Introduction
Osteoarthritis (OA) is a joint disease,
characterized by the degeneration of articular cartilage and bone
remodeling that clinically results in pain and joint stiffness,
which involves mechanical and biological factors (1,2).
Adult articular cartilage consists of a relatively sparse
population of non-proliferating chondrocytes which produce a large
amount of extracellular matrix (ECM), mainly composed of two major
types of macromolecules, collagens (types II, IX and XI) and
proteoglycans. Chondrocytes are responsible for tissue homeostasis,
including the synthesis and degradation of ECM components (3). Therefore, chondrocyte proliferation
is important in maintaining cellular function.
The cell cycle represents a highly regulated series
of events that leads to eukaryotic cell reproduction. During the
early stages of the cell cycle, DNA is replicated and the
chromosomes are duplicated during the transition through to the S
phase. This process begins at specific DNA sites known as
replication origins. At these sites, the DNA replication licensing
machinery opens the DNA double helix, exposing it to enzymes that
conduct DNA synthesis (4). The S
phase is followed by chromosomal segregation and nuclear and cell
division, which is collectively termed the M phase. The majority of
cell cycles contain additional gap phases between the G1 and S
phase, which provide more time for growth and also serve as
important regulatory transitions, during which progression to the
next cell cycle stage is controlled by intracellular and
extracellular signals (5,6). G1 is a particularly important
regulatory period, since it is during this phase that most cells
either become committed to continued division or exit from the cell
cycle (7,8).
The destiny of a cell is strictly controlled by a
set of cell cycle factors. In particular, the Ser/Thr protein
kinases CDK4 and CDK6 are important for cell cycle progression at
the G1 phase. Cyclin D1 is also a positive regulator of the G1/S
transition and functions as one of the key restriction points in
the cell cycle by binding to CDK4 or CDK6 to control cell cycle
progression from the G1 to the S phase (9).
Bauhinia championii (Benth.)
Benth. polysaccharide (BCBP) treatment
promotes chondrocyte proliferation and may be associated with the
upregulation of cyclin D1, CDK4 and CDK6 levels, which are
important targets in the G1/S transition of the cell cycle.
Natural products have been considered as alternative
medicine for several years. Numerous plants and their constituents
have been shown to possess beneficial therapeutic effects for
various diseases (10).
Polysaccharides isolated from plants have attracted a large amount
of research attention due to their broad spectrum of therapeutic
properties and their relatively low toxicity (11–13).
In modern pharmacological studies, polysaccharides isolated from
plants have been shown to carry out anti-inflammatory, anti-oxidant
and antitumor activities (14–16).
However, to the best of our knowledge, no previous studies have
been conducted on the effects of BCBP on chondrocytes.
In the present study, polysaccharides from
Bauhinia championii (Benth.) Benth. were obtained using
water extraction and ethanol precipitation. To investigate the
effects of BCBP on the proliferation and cell cycle of
chondrocytes, an MTT assay was used to evaluate the proliferative
activity of BCBP. In addition, the cell cycle distribution was
detected by flow cytometry. Furthermore, the mRNA and protein
expression levels of cyclin D1, CDK4 and CDK6 were determined using
reverse transcription polymerase chain reaction (RT-PCR) and
western blot analysis, respectively.
Materials and methods
Drug preparation. Bauhinia championii
(Benth.)
Benth. was ground and dried and the powder
(100 g) was extracted with pure water (1:15, w/v) at 85°C for 6 h,
followed by centrifugation at 3,000 r/min for 15 min. Water
extracts were collected and the dregs extracted. The combined
extracts were pooled and condensed to ~100 ml under a reduced
pressure. Subsequently, 400 ml of 95% alcohol by volume was slowly
added by stirring to precipitate the polysaccharides. This
polysaccharide sediment was further refined by repeated dissolution
and precipitation 3 times, followed by a wash with ethanol, acetone
and ether (17).
Animals
Healthy and clean 4-week-old male Sprague-Dawley
rats (n=30; weight, 90–110 g) were purchased from the Shanghai
Laboratory Animal Commission (SLAC, Shanghai, China). The animal
license number for the rats was SCXK (Shanghai) 2008–0005.
Experiments involving the animals complied with the Guidance
Suggestions for the Care and Use of Laboratory Animals 2006
administered by the Ministry of Science and Technology of the
People’s Republic of China (18).
Reagents
Fetal bovine serum (FBS), Dulbecco’s modified
Eagle’s medium (DMEM) and Trypsin-EDTA were obtained from HyClone
(Logan, UT, USA). Type II collagenase and
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma-Aldrich (St. Louis, MO, USA). The TRIzol
reagent was obtained from Invitrogen (Carlsbad, CA, USA). A reverse
transcription test kit was obtained from Promega (Madison, WI, USA)
and the cell cycle test kit was purchased from Becton-Dickinson
(San Jose, CA, USA). A total protein extraction kit was purchased
from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Polyvinylidene fluoride (PVDF) membranes were purchased from
Millipore (Billerica, MA, USA). Rabbit anti-cyclin D1, -CDK4,
-CDK6 and -β-actin and HRP secondary goat anti-rabbit
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Any additional chemicals, unless otherwise
stated, were obtained from Sigma-Aldrich.
