Introduction
Numerous diseases cause breakdown of the
blood-retinal barrier (BRB) and macular edema. Hypoxia, altered
blood flow and retinal ischemia are closely correlated with the
development and progression of macular edema (1,2).
Currently, glucocorticoids (GCs) are the most
commonly used medicine for ocular applications. Recent studies have
demonstrated the efficacy and complications associated with
intravitreal GC injection for diabetic macular edema (3,4). GCs
may act by suppressing inflammation and directly affecting
endothelial cells, through regulation of the phosphorylation,
organization and content of tight junction proteins. GCs have also
been demonstrated to reduce the breakdown of the BRB (5). It has been identified that GCs
upregulate the tight junction transmembrane proteins, occludin and
claudin 5, in primary retinal endothelial cells through
transactivation of the promoters for these genes (6).
Although GCs have been demonstrated to ameliorate
macular edema, the side-effects accompanying treatment with GCs
cause their frequent use to be problematic. One of the major
side-effects of intravitreal injection of triamcinolone acetonide
(TA), a synthetic corticosteroid, is steroid-induced increases in
intraocular pressure (IOP). One study demonstrated that a rise in
IOP to values >21 mmHg may be expected to occur in ~50% of
treated eyes (7). The frequency of
cataract surgery following intravitreal injection of high-dose TA
in elderly patients is increasing (8).
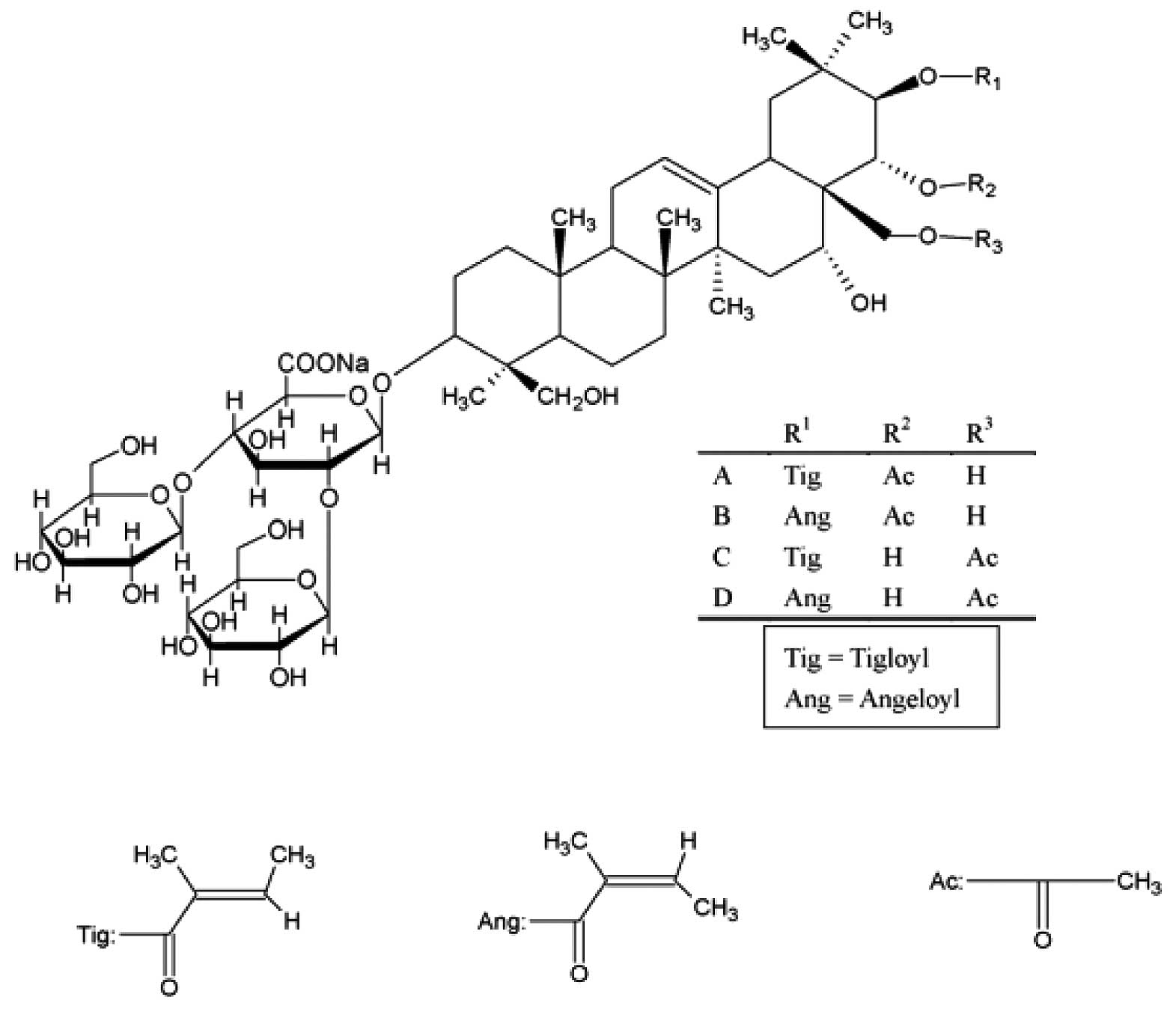
Escin is a natural mixture of triterpene saponins,
mainly consisting of A-, B-, C- and D-escin (Fig. 1). Accumulating experimental
evidence suggests that escin exerts anti-inflammatory and
anti-edematous effects. Escin inhibits acetic acid-induced
increases in capillary permeability and adhesion formation in
animal models. It also attenuates cognitive deficits and
hippocampal injury following transient global cerebral ischemia in
mice, by regulating certain inflammatory genes (9,10).
Furthermore, escin has been demonstrated to be a safe and potent
anti-inflammatory drug with long-term, effective anti-inflammatory
action without immunosuppression (11,12).
Escin also exerts synergistic anti-inflammatory effects with low
doses of GCs in vivo and in vitro(13). In China, escin is widely used
(clinically) for the treatment of retinal vein occlusion and
central serous chorioretinopathy (14–16).
In the present study, we investigated whether the
