Introduction
Microglial cells, the resident brain macrophages of
the central nervous system (CNS), play a prominent role in the
pathogenesis of neurodegenerative disorders (1). In response to environmental stress or
lipopolysaccharide (LPS) exposure, microglia are activated and
become involved in the innate and adaptive immune responses via the
production of proinflammatory mediators, including nuclear
factor-κB (NF-κB), caspase 3 and heat shock protein 60 (HSP60)
(2–6). It has been demonstrated that
extracellular HSP60 increases the production of proinflammatory
factors in microglia, which causes neuronal damage (7,8).
Therefore, the control of HSP60 production may be an effective
therapeutic option for the treatment of neurodegenerative diseases.
Currently, a limited number of disease-modifying treatment
approaches are used or are under investigation as therapeutic
interventions in CNS injuries and neurodegenerative diseases
(9,10). For example, it has been shown that
minocycline possesses anti-apoptotic and anti-inflammatory actions
in animal models of CNS injuries and neurodegenerative diseases
(11,12). However, the complexity of current
pharmacological agents has delayed their development for the
treatment or prevention of CNS diseases.
Lycium barbarum, a traditional Chinese
medicine, has been used as a food supplement for centuries.
Previous studies have shown that Lycium barbarum possesses
eye-protective (13), anti-aging
(14), immunoregulating (15) and antitumor properties (15,16).
Polysaccharides are the major active ingredient in Lycium
barbarum (LBPs). Although the benefits of Lycium
barbarum and LBPs have been recognised, their functions in the
CNS are poorly understood. A limited number of studies have shown
that LBPs prevent β-amyloid peptide- or glutamate-induced
neurotoxicity in cultured cortical neurons (17,18).
The effects of LBPs on neuroimmune diseases have not yet been fully
elucidated.
In the present study, we aimed to investigate the
effects of LBPs on LPS-induced inflammatory injury in BV2 microglia
cells. Our results showed that LBPs suppress the increased levels
of caspase 3, TNF-α and HSP60 caused by LPS treatment, possibly via
NF-κB inhibition. Therefore, the extract from Lycium
barbarum may be used to control the activation of microglia and
their induction of the inflammatory response in the CNS.
Materials and methods
Chemicals
LBPs (purity >95%, data not shown) were purchased
from Ningxia Qiyuan Pharmaceutical Company (Yinchuan, China).
Antibodies against GAPDH, lamin B and NF-κB were from Abcam
(Cambridge, MA, USA). The anti-HSP60 antibody was from Stressgen
(San Diego, CA, USA) and the anti-caspase 3 antibody was from Cell
Signaling Technology, Inc. (Beverly, MA, USA). The proteinase
inhibitor cocktails were from Merck Chemicals (Whitehouse Station,
NJ, USA). HSP60 and TNF-α enzyme-linked immunosorbent assay (ELISA)
kits were from Yaji (Shanghai, China) and eBioscience (San Diego,
CA, USA), respectively. BCA and ECL kits were from Pierce
(Rockford, IL, USA). Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum (FBS) were from Gibco (Grand Island, NY, USA).
All the additional chemicals were purchased from Sigma (St. Louis,
MO, USA), unless otherwise stated.
Microglia cell culture
BV2 mouse microglial cells were cultured in DMEM
supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin
(100 g/ml). Cultures were maintained at 37°C in a humidified
incubator gassed with 95% O2 and 5% CO2. LBPs
were dissolved in phosphate-buffered saline (PBS). The final
concentration of PBS in the medium was <0.05% (vol/vol) and this
demonstrated no effect on cell growth (data not shown). The cells
were treated with the indicated concentrations of LBPs for 24 h
following the administration of LPS (200 ng/ml).
Cell viability assay
Cells (3×104 cells in 200 μl/well) were
seeded in 96-well plates. An MTT assay was used to assess the
percentage of viable cells following pharmacological treatments, as
previously described (19).
Briefly, the culture medium in the 96-well plates was replaced by
100 μl of fresh MTT solution (0.5 mg/ml) following the treatments.
After 3 h of incubation at 37°C, the supernatant was removed and
formazan salt crystals dissolved in 150 μl dimethyl sulfoxide
(DMSO) were added to the cells. Cell viability was measured by the
absorbance at 570 nm. The cell survival ratio was calculated by
normalization to the control.
