Introduction
Bee pollen is the male gametophyte of gymnosperms
and angiosperms, which contains all the necessary nutrients for
plant growth and development. Bee pollen is known to be a natural
and healthy food that improves human immunity (1). Bee pollen polysaccharides exhibit
antitumor (2) and immunomodulating
activities (3,4). Previous studies have demonstrated
that pollen significantly inhibits tumor growth and enhances
immunomodulating activity, while alleviating the distress of
chemotherapy in cancer patients (5,6).
Polysaccharides constitute one group of the main
components of plants, and have complex structural features
including type I (RG-I) and type II rhamnogalacturonan (RG-II),
homogalacturonan (HG) and arabinogalactan (AG). Polysaccharides
have been reported to possess a broad spectrum of biological
activities, particularly antitumor (7–10)
and immunomodulating activities (11–16).
Rosa rugosa is a common type of plants in China, and its bee
pollen has been used as an immunological food for a long time.
Polysaccharides are the main active components in bee pollen.
However, there is limited information regarding the fractionation
and antitumor activity of bee pollen polysaccharides. To the best
of our knowledge, this is the first study to investigate the total
fractionation and antitumor activity of bee pollen polysaccharides
from Rosa rugosa.
Materials and methods
Materials
Bee pollen from Rosa rugosa was kindly
provided by the Feed Research Institute Chinese Academy of
Agricultural Sciences (Beijing, China). Sepharose® CL-6B
was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s
modified Eagle’s medium:Nutrient Mixture F12 (DMEM/F12) medium and
calf serum were purchased from Gibco (Carlsbad, CA, USA). Trypsin
was obtained from Amersco (Framingham, MA, USA), 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) from
Sigma (St. Louis, MO, USA), and penicillin/streptomycin from the
Tianjin Hao Yang Biological Manufacture Co., Ltd. (Tianjin, China).
All the additional analytical grade chemicals and reagents were
produced in China.
Analytical methods
Total carbohydrate contents were determined using
the phenol-sulphuric acid method (16,17).
Uronic acid contents were determined using the m-hydroxydiphenyl
method (16,18). All the gel permeation and anion
exchange chromatographies were monitored by assaying total sugar
and uronic acid contents. Sugar composition analysis was performed
as described by Zhang et al(15) and Yu et al(19). Each polysaccharide sample (2 mg)
was hydrolyzed with 2 M trifluoroacetic acid (TFA) at 120°C for 2
h. The monosaccharide derivatives were released using
1-phenyl-3-methyl-5-pyrazolone and analyzed on a DIKMA Inertsil
ODS-3 column (4.6×150 mm) connected to a Shimadzu high performance
liquid chromatography (HPLC) system (LC-10ATvp pump and SPD-10AVD
UV-VIS detector).
Cell culture
The cell lines HT-29 and HCT116 were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
HT-29 cells were cultured in DMEM/F12 medium supplemented with 10%
calf serum and 100 IU/ml penicillin and streptomycin. HCT116 cells
were cultured in Iscove’s modified Dulbecco’s medium (IMDM)
supplemented with 10% calf serum and 100 IU/ml penicillin and
streptomycin. The cells were incubated at 37°C in a humidified
atmosphere of 5% CO2 in air.
Cell proliferation assay
HT-29 and HCT116 cells were seeded in a 96-well
plate (Costar®) at a density of 1×104
cells/well, adhered for 24 h, and then treated for 72 h with the
polysaccharide fractions at varying concentrations (7). Control cells were similarly treated
without the polysaccharides. The media were removed and 100 μl/well
of MTT solution (0.5 mg/ml) were added. The supernatants were
discarded after a 4-h incubation. The resulting formazan was
dissolved in 100 μl DMSO and the absorbance at 570 nm was measured
using a microplate reader (Bio-Rad, Hercules, CA, USA). All the
experiments were performed in triplicate and repeated at least
three times. Cell proliferation under all the conditions was
expressed as a percentage of the control, which was set at
100%.