combination of escin and GCs was able to produce synergistic
protective effects on BRB breakdown in a rat model of acute retinal
ischemia.
Materials and methods
Subjects and drugs
Male Sprague-Dawley rats (weight, 180±20 g) were
obtained from the Experimental Animal Center of Luye Pharma Group
(Yantai, China). Rats were kept in an air-conditioned room
(temperature, 23±2°C) with a 12-h light-dark cycle, with free
access to food and water. Animal experimental procedures were
conducted in strict accordance with the National Institutes of
Health regulations on the use and care of animals for scientific
purposes (NIH Publication No. 0-23, revised 1996). Surgical
procedures and the sacrifice of animals (at the end of the
observation period) were performed under general anesthesia induced
by an intraperitoneal (i.p.) injection of chloral hydrate (350
mg/kg).
Sodium salt of escin (sodium escinate), consisting
of A-, B-, C- and D-escin, was obtained from Shandong Luye
Pharmaceutical Co., Ltd. (batch no. 1206043; Yantai, China) and TA
injections were provided by Kunming Jida Pharmaceutical Co., Ltd.
(batch no. 120105; Kunming, China). Evans blue dye (Sinopharm
Chemical Reagent Co., Ltd, Shanghai, China) was prepared by
dissolving the dye in normal saline (45 mg/ml).
Retinal ischemia-reperfusion study
protocol
Rats were anesthetized intraperitoneally with
chloral hydrate (350 mg/kg) and chlorpromazine (3 mg/kg). Five
minutes following administration of the anesthetics, the anterior
chamber of the left eye was cannulated with a 30-gauge needle
connected by silicone elastomer tubing to a reservoir of balanced
oxyglutathione solution. The IOP was raised to 120 mmHg by
increasing the height of reservoir. The eye was examined with an
ophthalmoscope to confirm that the retinal vessels had collapsed
and the retina appeared gray, indicating that the retina was
ischemic. After 50 min of retinal ischemia, the IOP had returned to
normal and ophthalmoscopy was used to confirm that the circulation
had also returned to normal. The body temperature of the rats was
maintained at 37°C with a heating pad during ischemia, until the
rats had recovered from anesthesia.
Following surgery, the rats were divided randomly
into four groups: the ischemia-reperfusion (IR); TA (2 and 5 μl)
treatment; escin (0.9 and 1.8 mg/kg) treatment; and combined escin
(0.9 mg/kg) and TA (2 μl) treatment groups. Following the induction
of general anesthesia, pupils were dilated with 0.5% tropicamide.