Isolation and culture of rat
chondrocytes
Articular cartilage obtained from rat knee joints
was rinsed in PBS and DMEM three times. The cartilage was sectioned
into 1-mm3 slices, digested with 0.2% type II
collagenase and then transferred to a 37°C incubator to isolate the
chondrocytes. The supernatant was collected every 2 h and
centrifuged at 1,000 r/min for 5 min to obtain the cell pellet. The
cells were then filtered through 200 mesh stainless steel filters,
seeded (5×105 cells/ml) in 6-well plates in DMEM
containing 10% FBS and cultivated in a CO2 incubator (5%
CO2 at 37°C) (19). The
primary cultured cells were observed under an inverted microscope
and passaged upon reaching 80% confluence.
Identification of chondrocytes using
immunohistochemical staining
The second generation chondrocytes were seeded onto
cover slips and cultured for 72 h. The cells were washed with PBS
and fixed in 4% neutral formalin for 30 min. Subsequent steps were
performed according to the manufacturer’s instructions. The
expression of type II collagen was observed using
immunohistochemical staining. Images were captured at a
magnification of ×40.
Evaluation of cell viability using the
MTT assay
The viability of chondrocytes was assessed using the
MTT colorimetric assay. The second passage chondrocytes were seeded
in 96-well plates at a density of 1.0×104 cells/well in
0.1 ml of 10% FBS/DMEM. The chondrocytes were treated with various
concentrations of BCBP for 24, 48 or 72 h. The medium was then
removed and 20 μl of 0.5% MTT solution was added to each well,
followed by incubation at 37°C for 4 h. The purple-blue MTT
formazan precipitate was dissolved in 100 μl dimethyl sulfoxide
(DMSO). The absorbance was measured at 490 nm using an ELISA reader
(ELx800™; BioTek Instruments, Inc., Winooski, VT, USA).
Cell cycle analysis using flow cytometry
with PI staining
Chondrocytes were seeded in 35-mm petri dishes at a
density of 5×104 cells/ml. The chondrocytes were treated
with various concentrations of BCBP for 48 h. The cells were then
digested, collected and resuspended in PBS. The cell concentration
was adjusted to 1×106 cells/ml following centrifugation.
Solutions A, B and C were added according to the manufacturer’s
instructions. ModFit software was used to analyze the DNA content
and to count the cell numbers in the G0/G1, S and G2/M phases.
RNA extraction and RT-PCR analysis
Chondrocytes (1×105) were seeded in
6-well plates in 2 ml of medium and treated with various
concentrations of BCBP for 48 h. Total RNA was isolated with TRIzol
reagent. RNA (1 μg) was reverse transcribed into cDNA. The obtained
cDNA was used to determine the mRNA levels of cyclin D1, CDK4 and
CDK6. β-actin was used as an internal control. The sequences of the
primers used for amplification of cyclin D1, CDK4 and CDK6 were as
follows: Cyclin D1 forward, 5′-AAT GCC AGA GGC GGA TGA GA-3′ and
reverse, 5′-GCT TGT GCG GTA GCA GGA GA-3′, 189 bp; CDK4 forward,
5′-GAA GAC GAC TGG CCT CGA GA-3′ and reverse, 5′-ACT GCG CTC CAG
ATT CCT CC-3′, 109 bp; CDK6 forward, 5′-TTG TGA CAG ACA TCG ACG
AG-3′ and reverse, 5′-GAC AGG TGA GAA TGC AGG TT-3′, 151 bp; and
β-actin forward, 5′-CGT TGA CAT CCG TAA AGA CC-3′ and reverse,
5′-GGA GCC AGG GCA GTA ATC T-3′, 108 bp.
Western blot analysis
Chondrocytes were seeded in culture flasks and
treated with or without BCBP at 37°C for 48 h. Cells were scraped
from the culture, washed twice with PBS and then suspended in 30 μl
western blotting lysis buffer. The protein concentration was
determined using a bicinchoninic acid (BCA) protein assay. Samples
were loaded with 20 μg protein and separated by electrophoresis on
12% SDS-polyacrylamide gels. Following electrophoresis, protein
blots were transferred to PVDF membranes. The membranes were
blocked with 5% skimmed milk in TBST solution and incubated
overnight with the primary antibodies at 4°C. The membranes were
then washed in TBST and exposed to secondary antibodies. The
membranes were developed by ECL Plus Western Blotting Detection
Reagents (Molecular Imager ChemiDoc XRS System; Bio-Rad, Hercules,
CA, USA)
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) when appropriate. All the experiments were repeated at least
three times and the representative results are shown. Results were
analyzed by one-way ANOVA (control vs. treatments) followed by
Student’s t-test using SPSS 16.0 software. P<0.05 was considered
to indicate a statistically significant difference.
