Introduction
The pathogenesis of postmenopausal osteoporosis
involves increased bone turnover with a relative increase in bone
resorption, leading to a marked decline in bone mass with the loss
of estrogen following menopause. A number of bone diseases,
including osteopenia and osteoporosis, reflect an imbalance in the
differentiation and function of two cell types, the osteoblast and
osteoclast, which are responsible for bone formation and bone
resorption, respectively (1,2).
Osteoclasts are derived from bone marrow hematopoietic stem cells
(3). The number and activity of
osteoclasts is determined by cell lineage allocation, proliferation
and the differentiation of osteoclast precursors (4). Osteoclastic differentiation requires
macrophage colony-stimulating factor (M-CSF) and receptor for
activation of NFκB ligand (RANKL) (5). In the early stages of
osteoclastogenesis, binding of M-CSF to colony stimulating factor 1
receptor (c-fms) stimulates expression of RANK, the receptor of
RANKL, in hematopoietic osteoclast precursor cells (6). At later stages, binding of RANKL to
RANK activates c-fos, c-jun and nuclear factor of activated T cells
cytoplasmic 1 (NFAT c1) in osteoclast precursors, which then
differentiate into mononuclear osteoclasts (5).
Epidemiological and longitudinal studies have
revealed a positive correlation between the intake of n-3 long
chain polyunsaturated fatty acids (PUFAs) and bone mineral density
in postmenopausal women (7). In
animals, dietary supplementation with n-3 PUFA-rich oils, including
fish oil, has been linked to improved maintenance of bone mass
postovariectomy (8–10). In addition, endogenously produced
n-3 PUFAs have been revealed to protect against ovariectomy-induced
bone loss in fat-1 transgenic mice (11,12).
Administration of n-3 PUFAs for 16 weeks was observed to suppress
RANKL expression and NFκB activation in the activated splenic CD4
cells of ovariectomized mice (13). Previous in vitro studies
have revealed that n-3 PUFAs are linked to decreased NFκB
expression (14,15) and modulation of RANKL signaling in
RAW264.7 cells (16). These
studies indicated that n-3 PUFAs reduced bone resorption by
decreasing osteoclastogenesis. However, it remains unknown whether
n-3 PUFAs affect the early stages of osteoclastogenesis and which
genes or molecules these fatty acids target in vivo in
ovariectomized rats. By contrast, n-3 PUFAs also affect bone
formation in animal models (17,18)
and osteoblast functions by increasing Runx2 expression in MC3T3
cells (19). The effects of n-3
PUFAs on bone formation and osteoblasts remain poorly understood in
ovariectomized rats.
In the present study, the effects of fish oil on
bone metabolism and the expression of genes involved in
osteoclastogenesis were investigated in vivo using
ovariectomized rats. Fish oil reduced the activity and number of
osteoclasts without altering the activity and number of
osteoblasts. The decrease in the number of osteoclasts was found to
be caused by a reduction of osteoclastogenesis, which was
associated with the decreased expression of M-CSF in the early
stages of osteoclastic differentiation.
Materials and methods
Animals and diets
Female Wistar/ST rats (9 weeks old) were purchased
from Japan SLC, Inc. (Shizuoka, Japan) and housed individually in a
temperature-controlled room with a 12-h light/dark cycle. Following
a 1-week period of adaptation, the animals were subjected to
bilateral ovariectomy (Ovx) or sham-operation (Sham). The animals
were further divided into two groups and fed American Institute of
Nutrition (AIN)-76A-based semipurified diets; corn (C) or fish (F)
oil-containing (ShamC, ShamF, OvxC and OvxF; n=10 for each group).
C or F diets contained 5% corn or fish oil (4.5% menhaden oil with
0.5% corn oil), respectively (Table
I; Research Diets, Inc., New Brunswick, NJ, USA). F diet was
supplemented with 6.3 mg/kg α-tocopherol to match the concentration
of corn oil. The fatty acid composition of the oils used in the
diets are presented in Table II.