The rats were treated with escin through the caudal vein and with
TA through the vitreous body. A group of rats underwent surgery
without increased IOP, in order to serve as a sham group.
Twenty-four hours after surgery, the BRB permeability was
assayed.
Evaluation of BRB permeability
Following the induction of general anesthesia with
chloral hydrate (350 mg/kg), Evans blue was injected into the blood
through the caudal vein at a dosage of 45 mg/kg. When the dye had
circulated for 120 min, the chest cavity was opened and the rats
were perfused via the left ventricle with citrate buffer (0.05 M,
pH 3.5) for 2 min at 37°C. Immediately after perfusion, both eyes
were enucleated and bisected at the equator. The retinas were then
carefully dissected away. Following measurement of the retinal
weight, Evans blue was extracted by incubating each retina in 180
μl formamide for 18 h at 70°C. The extract was ultracentrifuged for
60 min at 12,000 × g and 25°C. In total, 60 μl supernatant was used
for spectrophotometry. The background-subtracted absorbance was
determined by measuring the absorbance of each sample at 620 nm
(the absorbance maximum for Evans blue in formamide) and at 740 nm
(the absorbance minimum). BRB breakdown was calculated as follows:
Evans blue weight (ng) × retinal weight (mg)-1.
Morphological analysis
Retinal thickness was evaluated in hematoxylin and
eosin-stained sections using the Metamorph/BX51 Microscopic image
analysis software (Molecular Devices, USA). The eyes were cut
vertically through the center of the cornea and optic nerve, and
the two halves were embedded face down. Care was taken to maintain
a consistent cutting angle for all the eyes measured for retinal
thickness. Images of retinal sections were captured and retinal
thickness was measured under the same conditions.
Immunohistochemistry
Twenty-four hours after renal ischemia, rats were
deeply anesthetized and transcardially perfused with saline
solution, followed by 4% paraformaldehyde in 0.1 M
phosphate-buffered saline (PBS) for 2 min. The eyes were enucleated
and post-fixed in 4% paraformaldehyde for 24 h. Coronal sections
(10 μm) were obtained using a Leica CM1950S cryostat (Leica
Microsystems, Wetzlar, Germany). Sections were blocked with 3%
normal goat serum (diluted in PBS containing 0.3% Triton X-100) for
1 h and incubated with primary antibodies (anti-occludin; 1:200;
Abcam, Cambridge, MA, USA) overnight at 4°C. Following rinsing with
PBS, sections were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (1:200; Beyotime Institute
of Biotechnology, Haimen, China) for 2 h at room temperature. The
images from five fields of each ischemic region, from six rats in
each group, were examined using the same brightness and exposure
settings.
Western blot analysis
Twenty-four hours after retinal ischemia, fresh
retinal tissues were homogenized on ice in cold lysis buffer
(Beyotime Institute of Biotechnology) with a 1:100 volume of
phenylmethyl sulfonylfluoride (PMSF). The homogenates were
centrifuged at 14,000 × g for 5 min at 4°C. The supernatants were
aliquoted and stored at −80°C following the removal of a small
aliquot for protein estimation. Protein concentrations were
determined using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology). The samples were thawed on
ice and mixed with 4X sample buffer (Invitrogen Life Technologies,
Carlsbad, CA, USA), before heating at 100°C for 5 min. Equivalent
quantities of proteins (50 μg) were loaded on 12% Tris-glycine
sodium dodecyl sulfate (SDS)-polyacrylamide gels for fractionation
at 160 V. Predetermined molecular weight standards (Beyotime
Institute of Biotechnology) were used as markers. Protein on the
gel was blotted onto nitrocellulose membranes at 106 V for 70 min
at 4°C. Following transfer, the membranes were incubated with
blocking buffer (5% skim milk in wash buffer) for 2 h at room
temperature and washed three times (5 min/wash) with 0.1%
Tris-buffered saline with Tween 20 (TBST). Incubation with occludin
antibody in diluent buffer (5% bovine serum albumin and 0.1% TBST)
was performed overnight at 4°C (1:1,000 dilution). The membranes
were then washed three times (5 min each) with TBST. The primary
antibody was probed with horseradish peroxidase-conjugated IgG goat
anti-rabbit secondary antibody (1:2,000) for 2 h, washed three
times in TBST and processed with enhanced chemiluminescence (ECL)
detection reagents (Beyotime Institute of Biotechnology). The
processed membranes were then exposed to photographic films for
visualization of the signals. β-actin western blot analysis was
performed for each membrane as an internal control of protein
loading.