Results
Morphological observation and
characterization of chondrocytes
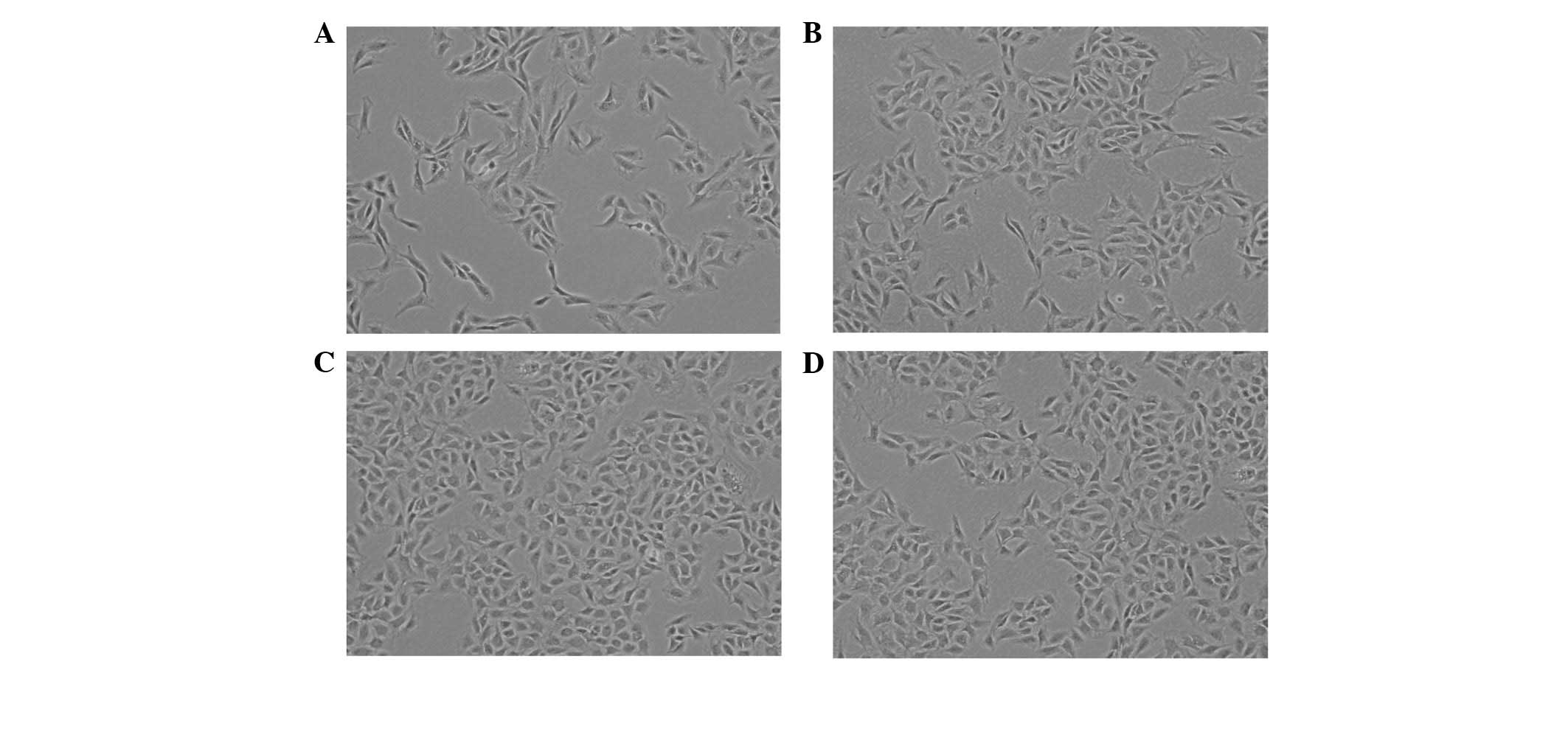
The second generation chondrocytes were treated with
various concentrations of BCBP for 48 h. BCBP treatment
significantly increased the total number of chondrocytes compared
with the untreated control cells (Fig.
1), indicating that BCBP promotes chondrocyte
proliferation.
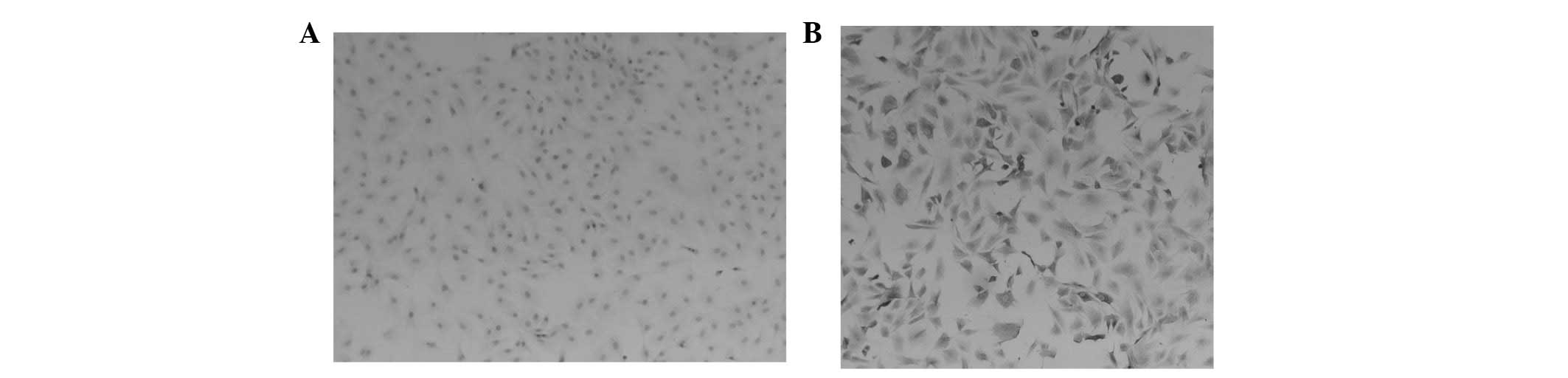
To characterize the chondrocytes, the effects of
BCBP on type II collagen expression were evaluated using
immunohistochemical staining. Type II collagen matrix is a
chondrocyte-specific protein and may be used as a biomarker to
identify chondrocytes (20,21).
