Introduction
Renal fibrosis, characterized by tubulointerstitial
fibrosis and glomerulosclerosis, is the final manifestation of
chronic kidney disease (CKD). With the progression of CKD, patients
inevitably reach endstage renal disease and require renal
replacement therapies, such as dialysis and transplantation. Renal
fibrosis is the final result of CKD. Transformation in the
phenotype of the cell epithelial-mesenchymal transition (EMT) is
considered to greatly contribute to renal fibrosis. Epithelial
cells that have undergone EMT are characterized by the loss of
epithelial cell markers, such as E-cadherin, and de novo
mesenchymal cell markers, such as α-smooth muscle actin
(α-SMA).
EMT, as an important physiological event, occurs
frequently during embryonic development (1). However, numerous studies have
demonstrated that EMT also occurs during pathological conditions
such as tumor metastasis and tissue fibrosis (2–4).
Various studies have confirmed that EMT plays a crucial role in the
development and progression of renal fibrosis (5).
Paired box 2 (PAX2) gene encodes a nuclear
transcription factor which is key to inducing the renal tubular
epithelial cell transition during embryonic development. This plays
an important role in regulating the embryonic development of the
kidney at various stages (6).
However, in the perinatal period, new nephron formation is hindered
when the expression of PAX2 is sharply suppressed. Aberrant PAX2
expression in renal tubular epithelial cells has been reported in
children with glomerular diseases, suggesting that restoration of
PAX2 may result in renal tubular epithelial cell
transdifferentiation (7). We have
previously observed that in a unilateral ureteral obstruction (UUO)
model, the reactivation of PAX2 occurs primarily in the renal
tubular epithelial cells, suggesting its role in regulating EMT
(8).
However, the effect of PAX2 on EMT has not yet been
elucidated. In the present study, we investigated whether PAX2
induces EMT in NRK52E cells in vitro in order to provide
experimental evidence for the potential role of PAX2 in the
prevention and treatment of renal interstitial fibrosis.
Materials and methods
Animals and UUO model
Four-week-old male Wistar rats (weighing 120–150 g)
were obtained from the Shengjing Hospital Laboratory Animal Center
(Shenyang, China). This study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee of Shengjing Hospital of China Medical
University. The rats were anesthetized via intraperitoneal
injection of 10% chloral hydrate (Shengjing Hospital, Shenyang,
China). The left ureter was exposed and ligated with 3-0 silk
through a midline abdominal incision. The rats were sacrificed 21
days following UUO.
Complementary DNA (cDNA) synthesis
Total RNA was extracted from the renal cortex of the
rats using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions. Total RNA (500 ng) was
reverse-transcribed into cDNA using the reverse transcription (RT)
reagent kit according to the manufacturer’s guidelines. The
full-length PAX2 gene was amplified by polymerase chain reaction
(PCR) from PAX2 cDNA, using the following primers: forward,
GAGGATCCCCGGGTACCGGTCGCCACCATGGATATG CACTGCAAAG and reverse,
CACCATGGTGGCGACCGG GTGGCGGTCATAGGCAGC containing AgeI sites.
The target sequence was amplified by PCR at 94°C for 5 min, 94°C
for 30 sec, 55°C for 30 sec, 72°C for 1.5 min for 30 cycles, and
72°C for 10 min.
Construction of pGC-FU-GFP-PAX2
plasmid
Following PCR, the PCR products of the PAX2 gene
were purified to generate the pGC-FU-GFP transfer vector (GeneChem
Co., Ltd., Shanghai, China). The ligated products were transformed
into TOP10 chemically competent Escherichia coli (Takara,
Otsu, Shiga, Japan) and incubated on Luria-Bertani plates
containing 100 μg/ml ampicillin at 37°C overnight. Subsequently, 8
putative ampicillin-resistant positive clones were selected to
amplify, extract and purify for PCR amplification and
electrophoresis detection. The sequencing was completed by Shanghai
ShengGong Biotechnology Co., Ltd. (Shanghai, China).