Evaluation of drug interactions
The interaction between escin and TA was analyzed
using Berenbaum’s method, to determine whether the combination was
synergistic. The method is performed based on the following
equation: E(da,db) > E(da) + E(db), where E is the observed
effect and da and db are the doses of agents a and b, respectively.
Synergism is indicated when the total effect of a combination is
greater than expected from the sum of its effects (17).
Statistical analysis
Quantitative data from the experiments were
expressed as the mean ± standard deviation and significance was
determined by one-way analysis of variance (ANOVA) followed by
Tukey’s test. In all cases, P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of escin and TA on BRB
permeability
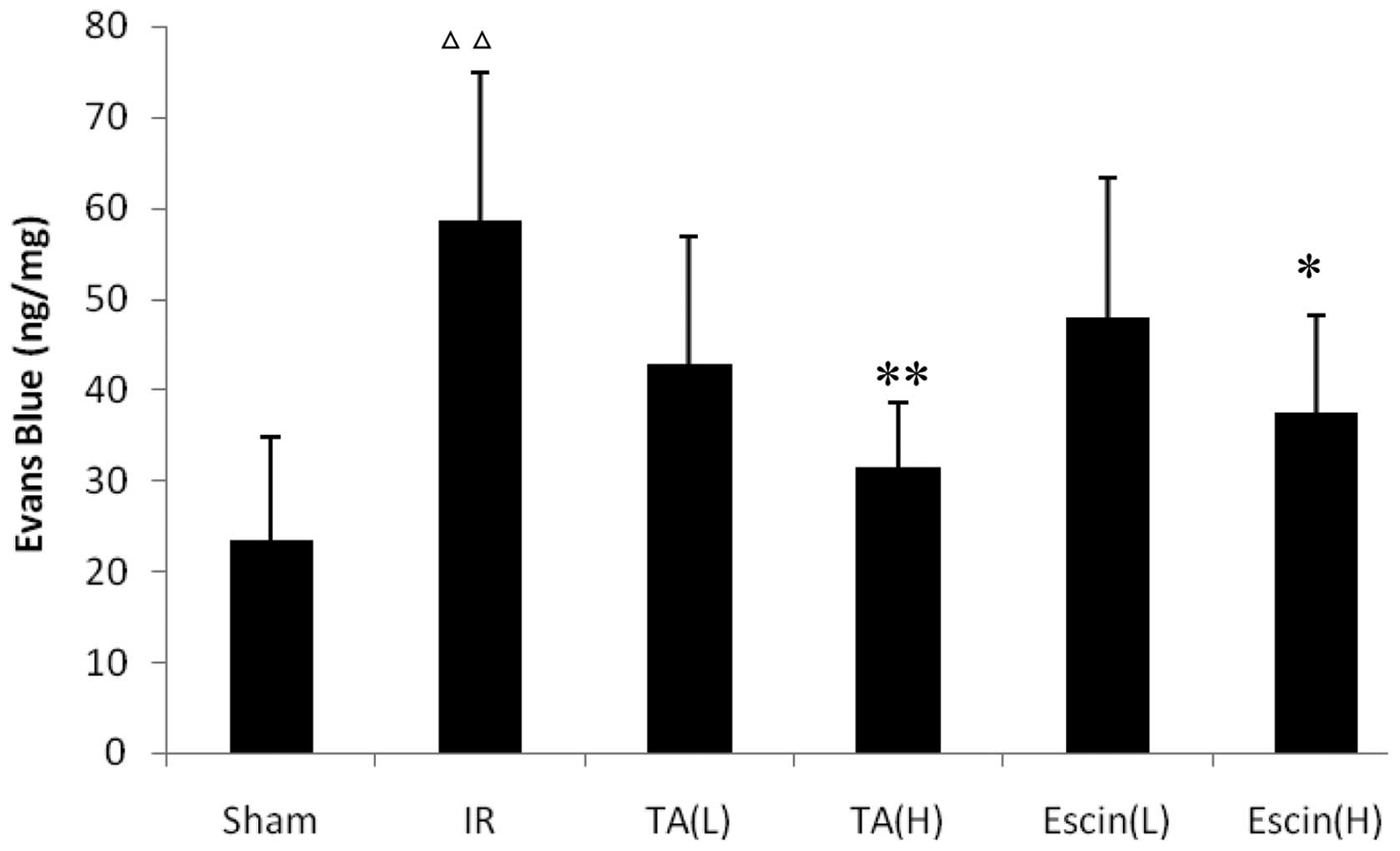
The IR rats demonstrated a greater level of retinal
Evans blue leakage than the sham rats (P<0.01). High doses (H)
of escin (1.8 mg/kg) and TA (5 μl) significantly inhibited retinal
Evans blue leakage compared with the IR group (P<0.05 and
P<0.01, respectively; Fig. 2).