The results demonstrated that the cytoplasm of the negative group
of cells was unstained (Fig. 2A),
whereas the cytoplasm of chondrocytes was stained a brown-yellow
color (Fig. 2B).
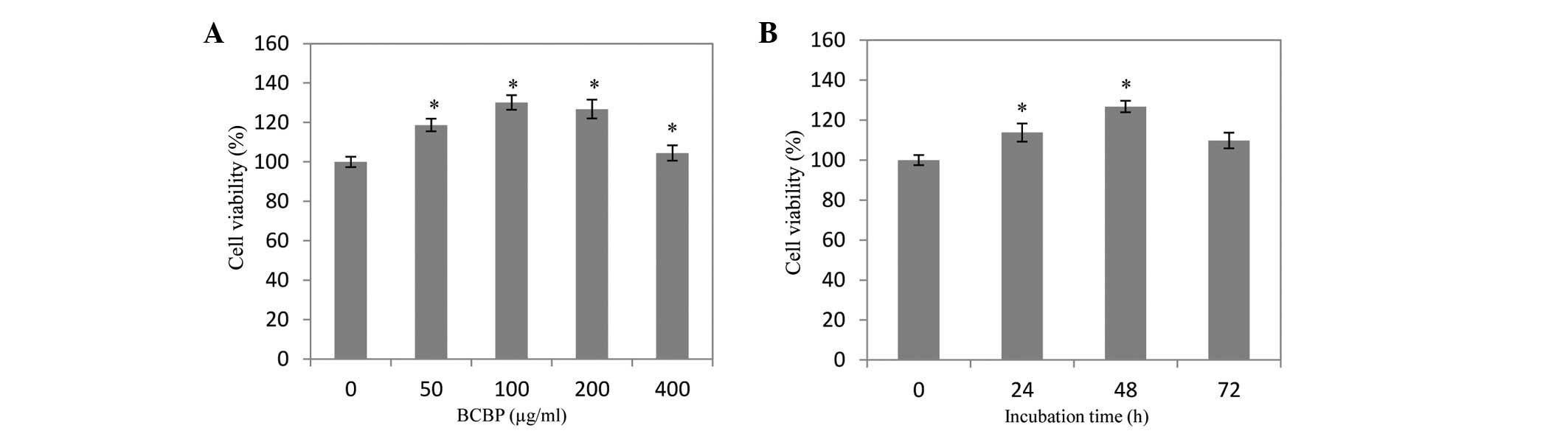
Effect of BCBP on the viability of
chondrocytes
The cells were treated with 50–200 μg/ml BCBP for 48
h to evaluate the effect of BCBP on the viability of chondrocytes.
As shown in Fig. 3, the viability
of chondrocytes was increased by 18.72±2.61, 30.13±3.24 and
26.87±3.77% when the cells were treated with 50, 100 and 200 μg/ml
of BCBP, respectively. Furthermore, the viability of chondrocytes
was increased by 3.24±2.06, 11.57±2.30 and 19.73±2.42% when the
cells were treated with 200 μg/ml BCBP for 24, 48 and 72 h,
respectively, compared with untreated cells (P<0.05).