Cell cultures and transient
transfection
The well-characterized normal rat renal tubular
epithelial cell line NRK52E was obtained from the American Type
Culture Collection (Rockville, MD, USA). The NRK52E cells were
cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) with
10% fetal calf serum and 1% penicillin/streptomycin solution (P/S;
cat. no. 0503) at 37°C in a 5% CO2 atmosphere. Cells
were plated onto 6-well plates at a density of 2×105
cells/well, grown overnight, and then transferred to serum-free
medium prior to PAX2 transfection. NRK52E cells were transfected
with 4 μg pGC-FU-GFP-PAX2 plasmids or empty vector using 5 μl
Lipofectamine™ 2000 transfection reagent (Invitrogen) according to
the manufacturer’s instructions. Cells were observed for green
fluorescent protein (GFP) using fluorescence microscopy after 24,
48, 72 and 96 h transfection. The control cells were grown in
serum-free medium alone. All the experiments were performed in
triplicate.
Phase contrast microscopy
After phase contrast microscopy was performed using
a charge-coupled device (CCD) video camera attached to a TMS phase
contrast microscope (Nikon, Tokyo, Japan), all the micrographs were
subsequently processed using Adobe Photoshop software.
Western blotting
Protein concentrations were determined using a BCA
protein assay kit (Sigma-Aldrich, Seelze, Germany). Samples were
heated at 100°C for 5–10 min before loading and were separated on
precast 10 or 5% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA,
USA). The proteins were electrotransferred onto nitrocellulose
membranes (Amersham, Arlington Heights, IL, USA) in transfer
buffer. Following transfer, all incubations were conducted on a
rocking platform at room temperature. The membranes were blocked in
5% skimmed milk/TBST overnight, then incubated for 1 h with PAX2
(1:700; Zymed, Carlsbad, CA, USA), E-cadherin, fibronectin, snail
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
α-SMA (1:1,500; Sigma, St. Louis, MO, USA) primary antibodies. The
membranes were washed with Tris-buffered saline (TBS) and incubated
with a goat anti-mouse horseradish peroxidase-conjugated secondary
antibody for 1 h. Membranes were then washed with TBS buffer, and
the signals were visualized using the enhanced chemiluminescence
system (Amersham).
Real-time PCR
Total RNA was extracted from the cells. cDNA was
synthesized using 500 ng total RNA with the PrimeScript™ RT-PCR kit
(Takara). Real-time PCR was performed using the SYBR-Green
real-time PCR Master mix (Takara). Primers of PAX2, E-cadherin,
α-SMA, fibronectin, snail and the internal control β-actin were
synthesized by Invitrogen (Table
I). Reactions were amplified in a Roche LightCycler®
(Mannheim, Germany) under the following conditions: initial
denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C
for 20 sec, annealing at 60°C for 20 sec, and polymerization at
72°C for 30 sec. PCR products were analyzed by agarose
electrophoresis. Standard curves were established using serial
dilutions of sample cDNA. These curves were used to measure the
expression levels of the target gene and the reference β-actin gene
using Roche LightCycler® software 4.05. Expression of
the target gene was normalized to β-actin expression.
 | Table IReal-time RT-PCR primers used in the
study and amplification product lengths. |
Table I
Real-time RT-PCR primers used in the
study and amplification product lengths.
| Target | Sequence (5′→3′) | Product length
(bp) |
|---|
| β-actin | F:
ACGTTGACATCCGTAAAGAC | 200 |
| R:
GAAGGTGGACAGTGAGGC | |
| PAX2 | F:
CAACGGTGAGAAGAGGAAACGAG | 195 |
| R:
TAATGCTGCTGGGTGAAGGTGTC | |
| α-SMA | F:
CTCATCCACGAAACCACCTAT | 211 |
| R:
CGCCGATCCAGACAGAATA | |
| E-cadherin | F:
AAAGCAGGAAGAAAACACCACTC | 172 |
| R:
AAAGGGCACGCTATCAACATTAG | |
Statistical analysis
SPSS13.0 software was used for statistical analysis.