ELISA
The levels of HSP60 and TNF-α in the culture medium
were quantified according to the manufacturer's instructions.
Absorbance was determined at 450 nm using a microplate reader.
Western blot analysis
The cells were washed with PBS three times and lysed
with RIPA buffer. The protein concentration was determined using a
BCA kit, according to the manufacturer's instructions. Equal
quantities of protein were loaded and run on SDS/polyacrylamide
gels and then transferred to PVDF membranes. The membranes were
blocked with 5% dried milk and incubated with the primary
antibodies in TBST overnight at 4°C. After being rinsed in
milk-TBST, blots were incubated in the horseradish
peroxidase-conjugated secondary antibodies. The target proteins
were detected using the enhanced chemiluminescence (ECL) detection
system and X-ray films.
Statistical analysis
Statistical differences were determined using a
one-way ANOVA test. P<0.05 was considered to indicate a
statistically significant difference. Data in the text and figures
are presented as the mean ± standard error of the mean (SEM). n
represents the number of experiments.
Results
LBPs promote the viability of BV2
microglia
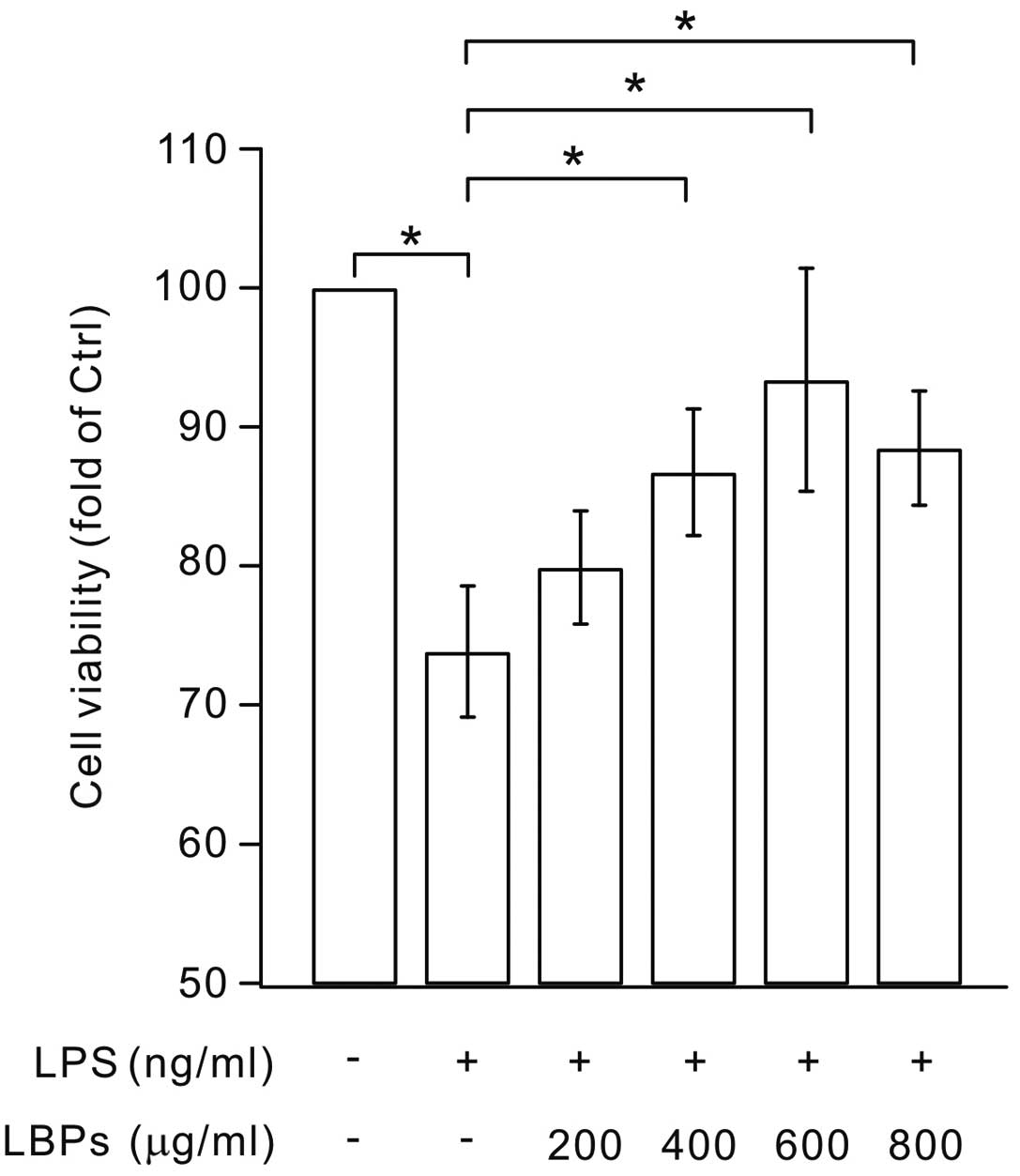
An MTT assay was performed to determine whether LBPs
had an effect on the apoptosis of LPS-stimulated BV2 cells. The
results demonstrated that the treatment of microglia with 1,000
μg/ml LBPs for up to 24 h did not increase the viability of
LPS-stimulated BV2 cells (data not shown). However, lower
concentrations of LBPs (200–800 μg/ml) significantly increased cell
viability compared with the LPS group (Fig. 1). These results indicate that LBPs
have a positive effect on the viability of LPS-stimulated BV2
microglia.