Statistical analysis
Data were presented as the means ± standard
deviation (SD). Statistical analysis was performed using SPSS
version 17.0 software. One-way analysis of variance (ANOVA) test
was used to perform a statistical comparison between the treatment
and control groups. P<0.05 and <0.01 were considered to
indicate a statistically significant difference.
Results
Isolation and fractionation of bee pollen
polysaccharides
The water-soluble polysaccharides were extracted
from the bee pollen of Rosa rugosa with hot water. Following
precipitation by the addition of 4 volumes of 95% ethanol, a total
polysaccharides fraction, referred to as WRPP (water-soluble
Rosa rugosa bee pollen polysaccharides), was obtained with a
yield of 4.6% (w/w). WRPP contained 58.1% total sugar and 13.3%
uronic acid. WRPP was a water-soluble light yellow powder. Sugar
composition analysis by HPLC indicated that WRPP consisted of
galactose (Gal) (21.4%), arabinose (Ara) (47.9%), rhamnose (Rha)
(3.4%), galacturonic acid (GalA) (12.1%), glucose (Glc) (11.6%),
mannose (Man) (2.6%) and glucoronic acid (GlcA) (1.0%).
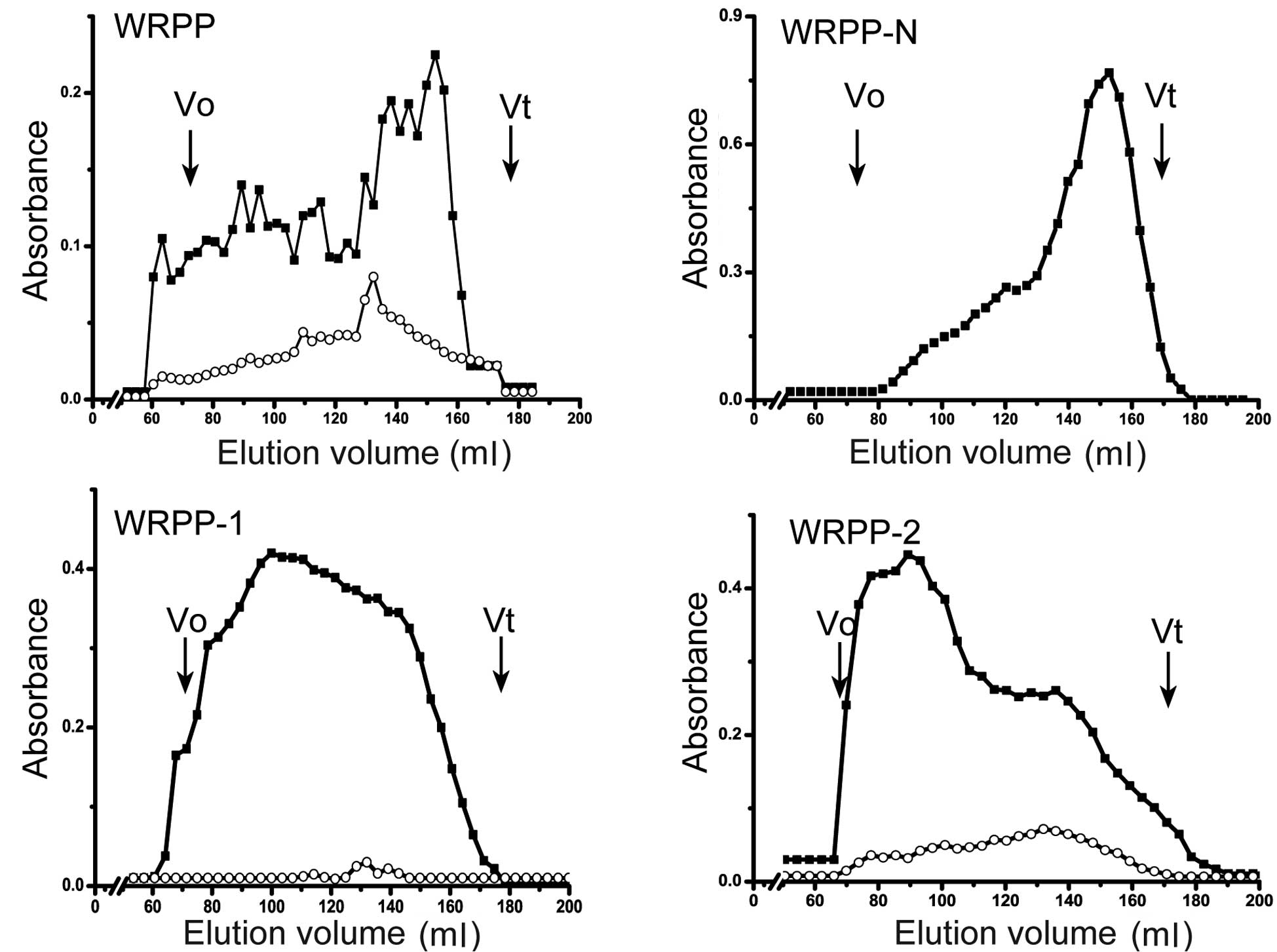
WRPP contain neutral and acidic polysaccharides and
showed a wide molecular weight distribution using Sepharose CL-6B
(Fig. 1). To fractionate WRPP, it
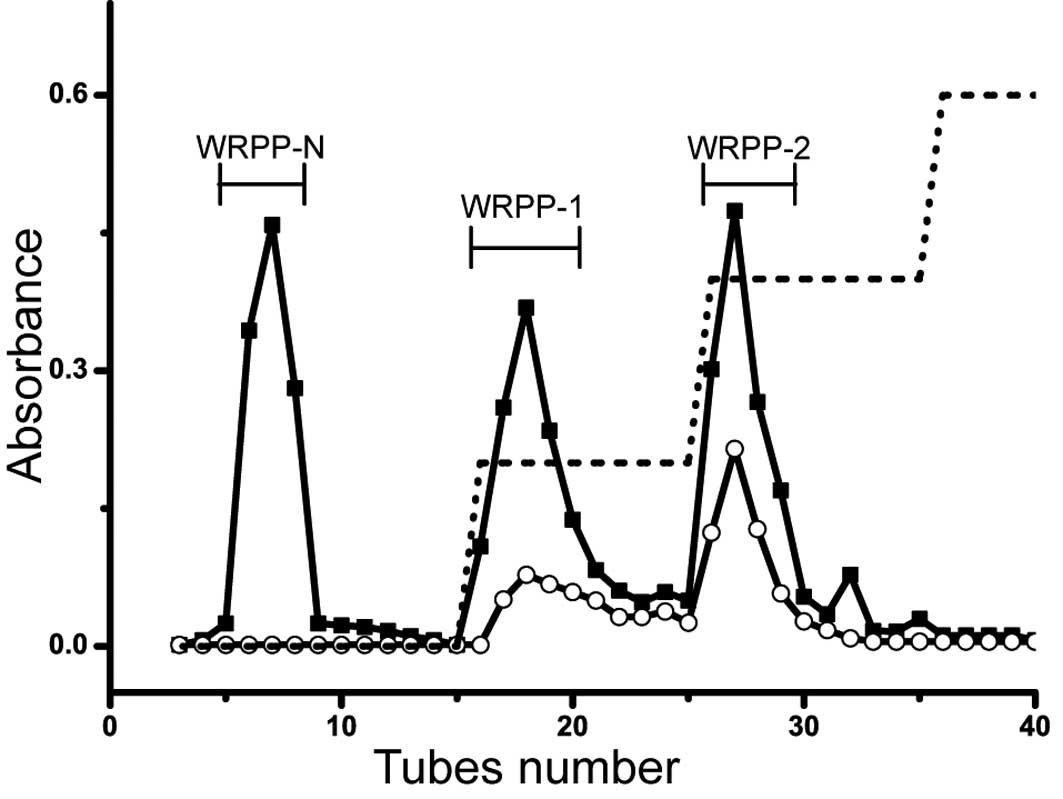
was applied on anion-exchange chromatography (Fig. 2). The elution profiles on
DEAE-Cellulose column are shown in Fig. 3. Three fractions of WRPP-N, WRPP-1
and WRPP-2 were eluted with distilled water, 0.2 and 0.4 M NaCl,
respectively. The molecular distribution on Sepharose CL-6B was
analyzed (Fig. 1). WRPP-N
exhibited one main peak near the total volume, while small curves
prior to the main peak also existed, suggesting a mixture of
polysaccharides with different molecular weights. WRPP-1 showed
wide distribution from the out volume to the total volume of
Sepharose CL-6B, while uronic acid showed a small peak. WRPP-2
exhibited a main peak near the out volume, while an additional peak
of rich uronic acid appeared near the total volume.