Low doses (L) of escin (0.9 mg/kg) and TA (2 μl) did not
significantly affect retinal Evans blue leakage compared with the
IR group; however, administered together, they significantly
inhibited retinal Evans blue leakage (Table I).
 | Table IEffects of the combination of escin
and TA on blood-retinal barrier permeability. |
Table I
Effects of the combination of escin
and TA on blood-retinal barrier permeability.
| Group | Evans blue
(ng/mg) | Inhibition rate
(%) |
|---|
| Sham | 14.1±10.2 | - |
| IR | 47.0±17.2a | - |
| TA (L) | 34.6±8.3 | 26.4 |
| Escin (L) | 37.9±16.3 | 19.3 |
| TA (L) and escin
(L) | 24.9±8.5b | 47.1c |
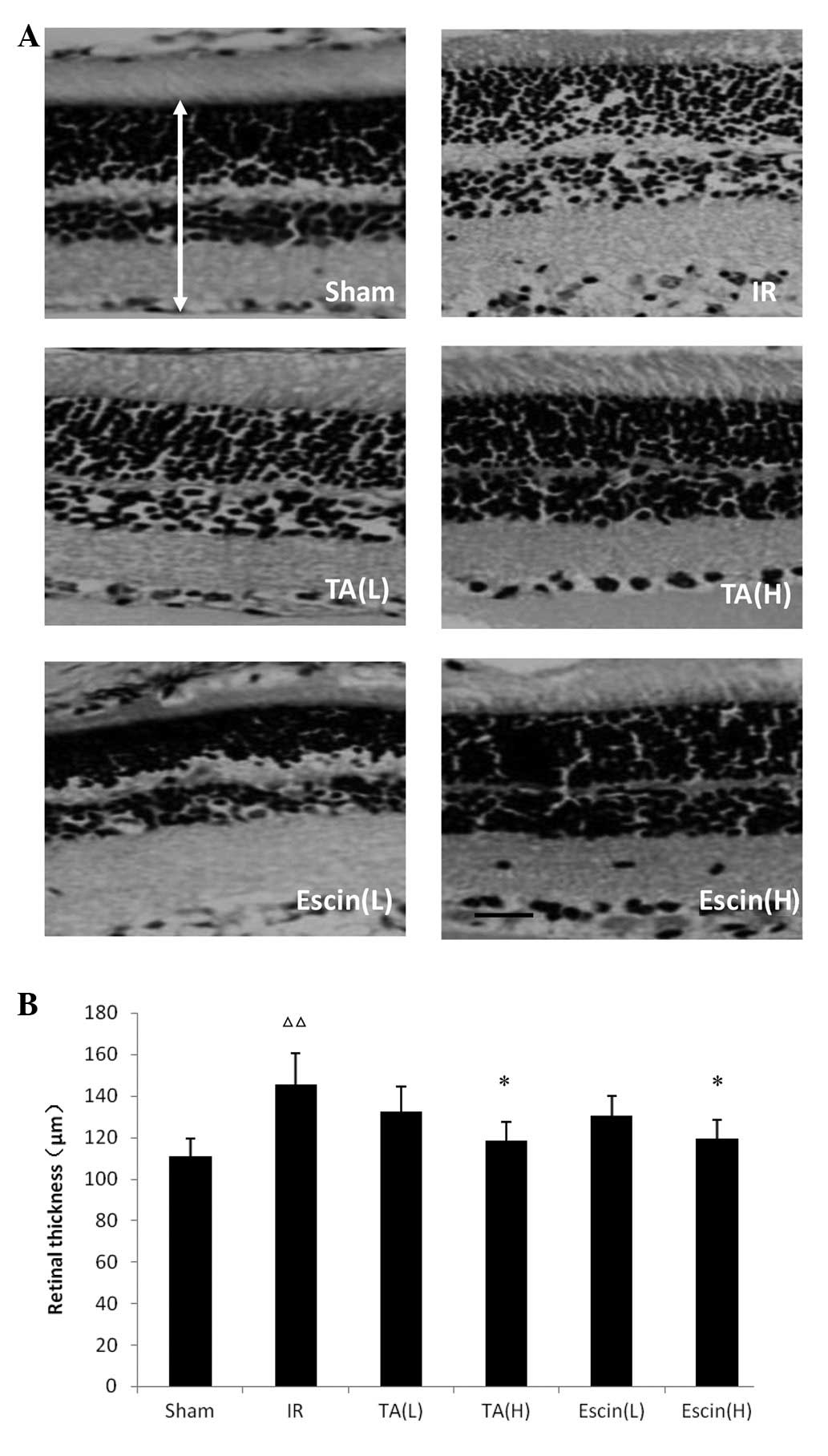
Effects of escin and TA on retinal
thickness
The retinal thickness in the IR group was
significantly increased compared with that of the sham group
(P<0.05). Escin (H) and TA (H) significantly reduced retinal
thickness compared with the IR group (P<0.05). There were no
significant differences in retinal thickness between the TA (L),
escin (L) and IR retinas (Fig.