The differences between the groups were assessed using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference. The following scheme was used throughout
the results: *P<0.05, **P<0.01 and
***P<0.001. Cells from three wells were analyzed for
each experiment, which was performed at least thrice.
Results
Construction and identification of
pGC-FU-GFP-PAX2 plasmid
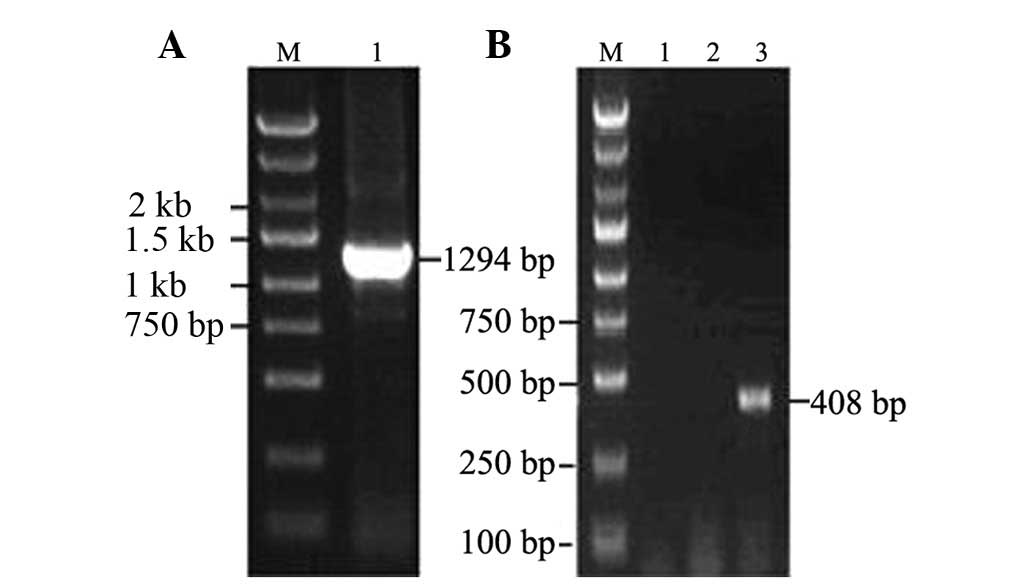
PAX2 mRNA was extracted from the kidney of a UUO
model rat. After PAX2 was digested by AgeI, a 1,294-bp
fragment of PAX2 was directly cloned into the pGC-FU-GFP vector.
Approximately 408-bp long DNA bands were observed in those positive
clones (Fig. 1). The positive