Composition analysis of the
polysaccharide fractions
The monosaccharide compositions and yields of the
collected fractions are listed in Table I. WRPP-N was composed of Glc
(34.3%), Gal (25.0%), Ara (36.7%) and Man (4.0%), while uronic acid
was not detected. The iodine test suggested that no starch was
present, while WRPP-N was composed of glucan, arabinogalactans
(AGs) and few mannoglucans.
 | Table IYields and structral features of the
collected fractions. |
Table I
Yields and structral features of the
collected fractions.
| | Monosaccharide
composition (%) |
|---|
| |
|
|---|
| Fraction | Yield (%) | Rha | GalA | Gal | Ara | Man | Glc | GlcA |
|---|
| WRPP | 4.6a | 3.4 | 12.1 | 21.4 | 47.9 | 2.6 | 11.6 | 1.0 |
| WRPP-N | 26.9b | 0 | 0 | 25.0 | 36.7 | 4.0 | 34.3 | 0 |
| WRPP-1 | 21.4b | 3.0 | 12.4 | 24.7 | 53.9 | 1.9 | 3.0 | 1.1 |
| WRPP-2 | 13.1b | 7.8 | 23.0 | 15.0 | 48.7 | 1.7 | 2.8 | 0.9 |
WRPP-1 consisted of Rha (3.0%), GalA (12.4%), Gal
(24.7%) and Ara (53.9%), with minor amounts of Man, Glc and GlcA
being detected (Table I). The
amounts of Gal and Ara were >70% of total sugars. The ratio of
Rha/GalA was 0.24, which was within the RG-I range of 0.05–1.0,
defined by Schols and Voragen (20) and Yu et al(19). These results indicated small
amounts of RG-I and HG domains, while a large proportion of AG and
arabinan fragments was evident.
WRPP-2 consisted of Rha (7.8%), GalA (23.0%), Gal
(15.0%) and Ara (48.7%), with minor amounts of Glc, GlcA and Man
being detected. The ratio of Rha/GalA was ~0.34, suggesting the
presence of RG-I, HG and AG.
WRPP-1 and WRPP-2 were mainly composed of Gal, Ara,
Rha and GalA. Both had a large proportion of Gal and Ara,
suggesting the existence of AG fragments. The amounts of GalA in
WRPP-1 and WRPP-2 were 12.4 and 23%, respectively; one explanatory
reason could be the elution order from the DEAE-Cellulose column.
WRPP-2 contained more RG-I and HG domains compared to WRPP-1, while
WRPP-1 contained more AG fragments compared to WRPP-2.