Effects of LBPs on NF-κB, caspase 3 and
HSP60 protein expression in LPS-stimulated BV2 microglia
NF-κB regulates the expression of numerous
proinflammatory factors (20).
Therefore, we examined the NF-κB level following LBPs treatment.
Fig. 2A shows that levels of the
p65 subunit of NF-κB increased with LPS treatment, but was markedly
inhibited by LBPs. Caspase 3 is the upstream protein of the NF-κB
signaling pathway. Inhibition of caspase 3 prevents neuronal loss
in brain diseases, including activated microglia. Thus, we examined
the effects of LBPs on caspase 3 expression (Fig. 2B). Our results showed that caspase
3 expression was suppressed following LBPs treatment. NF-κB has
been shown to initiate the transcription of the HSP60 stress
protein gene, which elicits a potent proinflammatory response in
innate immune cells. Results from a previous study have
demonstrated that LPS increases HSP60 expression in BV2 microglial
cells (6). The results of the
present study also showed that HSP60 expression markedly increased
following LPS treatment. However, LBPs treatment significantly
inhibited this HSP60 enhancement (Fig.
2C). These results suggest that the anti-inflammatory effects
of LBPs may be associated with the inhibition of NF-κB
signaling.
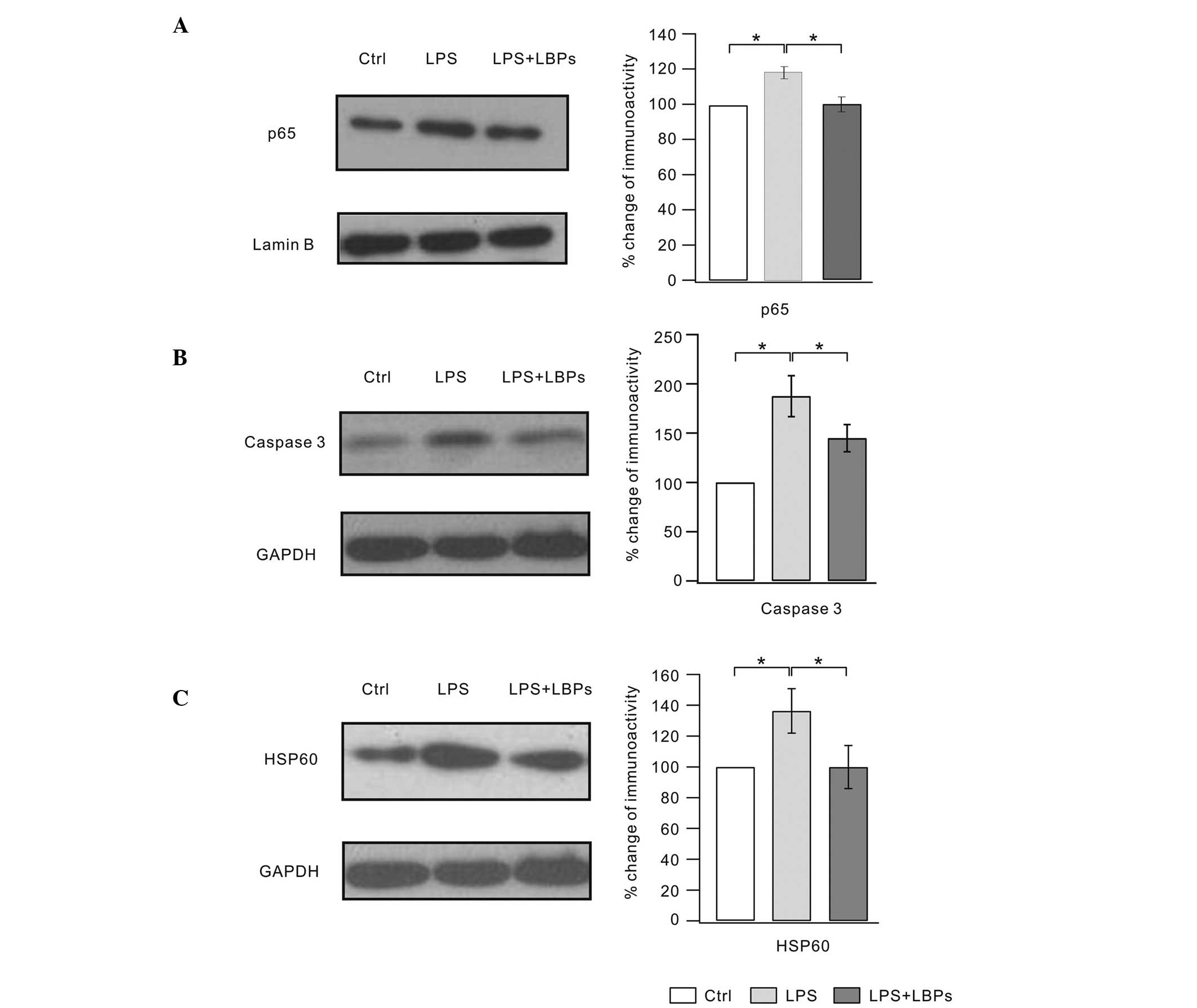 | Figure 2Lycium barbarum polysaccharides
(LBPs) inhibited the increased expression of NF-κB, caspase 3 and
HSP60 in lipopolysaccharide (LPS)-stimulated BV2 microglia. Cells
were pretreated with LPS for 1 h, followed by an incubation with
600 μg/ml LBPs for 24 h. (A) The lysates were probed by