clones were sent to Shanghai Invitrogen Biotechnology Co., Ltd.
After using BLAST in GenBank, PAX2 sequences were successfully
cloned into the eukaryotic expression vector pGC-FU-GFP.
Identification of pGC-FU-GFP-PAX2 plasmid
transfection
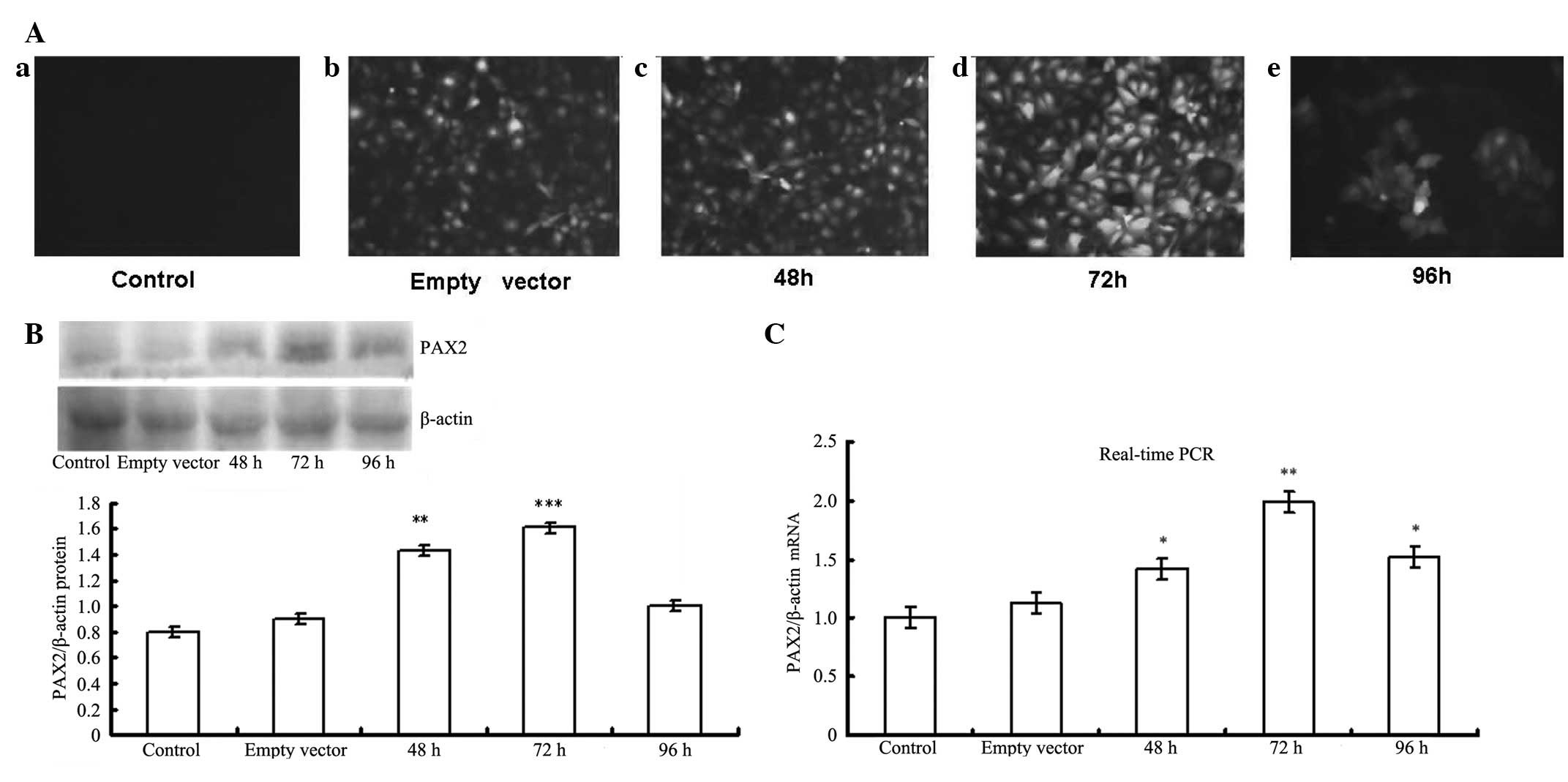
Fluorescence microscopy analysis showed that at 72 h
post-transfection of pGC-FU-GFP-PAX2, the intensity of GFP became
markedly stronger compared with that of controls and other
time-points. Expression of PAX2 protein began to increase 48 h
post-transfection. The expression level peaked at 72 h, and then
decreased at 96 h. Real-time PCR showed the same result (Fig. 2). These results indicate that 72 h
is the optimal time-point during PAX2 transfection.