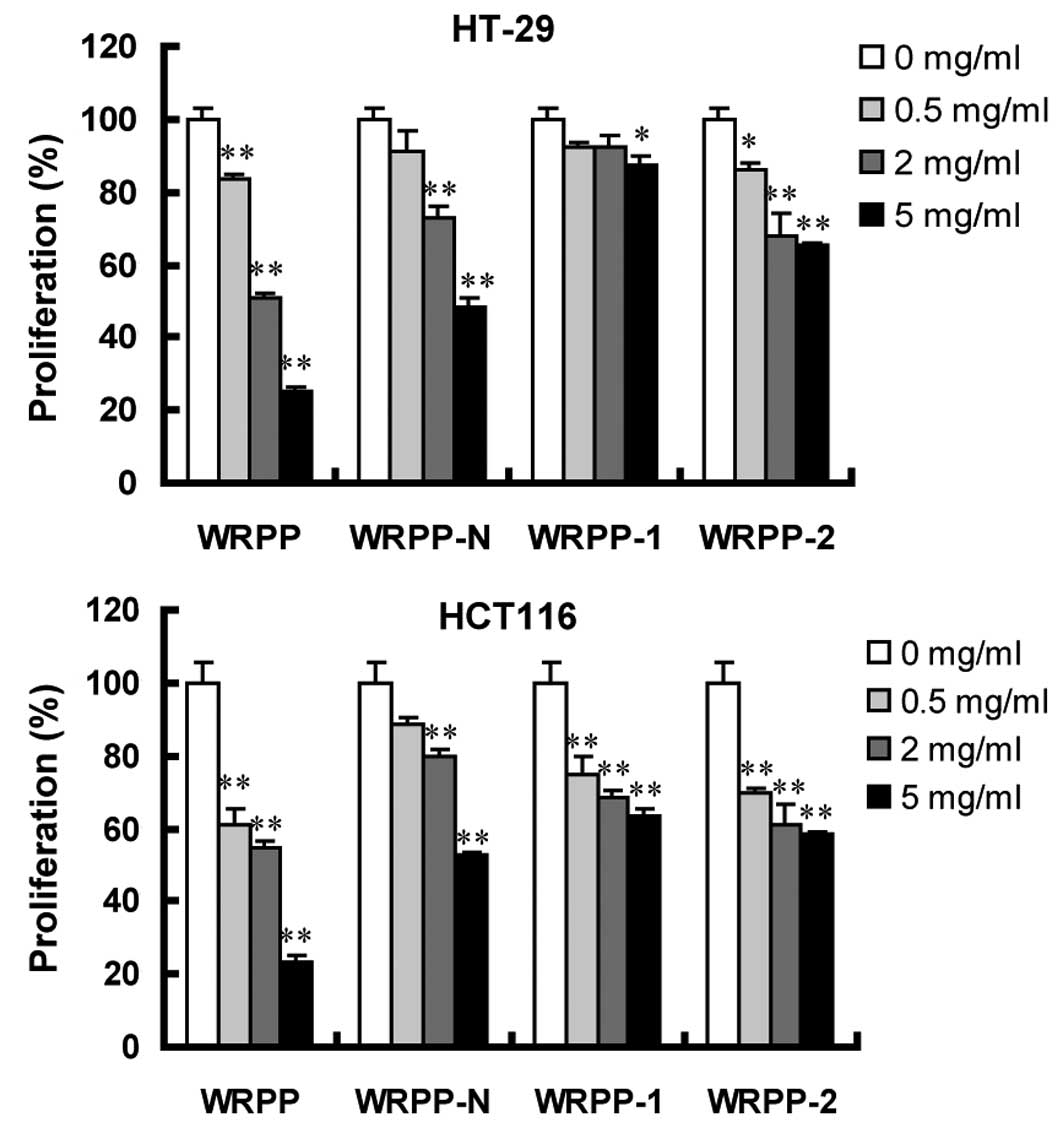
Antitumor effects in vitro
Human colon cancer HT-29 and HCT116 cells were
treated with varying concentrations of various bee pollen
polysaccharides from Rosa rugosa for 72 h. Cell
proliferation was assessed using MTT assay. Following a 72-h
treatment with total polysaccharides WRPP, cell proliferation was
significantly inhibited in a dose-dependent manner. All the
sub-fractions of WRPP also showed a concentration-dependent
proliferation-inhibitory effect on HT-29 and HCT116 cells (Fig. 4). Notably, all the sub-fractions
were less effective compared to the total fraction WRPP, suggesting
that sub-fractions have a synergistic effect, which accounted for
the anti-proliferative activity of bee pollen polysaccharides from
Rosa rugosa on HT-29 and HCT116 cells in vitro.
Discussion
In the present study, bee pollen polysaccharides
from Rosa rugosa were extracted and fractionated. Total WRPP
were purified to neutral (WRPP-N) and acidic polysaccharides
(WRPP-1, WRPP-2). Acidic fractions contained different amounts of
RG-I, HG and AG fragments. All the fractions inhibited the
proliferation of HT-29 and HCT116 cells in a dose-dependent manner
in vitro, indicating a potential antitumor activity.
