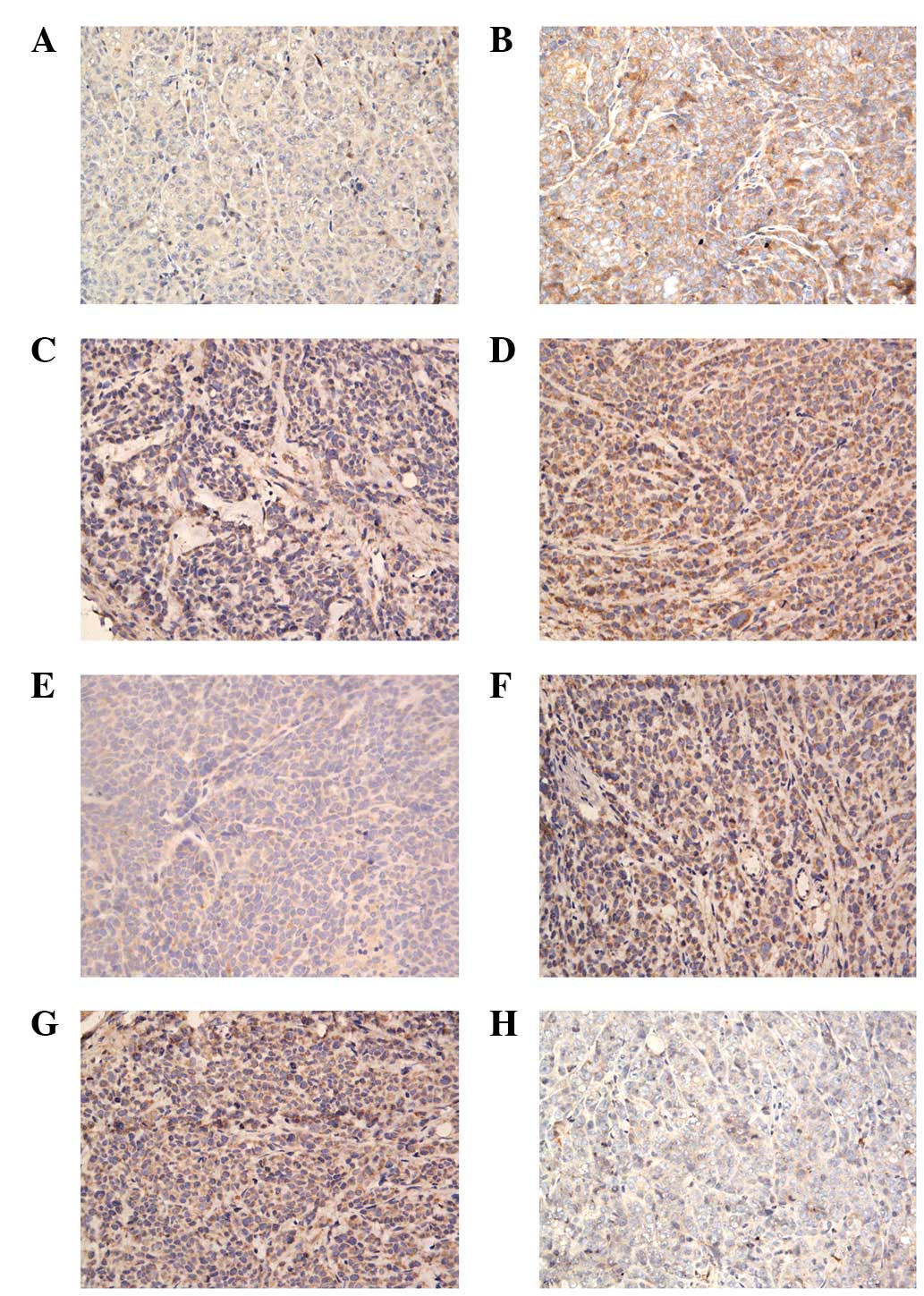
Expression of tumor apoptosis genes in
xenograft tumors
Bax, cyt c and caspase-3 expression levels
were increased in the xenografts of the P110 + DDP group compared
with those in the DDP group (Fig.