PAX2 induces mesenchymal morphology of
NRK52E cells
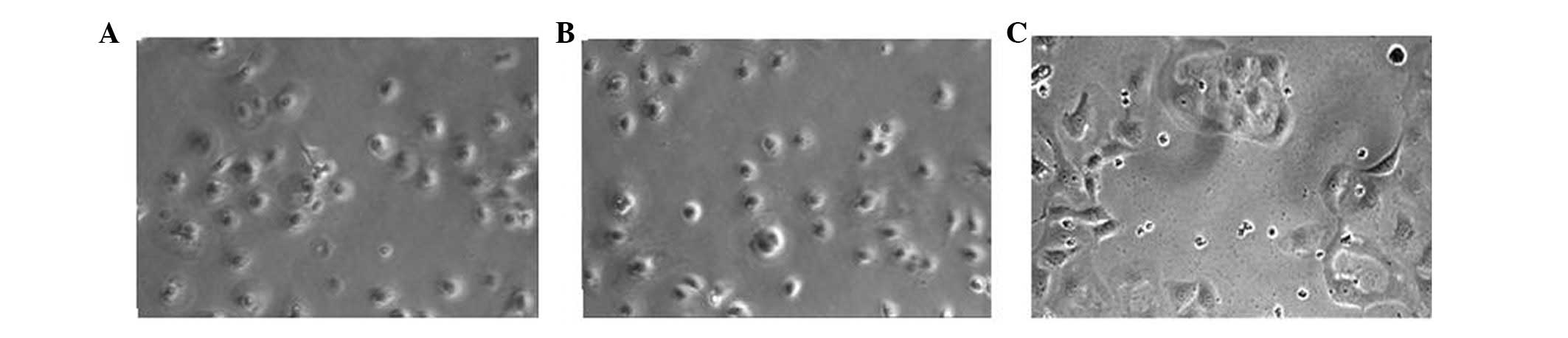
Control and empty vector NRK52E cells showed a
typical epithelial cuboidal shape, with cobblestone morphology.
Transfection with PAX2 caused distinct morphological changes, with
evidence of gross elongation, after 72 h of transfecting PAX2 in
NRK52E cells (Fig. 3).
PAX2 reduces the expression of E-cadherin
in tubular epithelial cells
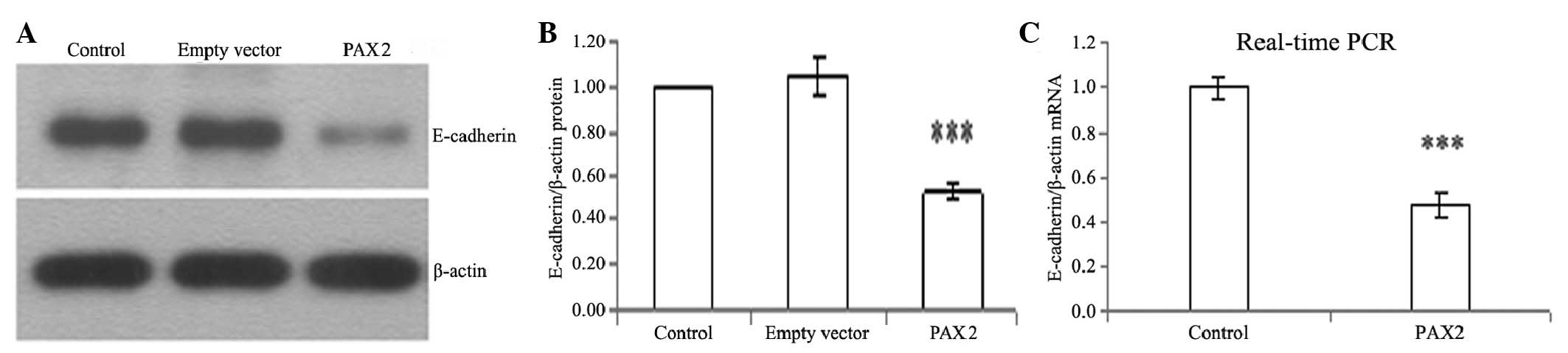
E-cadherin is a tubular epithelial cell-cell
adhesion receptor that is essential for the formation and
maintenance of the homeostasis and architecture of renal epithelia.
It was observed that PAX2 markedly repressed E-cadherin expression
in NRK52E cells. Western blotting and real-time PCR revealed
significant suppression of E-cadherin protein and mRNA expression
levels following PAX2 transfection for 72 h (Fig. 4).