Sub-fractions showed a significant synergistic effect. To the best
of our knowledge, this is the first study investigating the
fractionation and antitumor activity of bee pollen polysaccharides
from Rosa rugosa.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities (no. 09SSXT125).
References
|
1
|
Kroyer G and Hegedus N: Evaluation of
bioactive properties of pollen extracts as functional dietary food
supplement. Innov Food Sci Emerg Technol. 2:171–174. 2001.
View Article : Google Scholar
|
|
2
|
Yang X, Guo D, Zhang J and Wu M:
Characterization and antitumor activity of pollen polysaccharide.
Int Immunopharmacol. 7:427–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li F, Yuan Q and Rashid F: Isolation,
purification and immunobiological activity of a new water-soluble
bee pollen polysaccharide from Crataegus pinnatifida Bge.
Carbohydr Polym. 78:80–88. 2009. View Article : Google Scholar
|
|
4
|
Brecker L, Wicklein D, Moll H, Fuchs EC,
Becker WM and Petersen A: Structural and immunological properties
of arabinogalactan polysaccharides from pollen of timothy grass
(Phleum pratense L.). Carbohydr Res. 340:657–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Hu F, Zhu W and Li Y: Study on the
antitumor activity of propolis and bee pollen and royal jelly. J
Bee. 7:3–4. 2003.
|
|
6
|
Wang W, Hu J and Shen W: Antitumor
activity and mechanism of pollen. Chin Beekeep. 36:1–3. 1986.
|
|
7
|
Cheng H, Li S, Fan Y, et al: Comparative
studies of the antiproliferative effects of ginseng polysaccharides
on HT-29 human colon cancer cells. Med Oncol. 28:175–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Y, Cheng H, Liu D, et al: The
inhibitory effect of ginseng pectin on L-929 cell migration. Arch
Pharm Res. 33:681–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu C, Liu Y, Yuan G and Guan M: The
contribution of side chains to antitumor activity of a
polysaccharide from Codonopsis pilosula. Int J Biol
Macromol. 50:891–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao H, Li Y, Wang Y, et al: Antitumor and
immunostimulatory activity of a polysaccharide-protein complex from
Scolopendra subspinipes mutilans L. Koch in tumor-bearing
mice. Food Chem Toxicol. 50:2648–2655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inngjerdingen KT, Kiyohara H, Matsumoto T,
et al: An immunomodulating pectic polymer from Glinus
oppositifolius. Phytochemistry. 68:1046–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inngjerdingen M, Inngjerdingen KT, Patel
TR, et al: Pectic polysaccharides from Biophytum petersianum
Klotzsch, and their activation of macrophages and dendritic cells.
Glycobiology. 18:1074–1084. 2008.
|
|
13
|
Nergard CS, Matsumoto T, Inngjerdingen M,
et al: Structural and immunological studies of a pectin and a
pectic arabinogalactan from Vernonia kotschyana Sch. Bip. ex
Walp. (Asteraceae). Carbohydr Res. 340:115–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni W, Zhang X, Bi H, et al: Preparation of
a glucan from the roots of Rubus crataegifolius Bge. and its
immunological activity. Carbohydr Res. 344:2512–2518. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Li S, Sun L, et al: Further
analysis of the structure and immunological activity of an RG-I
type pectin from Panax ginseng. Carbohydr Polym. 89:519–525.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Yu L, Bi H, et al: Total
fractionation and characterization of the water-soluble
polysaccharides isolated from Panax ginseng C. A. Meyer.
Carbohydr Polym. 77:544–552. 2009. View Article : Google Scholar
|
|
17
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
18
|
Blumenkrantz N and Asboe-Hansen G: New
method for quantitative determination of uronic acids. Anal
Biochem. 54:484–489. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Zhang X, Li S, et al:
Rhamnogalacturonan I domains from ginseng pectin. Carbohydr Polym.
79:811–817. 2010. View Article : Google Scholar
|
|
20
|
Schols HA and Voragen A: Complex pectins:
structure elucidation using enzymes. Progress Biotechnol. 14:3–19.
1996. View Article : Google Scholar
|