4A-F), whereas the Bcl-2 expression level was higher in the
xenografts of the DDP-treated group than in the P110 + DDP group
(Fig. 4G and H).
Discussion
At present, although antitumor strategies are
continually updated and improved, chemotherapy remains the most
important method of cancer treatment. DDP is a genotoxic anticancer
drug which functions by inducing DNA damage and has been
successfully used in tumor treatment for several decades. However,
genotoxic drugs are non-selective and require administration at
high doses, leading to toxicity in normal tissue. The issue of
toxicity remains a major obstacle for the development of successful
cancer chemotherapies (6,7) and the identification of methods for
increasing tumor specificity and reducing the side-effects of
antitumor drugs is an extremely important area of cancer
research.
G3BP is a specific binding protein which binds the
SH3 domain of RasGAP via the nuclear transport factor 2 (NTF2)-like
domain. Guitard et al(17)
reported that G3BP is expressed at high levels in a number of types
of human tumor cells. In addition, previous studies have confirmed
that G3BP is overexpressed in colon, gastric, lung and breast
cancer (18–20). The anti-G3BP antibody selectively
kills cancer cells without affecting normal tissues (21). G3BPs have also been found to be
closely associated with a negative regulatory protein of RasGAP in
the Ras signaling pathway and cell proliferation. G3BPs play a role
in signal transduction via an N-terminal NTF2-like domain binding
to an N-terminal SH3 domain in RasGAP (22,23)
The G3BP protein family is closely associated with cancer and
specific fragments of the RasGAP SH3 domain may exhibit a
therapeutic effect by combining with G3BPs. Therefore, studies of
the protein structure of G3BP and the relationship between Ras-GAP
and G3BP are important in the development of methods for screening
G3BP-overexpressing cancers and the design of targeted antitumor
drugs
In 2004, Michod et al(24) reported that a specific peptide
derived from the RasGAP protein increased the sensitivity of tumor
cells to DDP and other anticancer drugs and thus induced the
apoptosis of tumor cells. This sensitizing effect did not occur in
normal cells. Following this, Cui et al(12)used molecular dynamics simulation to
study the potential interaction between G3BP and RasGAP and
employed protein homology modeling to determine the
three-dimensional structure of the NTF2 domain of G3BP. Protein
docking studies were performed to determine the structure of the
RasGAP SH3 domain based on the Monte Carlo method and three
possible protein-protein interaction models were obtained.
Molecular dynamics simulations and binding free energy calculations
were performed on the three models to reveal the most stable model
of binding. Energy decomposition calculations in each model
revealed that the Ile308, Val309, His310, Asn311 and Thr321
residues of the SH3 domain of RasGAP form marked interactions with
the Asp28, Met29, His31 and Arg32 residues of the NTF2-like domain
of G3BP. Following this, two novel antitumor peptides, P110 and
P109, were synthesized and the effects of the two sequences alone
and in combination with platinum drugs on HeLa cells were
confirmed. P110 contains the key residues of the SH3 domain of
RasGAP that are involved in binding the NTF2-like domain of
G3BP.
In the present study, the inhibition rate of P110
combined with DDP on SGC-7901, HCT-116, HeLa and A-549 cells was
higher than that of DDP or P110 alone. At DDP concentrations of 10,
30 and 90 μM, the difference between the combined and single drug
groups was found to be statistically significant. Apoptosis induced
by P110 (20 μM) and DDP (10 μM) was also examined and the results
demonstrated that P110 combined with DDP at lower concentrations
induced apoptosis of various types of cancer cells and the effect
was stronger than that of P110 and DDP alone. In animals, xenograft
experiments also revealed that the tumor growth inhibition rate of
P110 + DDP was higher than that of DDP alone and the CI of the P110
+ DDP treatment was <1. These results demonstrate that P110 and
DDP exhibit an excellent synergistic relationship and indicate that
peptide P110 enhances the proliferation inhibiting effect of DDP on
various types of cancer cells and increases the antitumor effect
in vivo. Therefore, P110 may be a DDP-sensitizing agent
which increases the antitumor effect of DDP. Peptide P110 may
specifically bind to G3BP and affect signal transduction in cancer
cells to induce apoptosis, however, the specific mechanism of the
sensitizing effect of P110 requires further study.