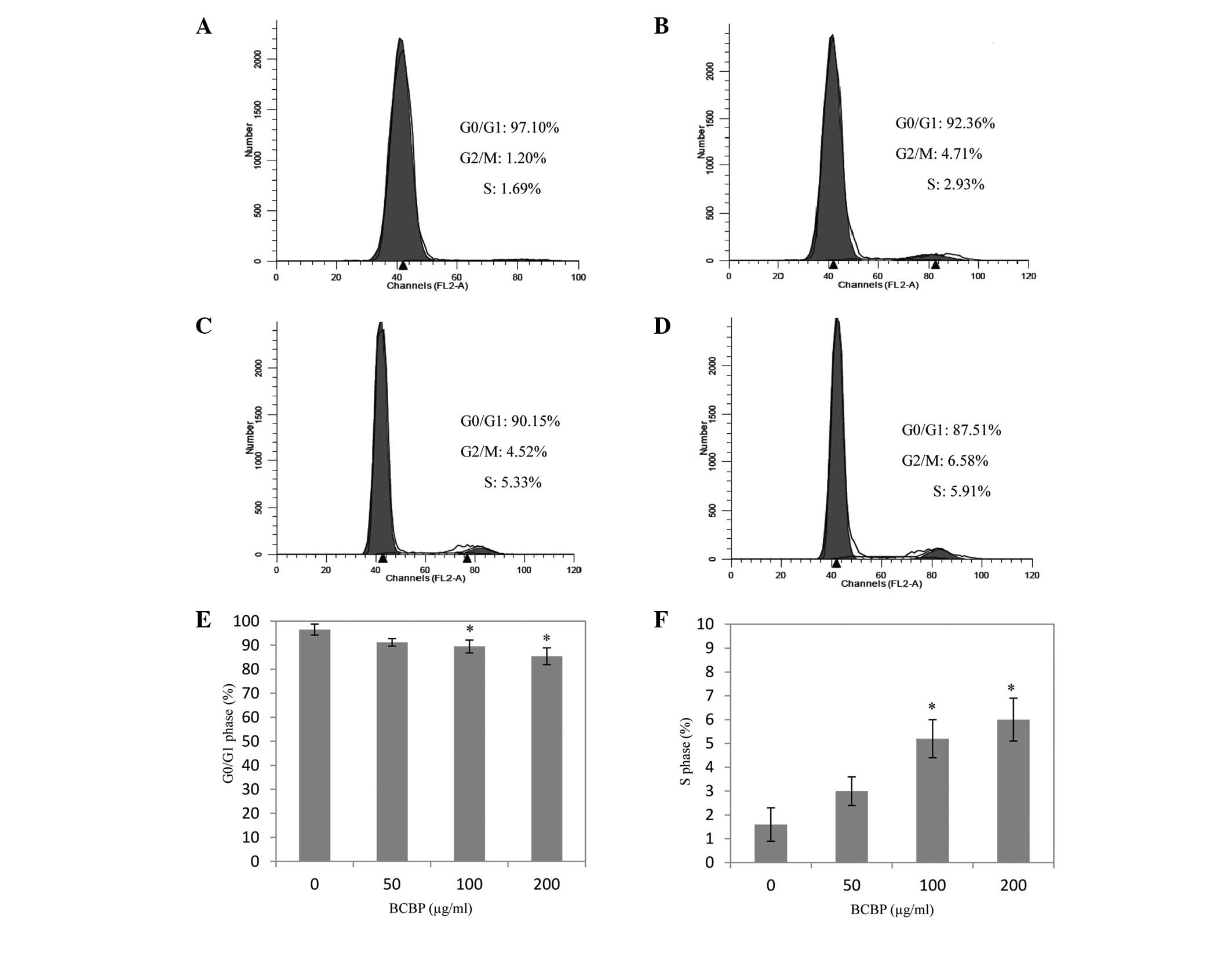
Effect of BCBP on the cell cycle of
chondrocytes
Chondrocytes were treated with 50, 100 and 200 μg/ml
of BCBP for 48 h to explore the effect of BCBP on cell cycle
progression. As shown in Fig. 4,
BCBP treatment decreased the percentage of cells in the G0/G1
phase, while the percentage of cells in the S phase was increased
(P<0.05). Taken together, the results suggest that BCBP enhances
cell cycle progression of the chondrocytes by promoting the G1 to S
phase transition.
Effect of BCBP on the expression of
cyclin D1, CDK4 and CDK6 in chondrocytes
Following BCBP treatment for 48 h, the mRNA and
protein expression levels of cyclin D1, CDK4 and CDK6 were
determined using RT-PCR and western blot analysis, respectively.