3).
Effects of escin and TA on occludin
expression following retinal ischemia
Escin (L) and TA (L) did not enhance occludin
expression compared with IR alone. However, when escin and TA were
administered together, occludin expression in the ganglion cell
layer increased significantly, as demonstrated by the
immunohistochemistry results (Fig. 4A
and B). Western blot analysis produced consistent results
(Fig. 4C).
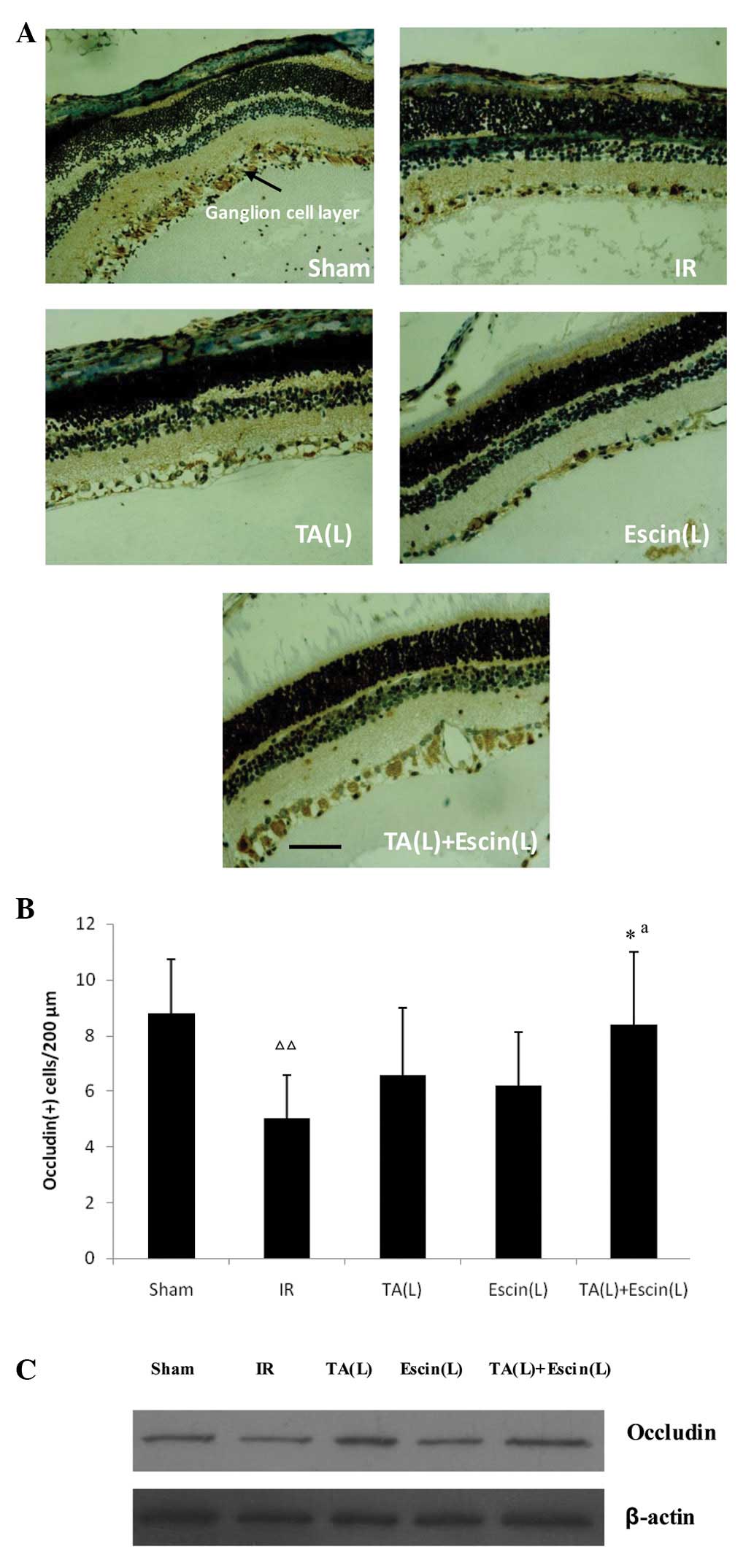 | Figure 4Effects of escin and triamcinolone
acetonide (TA) on occludin expression in the retina following
ischemia. Immunohistochemistry results: (A) Positive signals for
occludin were localized in the retinal ganglion cell layer; and (B)
low doses of escin and TA did not increase occludin expression
compared with the IR group; however, when escin and TA were
administered together, the occludin expression in the ganglion cell
layer significantly increased. Western blot analysis results: (C)
When escin and TA were administered together, the occludin
expression increased significantly compared with when they were
administered alone. Data are expressed as the mean ± standard
deviation; n=3 per group. *P<0.05 vs. the IR group
and ΔΔP<0.01 vs. the sham group. aE(da,db)
> E(da) + E(db). Scale bar, 40 μm. IR, ischemia-reperfusion; TA,
triamcinolone acetonide; L, low-dose; E, observed effect; da, dose
of agent a; db, dose of agent b. |
Discussion
The retina is a highly specialized neural tissue
that requires a unique vascular structure and tight control of
permeability to allow correct visual function. Regulation of the
flux of blood-borne metabolites into the retina is controlled by
the BRB. Hypoxia, altered blood flow and retinal ischemia may lead
to BRB breakdown and the development of macular edema. Numerous
experimental studies have demonstrated that ischemia is capable of
inducing retinal damage (18). The
retina is highly vulnerable to ischemic injury and its ischemic
tolerance is ≤60 min (19).