At present, a number of anticancer drugs kill tumor
cells by inducing apoptosis. The majority of studies agree that
there are two common pathways in cell apoptosis (25–27),
the mitochondria-independent death receptor and mitochondrial
pathways. It was previously reported that DDP induces cell
apoptosis by the mitochondrial pathway (28). At the transcriptional level, Bcl-2
family members are regulated by p53. p53 is able to reduce the
expression of Bcl-2, increase the expression of Bax and activate
the mitochondria to release cyt c, then further activate caspase-9
and caspase-3. Following this, the caspase cascade occurs and cell
apoptosis is induced. Zhang et al(29) reported that 38GAP
(RasGAP301–326) increased the effect of DDP on HCT-116
colon cancer cells and that the expression of Bcl-2 was
downregulated, while Bax was upregulated in 38GAP-treated cells.
The P110 peptide used in the present study is extremely similar in
structure to 38GAP and, when used in combination with DDP to treat
xenograft-bearing mice, the expression levels of Bax, cyt c
and caspase-3 were higher and the expression levels of Bcl-2 were
lower than in the DDP-treated mice. Thus, we hypothesize that the
inhibitory effect of P110 + DDP on colon carcinoma xenograft tumors
is associated with the apoptosis-inducing mechanism of DDP.
This study also indicates that the toxicity in
HUVECs is concentration-dependent. When the concentration of DDP
was 10 μM, the inhibitory effect of P110 + DDP on the proliferation
of HCT-116 and HeLa cells reached a plateau. The inhibition rate of
the combination was higher than that of DDP alone when the dose of
DDP was 90 μM. DDP (10 μM) + P110 was demonstrated to be effective
for the inhibition of tumor cells and its inhibition rate in HUVECs
was lower than that of high doses of DDP and of other
concentrations of P110 + DDP. Thus, P110 is able to enhance the
antitumor effect of DDP, thereby reducing the dose of DDP required
for efficacy and alleviating toxicity in normal tissues to result
in an improved clinical chemotherapeutic effect and quality of
life.
In conclusion, P110 sensitizes cancer cells to the
antitumor effect of DDP, enabling them to be killed selectively,
which indicates its potential clinical value.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Hubei Province (no. 2010CDB06908)and the
Wuhan Science and Technology Bureau Fund (no. 201060938363-05).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Yamaoka Y, Kato M and Asaka M: Geographic
differences in gastric cancer incidence can be explained by
differences between Helicobacter pylori strains. Intern Med.
47:1077–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Legge F, Fuoco G, Lorusso D, et al:
Pharmacotherapy of cervical cancer. Expert Opin Pharmacother.
11:2059–2075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar
|
|
6
|
Schiff D, Wen PY and van den Bent MJ:
Neurological adverse effects caused by cytotoxic and targeted
therapies. Nat Rev Clin Oncol. 6:596–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaushal GP, Kaushal V, Herzog C and Yang
C: Autophagy delays apoptosis in renal tubular epithelial cells in
cisplatin cytotoxicty. Autophagy. 4:710–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee S, Kim W, Moon SO, et al:
Rosiglitazone ameliorates cisplatin-induced renal injury in mice.
Nephrol Dial Transplant. 21:2096–2105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao X, Panichpisal K, Kurtzman N and
Nugent K: Cisplatin nephrotoxicity: a review. Am J Med Sci.