immunoblotting with antibodies against p65 and lamin B. The right
panel shows ratios of the signal intensity of p65/lamin B (Ctrl,
100%; LPS, 119.3±7%; LPS + LBP, 107.3±9%). (B) The lysates were
probed by immunoblotting with antibodies against caspase 3 and
GAPDH. The right panel shows ratios of the signal intensity of
caspase 3/GAPDH (Ctrl, 100%; LPS, 187.4±20.8%; LPS + LBPs,
144.9±13.7%). (C) The lysates were probed by immunoblotting with
antibodies against HSP60 and GAPDH. The right panel shows ratios of
the signal intensity of HSP60/GAPDH (Ctrl, 100%; LPS, 136.5±14.4%;
LPS + LBP, 100±18%). Each experiment was derived from 10
independent cultures. *P<0.05. Ctrl, control. |
Effects of LBPs on the expression of
TNF-α and HSP60 by LPS-stimulated BV2 microglia
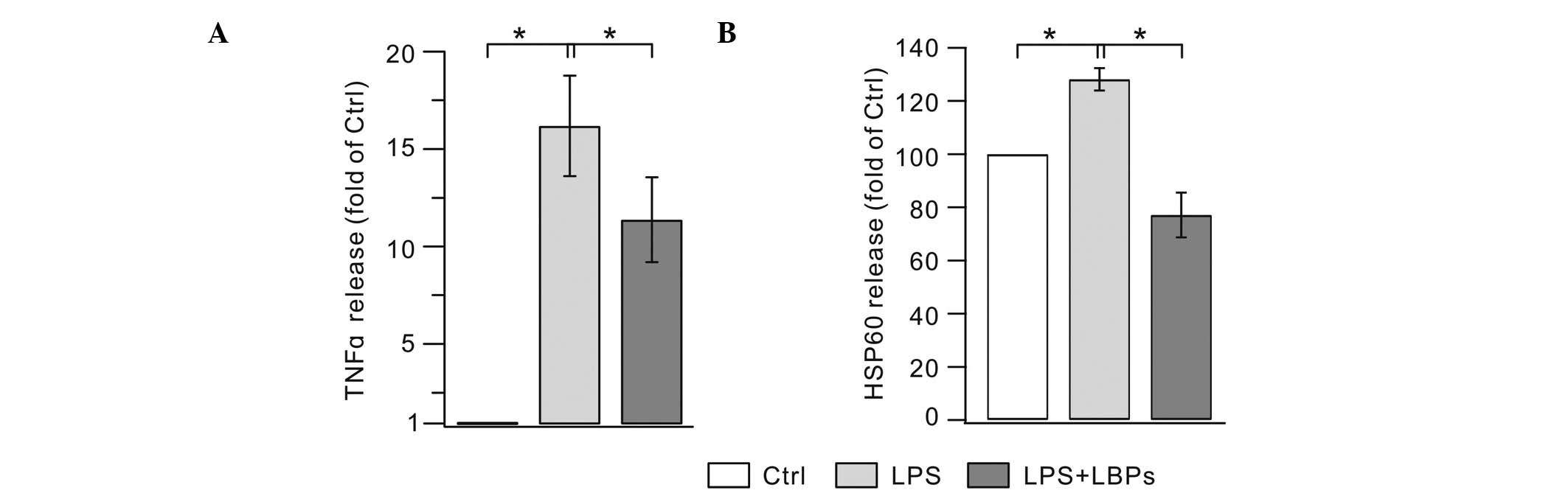
An ELISA assay was used to investigate whether LBPs
suppressed the release of TNF-α from LPS-stimulated BV2 cells. As
shown in Fig. 3A, the 24-h LPS
treatment of BV2 cells resulted in a marked increase in TNF-α
levels compared with the controls. The application of LBPs
significantly decreased the concentration of TNF-α in the culture
media. Previous studies suggest that upon stress, HSP60 is
upregulated and released into the extracellular space, where it
exerts injury effects (21,22).
We hypothesized that LPS also induces the increase of HSP60 in the
extracellular space and this was confirmed by our experiment
(Fig. 3B). LPS alone (200 ng/ml)
markedly increased the HSP60 release compared with the controls.
LBPs significantly reduced the HSP60 concentration in the medium.