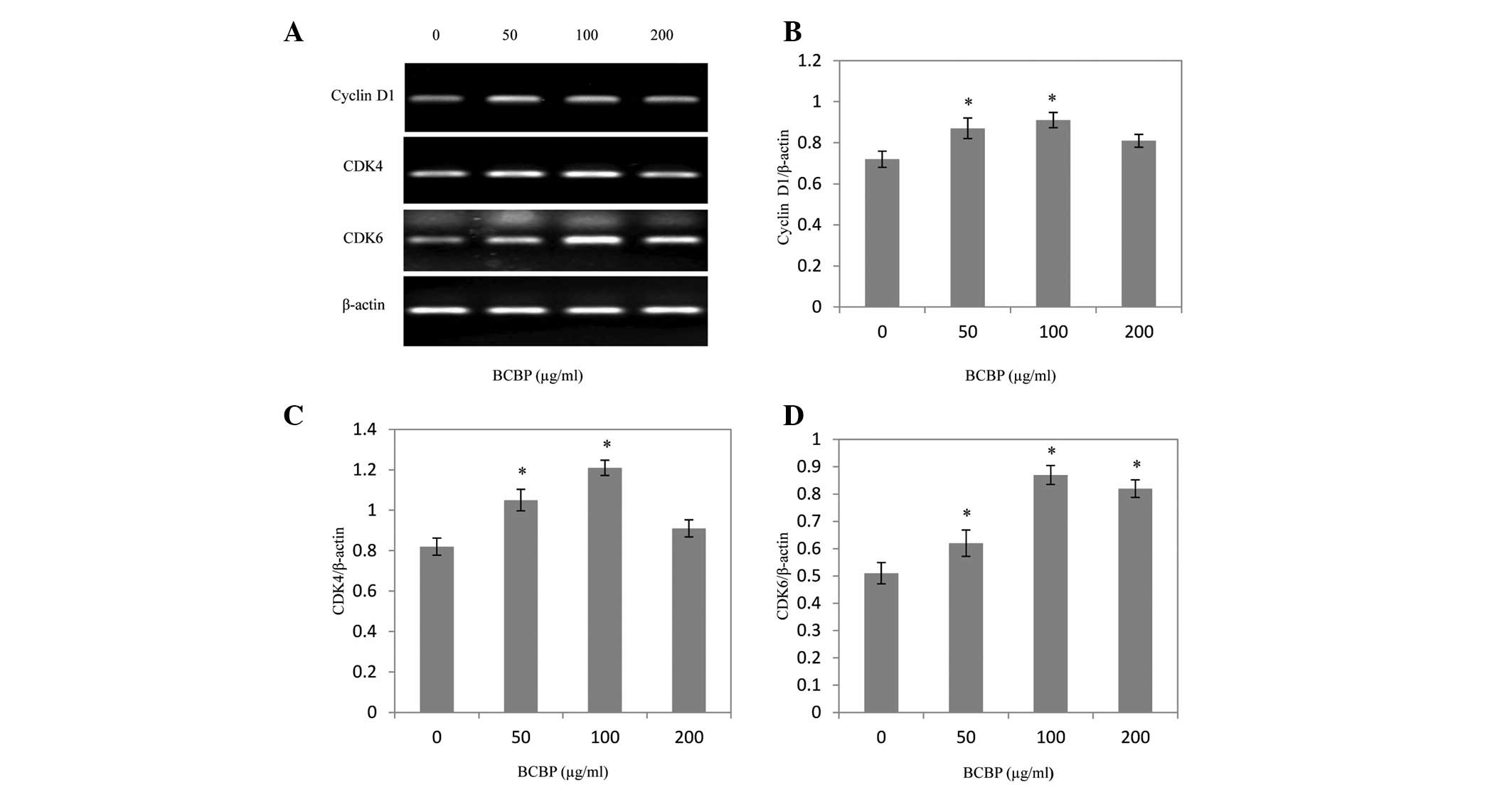
The results of the RT-PCR assay showed that BCBP treatment
significantly increased cyclin D1, CDK4 and CDK6 mRNA expression
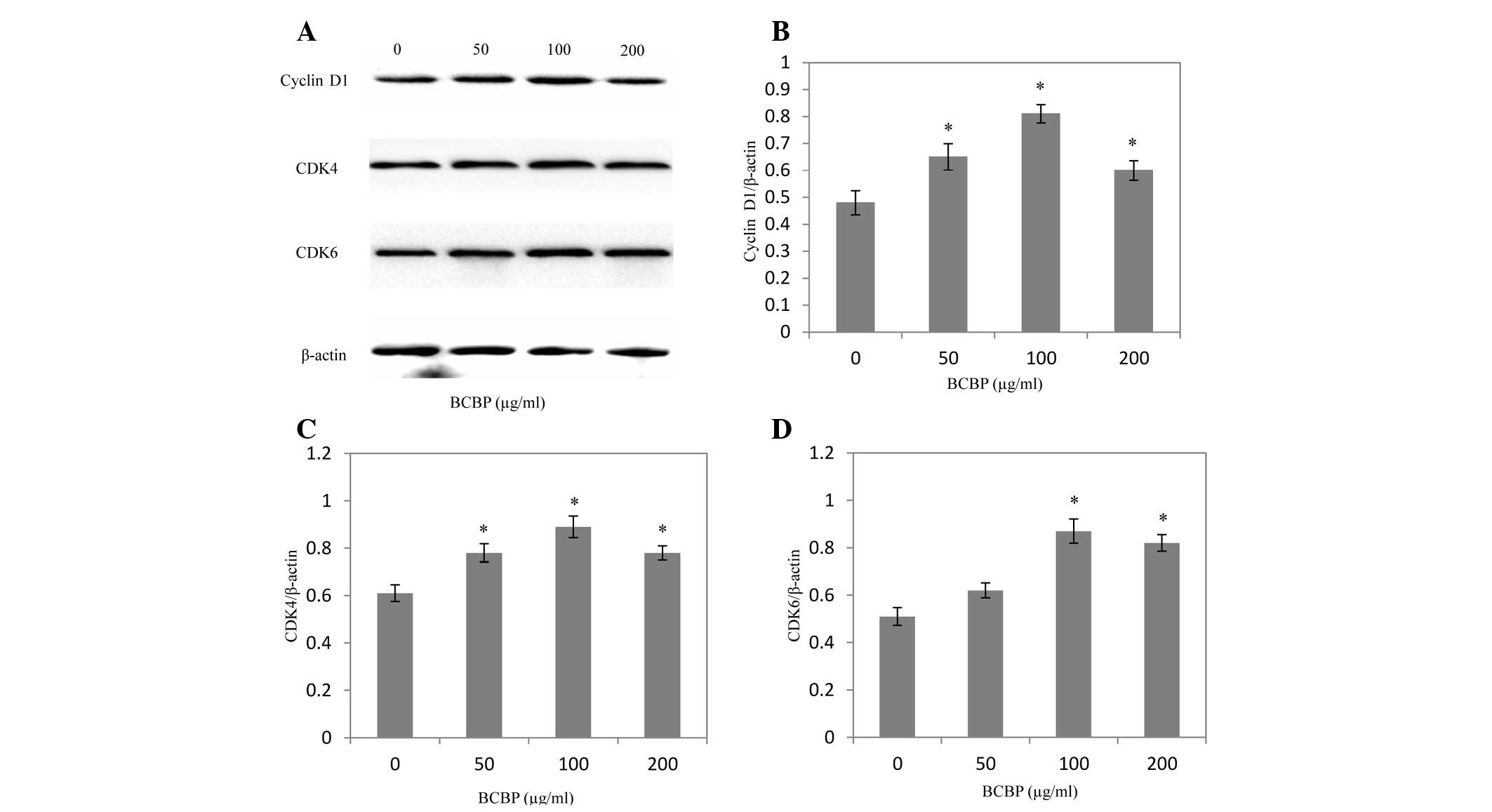
levels in chondrocytes (P<0.05, Fig. 5). The protein expression patterns
of cyclin D1, CDK4 and CDK6 were similar to those of their
respective mRNA levels (P<0.05, Fig. 6).
Discussion
OA, the most common age-related cartilage and joint
disorder, is a slow progressing degenerative disease characterized
by the degradation of the ECM and cell death resulting in a gradual
loss of articular cartilage integrity (22–24).
The only cell type present in mature cartilage is chondrocytes,
which are responsible for repairing damaged cartilage tissue.
Low proliferative activity in osteoarthritic
chondrocytes has recently been demonstrated; therefore, a treatment
that promotes the proliferative activity of chondrocytes may be
efficient to delay or even cease the progression of OA (25). The present study mainly focused on
exploring drugs that promote the proliferation of chondrocytes.
Bauhinia championii (Benth.)
Benth. is a herbal medicine demonstrated to
be clinically effective in treating OA. It possesses the functions
of expelling wind and removing dampness, in addition to promoting
blood circulation and relieving pain. However, the mode of action
of this treatment for OA remains to be elucidated. Before
Bauhinia championii (Benth.) Benth. is further developed as
an agent for treating OA, its underlying molecular mechanism
requires investigation.
The cell cycle is composed of four different stages;
the G1, S, G2 and M phases. Preparation for DNA synthesis occurs
during the G1 phase, DNA synthesis is carried out during the S
phase, preparation for mitosis occurs in the G2 phase and mitosis
is achieved in the M phase. The amount of DNA in a cell changes
during the cell cycle, allowing the different stages of the cycle
to be identified by analyzing the DNA content. The DNA content is