In the present study, a retinal ischemia model was
established in rats, in which ischemia was induced by increasing
the IOP. The results revealed that BRB permeability and retinal
thickness significantly increased following retinal ischemia.
Treatment with escin (L) or TA (L) alone did not inhibit BRB
permeability; however, when administered together, they
significantly reduced BRB permeability following ischemia. This
indicates that escin and TA have synergistic effects on reducing
BRB permeability.
The BRB is composed of tight and adherent junction
complexes. Retinal vascular endothelium and pigment epithelium have
well-developed tight junctions that confer a high degree of control
on solute and fluid permeability, thus maintaining the neural
environment of the retina. Occludin is an important transmembrane
protein in tight junctions that is responsible for forming the
permeability barrier (20). In
rats, the tight junction protein content is reduced following
retinal ischemia (21). There is
an inverse correlation between the tight junction protein content
and endothelial permeability. Therefore, in vivo reduction
of the tight junction protein content correlates with increased
vascular permeability. The immunohistochemistry and western blot
analysis results of the present study demonstrated that escin and
glucocorticoids have synergistic protective effects on
occludin.
In conclusion, escin and GCs were demonstrated to
have synergistic protective effects on BRB breakdown; the molecular
mechanism of which is correlated with the upregulation of occludin.
Administration of escin may allow a reduction in the dose of GCs
for the treatment of macular edema. The combination of escin with
GCs is a potentially beneficial method of treatment for BRB
breakdown and requires further study.
References
|
1
|
Williams R, Airey M, Baxter H, Forrester
J, Kennedy-Martin T and Girach A: Epidemiology of diabetic
retinopathy and macular oedema: a systematic review. Eye (Lond).