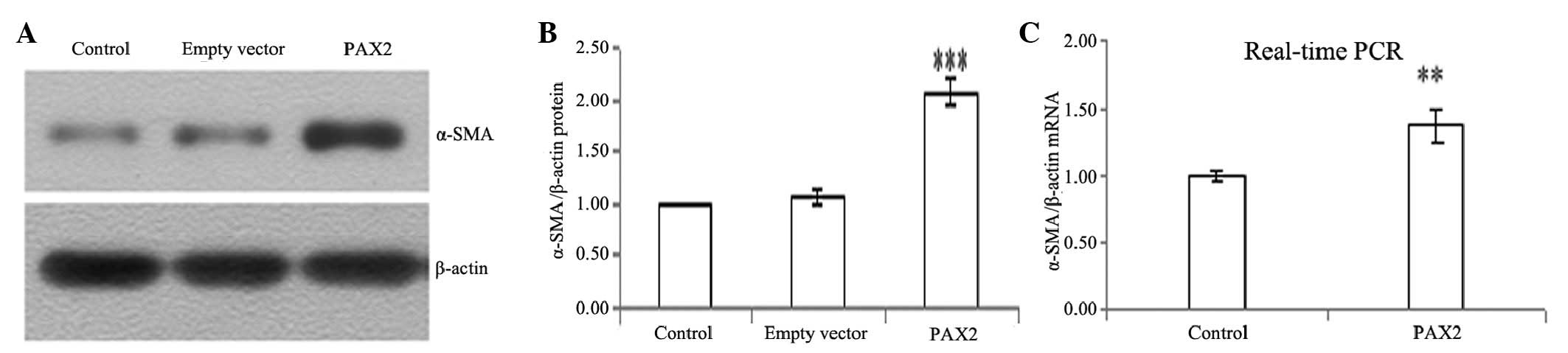
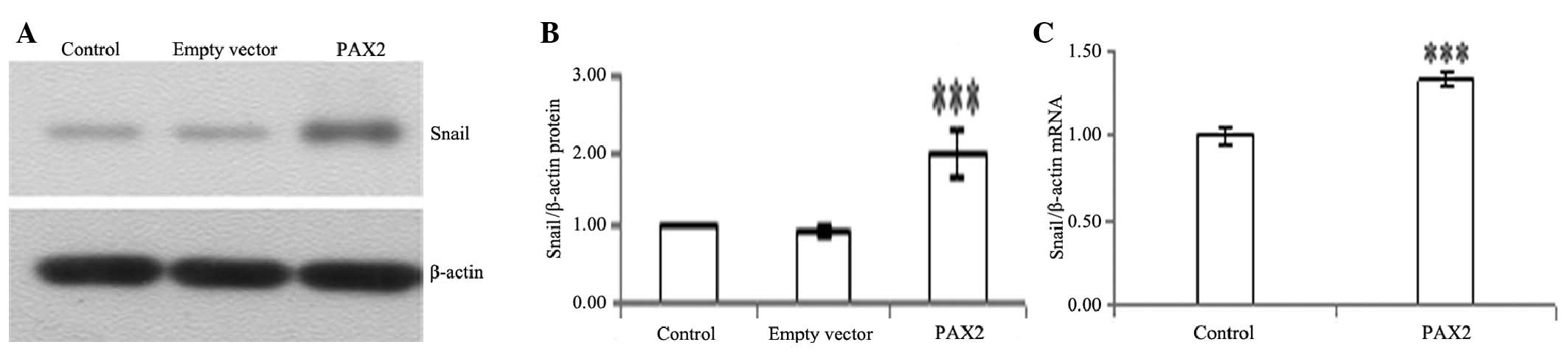
PAX2 induces the expression of α-SMA,
fibronectin and snail
To determine whether tubular epithelial cells
undergo conversion into myofibroblasts in vitro, we used
NRK52E cells as a model system to examine the ability of PAX2 to
induce the de novo expression of α-SMA, fibronectin and
snail, which are the phenotypic markers of myofibroblasts. PAX2
markedly induced protein and mRNA expression of α-SMA, fibronectin
and snail in NRK52E cells (Figs.
5–7).
Discussion
PAX2 is a member of the paired box class of nuclear
transcription factors, and it plays a pivotal role during the early
stages of renal epithelial differentiation. During kidney
development, PAX2 is present in the caudal mesonephric duct, the
ureteric bud, and at a later stage, in the mesenchymal condensates
that are induced by the bud (9).