334:115–124. 2007. View Article : Google Scholar
|
|
10
|
Zhang HZ, Liu JG, Wei YP, et al:
Expression of G3BP and RhoC in esophageal squamous carcinoma and
their effect on prognosis. World J Gastroenterol. 13:4126–4130.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ning JY, You JF, Pei F, et al: Monoclonal
antibody against G3BP: preparation, characterization and its
application in analysis of human tumors. Zhonghua Bing Li Xue Za
Zhi. 34:215–219. 2005.(In Chinese).
|
|
12
|
Cui W, Wei Z, Chen Q, et al:
Structure-based design of peptides against G3BP with cytotoxicity
on tumor cells. J Chem Inf Model. 50:380–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen JH, Huang YU, Xiong YU and Chen CH:
Peptides or derivatives which treat or prevent cancers and the
application CN Patent 200910163852. Issued August 11, 2009.
|
|
14
|
Zhang J, Tan SY, Chen JH, et al:
Polypeptide A28 enhances cytotoxic effect of cisplatin on colon
cancer cell line HCT-116. Chin J Cancer Biother. 17:318–321.
2010.(In Chinese).
|
|
15
|
Kaufmann SH, Peereboom D, Buckwalter CA,
et al: Cytotoxic effects of topotecan combined with various
anticancer agents in human cancer cell lines. J Natl Cancer Inst.
88:734–741. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC, Motzer RJ, Tong Y and Bosl GJ:
Computerized quantitation of synergism and antagonism of taxol,
topotecan and cisplatin against human teratocarcinoma cell growth:
a rational approach to clinical protocol design. J Natl Cancer
Inst. 86:1517–1524. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guitard E, Parker F, Millon R, et al: G3BP
is overexpressed in human tumors and promotes S phase entry. Cancer
Lett. 162:213–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Zheng J, Fang W, et al:
Identification of metastasis associated gene G3BP by differential
display in human cancer cell sublines with different metastatic
potentials G3BP as highly expressed in non-metastatic. Chin Med J
(Engl). 114:35–38. 2001.PubMed/NCBI
|
|
19
|
French J, Stirling R, Walsh M and Kennedy
HD: The expression of Ras-GTPase activating protein SH3
domain-binding proteins, G3BPs, in human breast cancers. Histochem
J. 34:223–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barnes CJ, Li F, Mandal M, et al:
Heregulin induces expression, ATPase activity and nuclear
localization of G3BP, a Ras signaling component, in human breast
tumors. Cancer Res. 62:1251–1255. 2002.PubMed/NCBI
|
|
21
|
Parker F, Kenigsberg M, Duchesne M, et al:
Monoclonal antibodies directed against the G3BP protein and uses US
Patent 7001980B1 [P]. Issued February 21, 2006.
|
|
22
|
Parker F, Maurier F, Delumeau I, et al: A
Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell
Biol. 16:2561–2569. 1996.PubMed/NCBI
|
|
23
|
Kennedy D, French J, Guitard E, et al:
Characterization of G3BPs: tissue specific expression, chromosomal
localisation and rasGAP(120) binding studies. J Cell Biochem.
84:173–187. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen M and Wang J: Initiator caspases in
apoptosis signaling pathways. Apoptosis. 7:313–319. 2002.
View Article : Google Scholar
|
|
27
|
Green DR: Apoptotic pathways: ten minutes
to dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Michod D, Yang JY, Chen J, et al: A
RasGAP-derived cell permeable peptide potently enhances
genotoxin-induced cytotoxicity in tumor cells. Oncogene.
23:8971–8978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Zhang S, He H, et al:
RasGAP-derived peptide 38GAP potentiates the cytotoxicity of
cisplatin through inhibitions of Akt, ERK and NF-κB in colon
carcinoma HCT116 cells. Cancer Lett. 308:62–70. 2011.PubMed/NCBI
|