These results indicate that LBPs effectively suppress the
production of TNF-α and HSP60 by over-activated microglia.
Discussion
Over-activated microglia produce cytokines and cause
neuronal inflammatory responses following LPS exposure (5). In the present study, we showed that
treatment with 600 μg/ml LBPs effectively suppressed the activation
of microglia. LPS treatment increased the expression of caspase 3
and HSP60, whereas LBPs treatment markedly suppressed their
expression. Treatment with LBPs also effectively prevented the
release of TNF-α and HSP60 into the culture medium. The effects of
LBPs may occur via NF-κB inhibition, since p65 expression was
decreased with LBPs treatment. Therefore, our data suggest that
LBPs may be a therapeutic agent for the treatment of inflammatory
diseases.
Microglia defend the brain by destroying invading
pathogens in the innate immune response of the CNS (23). Moderate microglia activation
promotes neuronal survival in ischemia (24). However, the over-activation of
microglia leads to the harmful activation of NF-κB signaling and
TNF-α release in inflammatory responses and organ failure (25). Therefore, the inhibition of
proinflammatory enzymes and cytokines may be an effective
therapeutic approach against neurodegenerative disorders and it is
of therapeutic importance to seek new strategies to decrease the
over-activation of microglia. Previous studies have investigated
the neuroprotective effects of several natural extracts, including
curcumin (26) and fucoidan
(27). The present study suggests
that the extract from Lycium barbarum is a potential
therapeutic tool.