As the mesenchymal condensates convert into nephron epithelia, PAX2
expression is repressed. Its suppression is essential for the
terminal differentiation of mesenchymal-epithelial transformation.
The increase in PAX2 in transgenic mice leads to severe kidney
abnormalities (10), and PAX2-null
mice lack kidneys, ureters and genital tracts (11). Over- or underexpression of PAX2 in
humans results in renal diseases. Murer et al(12) showed that PAX2 is overexpressed in
juvenile nephronophthisis. It was also observed that PAX2 was
expressed in immature dysplastic tubules, and that it was repressed
in mature renal tubular cells. In addition, PAX2 was present in
severe secondary interstitial fibrosis. The failure of PAX2
repression or the reactivation of PAX2 in juvenile nephronophthisis
probably leads to a primary defect along the cascade of mesenchymal
epithelial differentiation and may be involved in interstitial
fibrosis and cyst formation. Huang et al(13) observed the re-expression of PAX2 in
5/6 nephrectomized rats. These results indicated that the
re-expression of PAX2 may be significant in the process of EMT.
Similarly to those results, in 2010, we showed that PAX2 was
re-expressed in the renal tubular epithelium in a rat UUO
obstructive kidney model (8). PAX2
expression may have resulted from the initiation of renal fibrosis
regulatory mechanisms, and then activated the renal tubular
epithelial cell transdifferentiation. However, to date, PAX2 has
not been shown to be directly involved in renal tubular EMT.
During EMT, the following four key events are
crucial (14–17): loss of epithelial adhesion
properties, such as in E-cadherin; acquired expression of α-SMA
reorganization; disruption of the tubular basement membrane; and
enhanced cell migration and invasion. Our studies showed that PAX2
is capable of inducing tubular epithelial cells to undergo
transferation, which involves three of these events. Although we
did not study cell motility and migration, the PAX2-induced
morphologic alterations, which included the elongation observed
using phase contrast microscopy, are consistent with the improved
migratory capability of the transformed cells. We observed that the
expression of the epithelial cell adhesion molecule E-cadherin
decreased 72 h post-transfection, whereas the expression of α-SMA,
fibronectin and snail mesenchymal markers increased in NRK52E cells
72 h post-transfection, indicating that these cells lost their
epithelial characteristics and acquired mesenchymal cell
properties. These results showed that PAX2 induces the phenotypic
and morphologic alterations of renal tubular epithelial cells in
vitro.
Doberstein et al(18) reported that PAX2 directly binds to
ADAM10 promoter and regulates the expression of ADAM10. Moreover,
PAX2 has been shown to be a regulator of L1-CAM expression. The
downregulation of PAX2 may lead to EMT of renal cancer cells by
downregulation of ADAM10 or the induction of release of soluble
L1-CAM. These results appear to be contradictory to the findings of
the present study. A potential explanation for this result is that
the studies were carried out in different cells. The different
transdifferentiation effects of PAX2 may be due to the different
intracellular environments of the renal carcinoma cells and normal
renal tubular epithelial cells of rats.
In our previous study, it was demonstrated that PAX2
was re-expressed in rats with obstructive nephropathy and may
participate in the pathogenesis of renal tubular damage and renal
interstitial fibrosis (8). Huang
et al(8) showed that
silencing of PAX2 by RNA interference blocked the
interleukin-1-induced EMT in NRK52E cells, as reflected in the
suppression of α-SMA, the restoration of E-cadherin expression and
normal cell morphology (19).
Thus, it is reasonable to speculate that re-expression of PAX2
activates the renal tubular epithelial cell transdifferentiation
procedure and promotes the renal fibrosis process. Blocking PAX2
may reverse EMT and prevent or alleviate renal fibrosis. Zhou et
al(20) demonstrated that
prohibitin and PAX2 are associated with the development of renal
interstitial fibrosis. Decreased expression of prohibitin is
associated with increased PAX2 gene expression and renal
interstitial fibrosis index in UUO rats. In conclusion, the
mechanism by which PAX2 induces EMT requires further
investigation.