18:963–983. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ehrlich R, Harris A, Ciulla TA, Kheradiya
N, Winston DM and Wirostko B: Diabetic macular oedema: physical,
physiological and molecular factors contribute to this pathological
process. Acta Ophthalmol. 88:279–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart MW: Corticosteroid use for
diabetic macular edema: old fad or new trend? Curr Diab Rep.
12:364–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu L, Wu X, Geng J, Yuan Z and Chen L:
IVTA as adjunctive treatment to PRP and MPC for PDR and macular
edema: a meta-analysis. PLoS One. 7:e446832012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson CA, Berkowitz BA, Sato Y, Ando N,
Handa JT and de Juan E Jr: Treatment with intravitreal steroid
reduces blood-retinal barrier breakdown due to retinal
photocoagulation. Arch Ophthalmol. 110:1155–1159. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Felinski EA, Cox AE, Phillips BE and
Antonetti DA: Glucocorticoids induce transactivation of tight
junction genes occludin and claudin-5 in retinal endothelial cells
via a novel cis-element. Exp Eye Res. 86:867–878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonas JB, Kreissig I and Degenring R:
Intraocular pressure after intravitreal injection of triamcinolone
acetonide. Br J Ophthalmol. 87:24–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonas JB, Degenring R, Vossmerbauemer U
and Kamppeter B: Frequency of cataract surgery after intravitreal
injection of high-dosage triamcinolone acetonide. Eur J Ophthalmol.
15:462–464. 2005.PubMed/NCBI
|
|
9
|
Fu F, Hou Y, Jiang W, Wang R and Liu K:
Escin: inhibiting inflammation and promoting gastrointestinal
transit to attenuate formation of postoperative adhesions. World J
Surg. 29:1614–1620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Fu F, Zhang X, Zhu M, Wang T and
Fan H: Escin attenuates cognitive deficits and hippocampal injury
after transient global cerebral ischemia in mice via regulating
certain inflammatory genes. Neurochem Int. 57:119–127. 2010.
View Article : Google Scholar
|
|
11
|
Wang T, Fu F, Zhang L, Han B, Zhu M and
Zhang X: Effects of escin on acute inflammation and the immune
system in mice. Pharmacol Rep. 61:697–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Wang H, Fan H, et al: The potent
anti-inflammatory agent escin does not increase corticosterone
secretion and immune cell apoptosis in mice. Fitoterapia.
82:861–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin W, Zhang L, Sun F, et al: Escin exerts
synergistic anti-inflammatory effects with low doses of
glucocorticoids in vivo and in vitro. Phytomedicine. 18:272–277.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong YY, Yu SQ, Wang H, et al: Clinical
therapy of retinal vein occlusion with aescuven forte. Chin J New
Drugs Clin Rem. 24:965–967. 2005.(In Chinese).
|
|
15
|
Wang WJ, Gong YY, Wang H, et al: Aescuven
forte in treating central serous chorioretinopathy. Chin J New
Drugs Clin Rem. 24:961–964. 2005.(In Chinese).
|
|
16
|
Yuan Y and Wu L: Clinical observation of
the central serous chorioretinopathy treated by aescinate sodium
tablets combined with argon laser. Guiding Journal of TCM and
Pharmacology. 15:58–59. 2009.(In Chinese).
|
|
17
|
Berenbaum MC: What is synergy? Pharmacol
Rev. 41:93–141. 1989.PubMed/NCBI
|
|
18
|
Tsujikawa A, Ogura Y, Hiroshiba N, et al:
Retinal ischemia-reperfusion injury attenuated by blocking of
adhesion molecules of vascular endothelium. Invest Ophthalmol Vis
Sci. 40:1183–1190. 1999.PubMed/NCBI
|
|
19
|
Yilmaz T, Celebi S and Kükner AS: The
protective effects of melatonin, vitamin E and octreotide on
retinal edema during ischemia-reperfusion in the guinea pig retina.
Eur J Ophthalmol. 12:443–449. 2002.PubMed/NCBI
|
|
20
|
Furuse M, Hirase T, Itoh M, et al:
Occludin: a novel integral membrane protein localizing at tight
junctions. J Cell Biol. 123:1777–1788. 1993. View Article : Google Scholar
|
|
21
|
Xu HZ and Le YZ: Significance of outer
blood-retina barrier breakdown in diabetes and ischemia. Invest
Ophthalmol Vis Sci. 52:2160–2164. 2011. View Article : Google Scholar : PubMed/NCBI
|