2N in the G0 and G1 phases. Following DNA synthesis in the S phase,
DNA content becomes 4N in the G2 and M phases (26,27).
Flow cytometric analysis, which measures the DNA content of cells,
is more sensitive to the changes during the cell cycle compared
with the MTT method. In the present study, cell cycle distribution
was carried out using flow cytometry. The results showed that after
BCBP treatment, the G0/G1 ratio was reduced and the S ratio
increased, indicating that BCBP treatment promotes cell
proliferation by enhancing G1 phase entry and accelerating the G1/S
transition.
G1/S and G2/M are two important checkpoints
regulating stage transition and cell cycle progression. Stage
transitions in the cell cycle are controlled by interactions among
the molecules of the cyclin-CDK-CDK inhibitor (CKI) axis. In this
system, cyclins interact with CDKs to positively regulate their
activity (28,29). Cyclin D1, CDK4 and CDK6 are
important in the G1/S transition of the cell cycle. The mRNA and
protein expression levels of cyclin D1, CDK4 and CDK6 in
chondrocytes were detected using RT-PCR and western blot analysis,
respectively. The results showed that BCBP treatment effectively
enhanced the mRNA and protein levels of cyclin D1, CDK4 and CDK6.
In agreement with these results, flow cytometric analysis showed
that the S ratio increased, while the G1 ratio decreased with BCBP
treatment.
In conclusion, the present study has demonstrated
that BCBP promotes chondrocyte proliferation by accelerating the
G1/S transition and upregulating the expression of cyclin D1, CDK4
and CDK6. These results suggest that BCBP is a potential novel
therapeutic agent for the treatment of knee OA.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81072826), the Natural Science
Foundation of Fujian Province (no. 2010Y0032) and the Chen Keji
Integrative Medicine Developmental Foundation (no. CKJ2011003).
Abbreviations:
|
BCBP
|
Bauhinia championii (Benth.)