Acknowledgements
This study was supported by grants from the
Scientific Technique Project of Shengyang (F10-205-1-29) and the
Doctor Excellent Project Fund of Shengjing Hospital of China
Medical University (ma26).
References
|
1
|
Potenta S, Zeisberg E and Kalluri R: The
role of endothelial-to-mesenchymal transition in cancer
progression. Br J Cancer. 99:1375–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strutz F, Okada H, Lo CW, et al:
Identification and characterization of a fibroblast marker: FSP1. J
Cell Biol. 130:393–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeisberg M, Yang C, Martino M, et al:
Fibroblasts derive from hepatocytes in liver fibrosis via
epithelial to mesenchymal transition. J Biol Chem. 282:23337–23347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KK, Kugler MC, Wolters PJ, et al:
Alveolar epithelial cell mesenchymal transition develops in vivo
during pulmonary fibrosis and is regulated by the extracellular
matrix. Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg EM, Potenta SE, Sugimoto H,
Zeisberg M and Kalluri R: Fibroblasts in kidney fibrosis emerge via
endothelial-to-mesenchymal transition. J Am Soc Nephrol.
19:2282–2287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narlis M, Grote D, Gaitan Y, Boualia SK
and Bouchard M: PAX2 and Pax8 regulate branching morphogenesis and
nephron differentiation in the developing kidney. J Am Soc Nephrol.
18:1121–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng WM, Yi ZW, He XJ, et al: Function of
PAX2 in tubular epithelium transdifferentiation. J Clin Pediatr.
25:284–287. 2007.(In Chinese).
|
|
8
|
Li L, Wu YB and Zhang WG: PAX2
re-expression in renal tubular epithelial cells and correlation
with renal interstitial fibrosis of rats with obstructive
nephropathy. Ren Fail. 32:603–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eccles MR, He S, Legge M, et al: PAX genes
in development and disease: the role of PAX2 in urogenital tract
development. Int J Dev Biol. 46:535–544. 2002.PubMed/NCBI
|
|
10
|
Torres M, Gómez-Pardo E, Dressler GR and
Gruss P: Pax-2 controls multiple steps of urogenital development.
Development. 121:4057–4065. 1995.PubMed/NCBI
|
|
11
|
Dziarmaga A, Eccles M and Goodyer P:
Suppression of ureteric bud apoptosis rescues nephron endowment and
adult renal function in Pax2 mutant mice. J Am Soc Nephrol.
17:1568–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murer L, Caridi G, Della Vella M, et al:
Expression of nuclear transcription factor PAX2 in renal biopsies
of juvenile nephronophthisis. Nephron. 91:588–593. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang B, Pi L, Chen C, et al: WT1 and Pax2
re-expression is required for epithelial-mesenchymal transition in
5/6 nephrectomized rats and cultured kidney tubular epithelial
cells. Cells Tissues Organs. 195:296–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rastaldi MP: Epithelial-mesenchymal
transition and its implications for the development of renal
tubulointerstitial fibrosis. J Nephrol. 19:407–412. 2006.PubMed/NCBI
|
|
15
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar
|
|
16
|
Rastaldi MP, Ferrario F, Giardino L, et
al: Epithelial-mesenchymal transition of tubular epithelial cells
in human renal biopsies. Kidney Int. 62:137–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J and Liu Y: Dissection of key events
in tubular epithelial to myofibroblast transition and its
implications in renal interstitial fibrosis. Am J Pathol.
159:146–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doberstein K, Pfeilschifter J and Gutwein
P: The transcription factor PAX2 regulates ADAM10 expression in
renal cell carcinoma. Carcinogenesis. 32:1713–1723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doberstein K, Wieland A, Lee SB, et al:
L1-CAM expression in ccRCC correlates with shorter patients
survival times and confers chemoresistance in renal cell carcinoma
cells. Carcinogenesis. 32:262–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou TB, Zeng ZY, Qin YH and Zhao YJ: Less
expression of prohibitin is associated with increased paired box 2
(PAX2) in renal interstitial fibrosis rats. Int J Mol Sci.
13:9808–9825. 2012. View Article : Google Scholar : PubMed/NCBI
|