Caspases are essential for apoptosis and
inflammation and caspase 3 is crucial in cell death and CNS
inflammation (28). It has been
reported that LPS-stimulated microglia are not toxic to neighboring
neurons when caspase 3/7 is inhibited (29). The activation of NF-κB by caspase 3
is critical in inflammation. NF-κB is the most important
transcription factor for inducing the transcription of
proinflammatory genes. For example, the activation of NF-κB leads
to ischemia-induced neuronal injury (30). Therefore, several anti-inflammatory
therapies aim to inhibit NF-κB activity in LPS models or
inflammatory diseases (31). Our
results demonstrate that LBPs treatment markedly inhibits caspase 3
and the NF-κB downstream mediator p65, suggesting that the
anti-inflammatory effects of LBPs may be a result of the inhibition
of the NF-κB pathway.
HSP60 is a member of the heat shock protein family.
HSP60 is normally cytoprotective, however, it may become toxic by
targeting self-reactive T cells in inflammatory diseases (32). HSP60 production is induced by the
NF-κB-p65 cascade and is released following oxidative stress
(33). The regulation of HSP60
gene expression via the binding of NF-κB to the HSP60 gene promoter
may be the mechanism via which these effects occur (34). TNF-α is also a mediator of NF-κB
signaling and triggers an increase in the expression of HSP60,
which has been shown to be reversed by p65 inhibition (34). We showed that the levels of HSP60,
NF-κB and TNF-α are decreased by LBPs. Combined with the data of
our previous studies, our results indicate that LBPs may act
through the inhibition of NF-κB to prevent the over-activation of
microglia.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30960108, 31060140, 31070945,
31100780, 81060034 and 31260243) and LY12C09002. This study was
also supported by SRF for ROCS, State Education Ministry (SEM) and
Program for New Century Excellent Talents in University ‘NCET’ for
Dr Yin Wang.
References
|
1
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: uncovering the molecular
mechanisms. Nature Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanisch UK and Kettenmann H: Microglia:
active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gehrmann J, Matsumoto Y and Kreutzberg GW:
Microglia: intrinsic immuneffector cell of the brain. Brain Res
Brain Res Rev. 20:269–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Innamorato NG, Lastres-Becker I and
Cuadrado A: Role of microglial redox balance in modulation of
neuroinflammation. Curr Opin Neurol. 22:308–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch MA: The multifaceted profile of
activated microglia. Mol Neurobiol. 40:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li YH, Teng P, Wang Y, Zhang YM, Ma CJ and
Pu J: Expression and regulation of HSP60 in activated microglia
cells. J Ningxia Med Univ. 8:712–714. 2011.
|
|
7
|
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J
and Gu J: Microglial LOX-1 reacts with extracellular HSP60 to
bridge neuroinflammation and neurotoxicity. Neurochem Int.
61:1021–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehnardt S, Schott E, Trimbuch T, Laubisch
D, Krueger C, Wulczyn G, Nitsch R and Weber JR: A vicious cycle
involving release of heat shock protein 60 from injured cells and
activation of toll-like receptor 4 mediates neurodegeneration in
the CNS. J Neurosci. 28:2320–2331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia W, Han J, Huang G and Ying W:
Inflammation in ischaemic brain injury: current advances and future
perspectives. Clin Exp Pharmacol Physiol. 37:253–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perry VH, Nicoll JA and Holmes C:
Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201.
2010. View Article : Google Scholar
|
|
11
|
Stirling DP, Khodarahmi K, Liu J, McPhail
LT, McBride CB, Steeves JD, Ramer MS and Tetzlaff W: Minocycline
treatment reduces delayed oligodendrocyte death, attenuates axonal
dieback, and improves functional outcome after spinal cord injury.
J Neurosci. 24:2182–2190. 2004. View Article : Google Scholar
|
|
12
|
Choi SH, Lee DY, Chung ES, Hong YB, Kim SU
and Jin BK: Inhibition of thrombin-induced microglial activation
and NADPH oxidase by minocycline protects dopaminergic neurons in
the substantia nigra in vivo. J Neurochem. 95:1755–1765. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides
reduce neuronal damage, blood-retinal barrier disruption and
oxidative stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar
|
|
14
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative
stress in aged mice. J Ethnopharmacol. 111:504–511. 2007.
|
|
15
|
Tang WM, Chan E, Kwok CY, Lee YK, Wu JH,
Wan CW, Chan RY, Yu PH and Chan SW: A review of the anticancer and
immunomodulatory effects of Lycium barbarum fruit.
Inflammopharmacology. 20:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong H, Shen P, Jin L, Xing C and Tang F:
Therapeutic effects of Lycium barbarum polysaccharide (LBP)
on irradiation or chemotherapy-induced myelosuppressive mice.