Benth. polysaccharide
|
|
OA
|
osteoarthritis
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Wattanachai T, Yonemitsu I, Kaneko S and
Soma K: Functional lateral shift of the mandible effects on the
expression of ECM in rat temporomandibular cartilage. Angle Orthod.
79:652–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gentili C and Cancedda R: Cartilage and
bone extracellular matrix. Curr Pharm Des. 15:1334–1348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brondello JM, Philipot D, Djouad F,
Jorgensen C and Noël D: Cellular Senescence is a Common
Characteristic Shared by Preneoplasic and Osteo-Arthritic Tissue.
Open Rheumatol J. 4:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grimmer C, Balbus N, Lang U, Aigner T,
Cramer T, Müller L, Swoboda B and Pfander D: Regulation of type II
collagen synthesis during osteoarthritis by proly-4-hydroxylases:
possible influence of low oxygen levels. Am J Pathol. 169:491–502.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuji K, Bandyopadhyay A, Harfe BD, Cox K,
Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ and Rosen V: BMP2
activity, although dispensable for bone formation, is required for
the initiation of fracture healing. Nat Genet. 38:1424–1429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Planas-Silva MD and Weinberg RA: The
restriction point and control of cell proliferation. Curr Opin Cell
Biol. 9:768–772. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zetterberg A and Larsson O: Kinetic
analysis of regulatory events in G1 leading to proliferation or
quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA.
82:5365–5369. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Xie R, Hou W, Wang B, Shen R,
Wang X, Wang Q, Zhu T, Jonason JH and Chen D: PTHrP prevents
chondrocyte premature hypertrophy by inducing cyclin-D1-dependent
Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal
degradation. J Cell Sci. 122:1382–1389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tzianabos AO: Polysaccharide
immunomodulators as therapeutic agents: structural aspects and
biological function. Clin Microbiol Rev. 13:523–533. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paulsen BS: Plant polysaccharides with
immunostimulatory activities. Curr Org Chem. 5:939–950. 2001.
View Article : Google Scholar
|
|
13
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI
|
|
14
|
Xu R, Ye H, Sun Y, Tu Y and Zeng X:
Preparation, preliminary characterization, antioxidant,
hepatoprotective and antitumor activities of polysaccharides from
the flower of tea plant (Camellia sinensis). Food Chem
Toxicol. 50:2473–2480. 2012. View Article : Google Scholar
|
|
15
|
Zhang BZ, Yan PS, Chen H and He J:
Optimization of production conditions for mushroom polysaccharides
with high yield and antitumor activity. Carbohydr Polym.
87:2569–2575. 2012. View Article : Google Scholar
|
|
16
|
Hua Y, Gao Q, Wen L, Yang B, Tang J, You L
and Zhao M: Structural characterisation of acid- and alkali-soluble
polysaccharides in the fruiting body of Dictyophora
indusiata and their immunomodulatory activities. Food Chem.
132:739–743. 2012. View Article : Google Scholar
|
|
17
|
Sui Z, Gizaw Y and BeMiller JN: Extraction
of polysaccharides from a species of Chlorella. Carbohydr
Polym. 90:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance Suggestions for the Care
and Use of Laboratory Animals. Beijing, China: 2006
|
|
19
|
Li X, Du M, Liu X, et al: Millimeter wave
treatment promotes chondrocyte proliferation by upregulating the
expression of cyclin-dependent kinase 2 and cyclin A. Int J Mol
Med. 26:77–84. 2010.PubMed/NCBI
|
|
20
|
Machida YJ, Hamlin JL and Dutta A: Right
place, right time, and only once: replication initiation in
metazoans. Cell. 123:13–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heinegård D, Bayliss MT and Lorenzo P:
Biochemistry and metabolism of normal and osteoarthritic cartilage.
Osteoarthritis. Brandt KD, Doherty M and Lohmander LS: Oxford
University Press; New York, NY: pp. 74–84. 1998
|
|
23
|
Pritzker KPH: Pathology of osteoarthritis.
Osteoarthritis. Brandt KD, Doherty M and Lohmander LS: Oxford
University Press; New York, NY: pp. 50–61. 1998
|
|
24
|
Kim HA and Blanco FJ: Cell death and
apoptosis in osteoarthritic cartilage. Curr Drug Targets.
8:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang SG, Song SM, Kim JR, Park CS, Song
WK and Chun JS: Regulation of type II collagen expression by
cyclin-dependent kinase 6, cyclin D1, and p21 in articular
chondrocytes. IUBMB Life. 59:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li TF, Chen D, Wu Q, et al: Transforming
growth factor-β stimulates cyclin D1 expression through activation
of β-catenin signaling in chondrocytes. J Biol Chem.
281:21296–21304. 2006.
|
|
28
|
Susaki E, Nakayama K, Yamasaki L and
Nakayama KI: Common and specific roles of the related CDK
inhibitors p27 and p57 revealed by a knock-in mouse model. Proc
Natl Acad Sci USA. 106:5192–5197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng A and Solomon MJ: Speedy/Ringo C
regulates S and G2 phase progression in human cells. Cell Cycle.
7:3037–3047. 2008. View Article : Google Scholar : PubMed/NCBI
|