Cancer Biother Radiopharm. 20:155–162. 2005.
|
|
17
|
Ho YS, Yu MS, Lai CS, et al:
Characterizing the neuroprotective effects of alkaline extract of
Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain
Res. 1158:123–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho YS, Yu MS, Yik SY, So KF, Yuen WH and
Chang RC: Polysaccharides from wolfberry antagonizes glutamate
excitotoxicity in rat cortical neurons. Cell Mol Neurobiol.
29:1233–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu J, Shao CY, Yang W, Zhang XM, Wu ZY,
Zhou L, Wang XX, Li YH, Xia J, Luo JH and Shen Y: Chronic zinc
exposure decreases the surface expression of NR2A-containing NMDA
receptors in cultured hippocampal neurons. PloS One. 7:e460122012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarkar FH, Li Y, Wang Z and Kong D:
NF-kappaB signaling pathway and its therapeutic implications in
human diseases. Int Rev Immunol. 27:293–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayoun D, Kapp T, Edri-Brami M, Ventura T,
Cohen M, Avidan A and Lichtenstein RG: HSP60 is transported through
the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells
and undergoes N-glycosylation. FEBS J. 279:2083–2095. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizzo M, Macario AJ, de Macario EC,
Gouni-Berthold I, Berthold HK, Rini GB, Zummo G and Cappello F:
Heat shock protein-60 and risk for cardiovascular disease. Curr
Pharm Des. 17:3662–3668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kreutzberg GW: Microglia: a sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai AY and Todd KG: Microglia in cerebral
ischemia: molecular actions and interactions. Can J Physiol
Pharmacol. 84:49–59. 2006.PubMed/NCBI
|
|
25
|
Chen J, Zhou Y, Mueller-Steiner S, Chen
LF, Kwon H, Yi S, Mucke L and Gan L: SIRT1 protects against
microglia-dependent amyloid-beta toxicity through inhibiting
NF-kappaB signaling. J Biol Chem. 280:40364–40374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karlstetter M, Lippe E, Walczak Y, Moehle
C, Aslanidis A, Mirza M and Langmann T: Curcumin is a potent
modulator of microglial gene expression and migration. J
Neuroinflammation. 8:1252011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HY, Han MH, Park C, Jin CY, Kim GY,
Choi IW, Kim ND, Nam TJ, Kwon TK and Choi YH: Anti-inflammatory
effects of fucoidan through inhibition of NF-κB, MAPK and Akt
activation in lipopolysaccharide-induced BV2 microglia cells. Food
Chem Toxicol. 49:1745–1752. 2011.
|
|
28
|
Soria JA, Arroyo DS, Gaviglio EA,
Rodriguez-Galan MC, Wang JM and Iribarren P: Interleukin 4 induces
the apoptosis of mouse microglial cells by a caspase-dependent
mechanism. Neurobiol Dis. 43:616–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burguillos MA, Deierborg T, Kavanagh E,
Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P,
Englund E, Venero JL and Joseph B: Caspase signalling controls
microglia activation and neurotoxicity. Nature. 472:319–324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu JH, Ge JB, Li M, Wu F, Zhang W and Qin
ZH: Inhibition of NF-κB activation is associated with
anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a
mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm
Sci. 47:652–660. 2012.
|
|
31
|
Woods DC, White YA, Dau C and Johnson AL:
TLR4 activates NF-κB in human ovarian granulosa tumor cells.
Biochem Biophys Res Commun. 409:675–680. 2011.
|
|
32
|
Kapitein B, Aalberse JA, Klein MR, de
Jager W, Hoekstra MO, Knol EF and Prakken BJ: Recognition of
self-heat shock protein 60 by T cells from patients with atopic
dermatitis. Cell Stress Chaperones. 18:87–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin L, Kim SC, Wang Y, Gupta S, Davis B,
Simon SI, et al: HSP60 in heart failure: abnormal distribution and
role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol.
293:H2238–H2247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Chen L, Hagiwara N and Knowlton
AA: Regulation of heat shock protein 60 and 72 expression in the
failing heart. J Mol Cell Cardiol. 48:360–366. 2010. View Article : Google Scholar : PubMed/NCBI
|