After 2 weeks, blood and femoral and tibial bone samples were
collected under sodium pentobarbital anesthesia after overnight
access to food (non-fasting). Blood samples were used to determine
the serum concentrations of estradiol, osteocalcin, TNFα,
interleukin (IL)-6 and prostaglandin E2 (PGE2) and the plasma fatty
acid composition. Following removal of muscle and tendons, the
tibial bone was used for biochemical and histological analyses.
Animal experiments were performed in accordance with protocols
approved by the Animal Care Research Committee of Nara Women’s
University.
 | Table IDiet ingredients. |
Table I
Diet ingredients.
| Diet |
|---|
|
|
|---|
| Ingredient
(g/kg) | Corn oil | Fish oil |
|---|
| Casein | 200 | 200 |
| DL-methionine | 3 | 3 |
| Corn oil | 50 | 5 |
| Fish oil (menhaden
oil) | 0 | 45 |
| Corn starch | 150 | 150 |
| Sucrose | 500 | 500 |
| Cellulose | 50 | 50 |
| Mineral mixa | 35 | 35 |
| Vitamin mixb | 10 | 10 |
| Choline
bitartrate | 2 | 2 |
| Total (g) | 1000 | 1000 |
 | Table IIFatty acid composition of oils. |
Table II
Fatty acid composition of oils.
| Fatty acid | (C:D) | Corn oil (g/100
g) | Fish oila (g/100 g) |
|---|
| Myristic acid | 14:0 | - | 6.2 |
| Pentadecanoic
acid | 15:0 | - | 0.4 |
| Palmitic acid | 16:0 | 11.0 | 14.4 |
| Palmitoleic
acid | 16:1 n-7 | - | 8.8 |
| Hexadecadienoic
acid | 16:2 n-6 | - | 1.4 |
| Hexadecatrienoic
acid | 16:3 n-3 | - | 1.4 |
| Hexadecatetraenoic
acid | 16:4 n-3 | - | 1.4 |
| Stearic acid | 18:0 | 2.0 | 2.6 |
| Oleic acid | 18:1 n-9 | 25.0 | 11.2 |
| Linoleic acid | 18:2 n-6 | 60.2 | 7.8 |
| α-Linolenic
acid | 18:3 n-3 | 1.4 | 1.4 |
| Octadecatetraenoic
acid | 18:4 n-3 | - | 2.8 |
| Arachidic acid | 20:0 | - | 0.2 |
| Eicosanoic
acid | 20:1 n-9 | - | 1.4 |
| Eicosadienoic
acid | 20:2 n-6 | - | 0.2 |
| Dihomo-γ-linolenic
acid | 20:3 n-6 | - | 0.4 |
| Arachidonic
acid | 20:4 n-6 | - | 1.8 |
| Eicosapentaenoic
acid | 20:5 n-3 | - | 12.8 |
| Henicosapentaenoic
acid | 21:5 n-3 | - | 0.6 |
| Docosenoic
acid | 22:1 n-9 | - | 0.2 |
| Docosatetraenoic
acid | 22:4 n-6 | - | 0.2 |
| Docosapentaenoic
acid | 22:5 n-3 | - | 2.6 |
| Docosahexaenoic
acid | 22:6 n-3 | - | 9.2 |
| Lignoceric
acid | 24:0 | - | 0.6 |
| Tetracosenoic
acid | 24:1 n-9 | - | 0.2 |
Biochemical analysis
Serum concentrations of estradiol, osteocalcin,
TNFα, IL-6 and PGE2 were measured using an Elecsys E2II assay
(Roche Diagnostics GmbH, Mannheim, Germany), a Rat Osteocalcin
ELISA DS kit (DS Pharma Biomedical Co., Ltd., Osaka, Japan),
Quantikine Rat TNFα and IL-6 Immunoassays (both R&D Systems,
Inc., Minneapolis, MN, USA) and a PGE2 Express EIA kit (Cayman
Chemical Co., Ann Arbor, MI, USA), respectively.
The activities of alkaline phosphatase (ALP),
tartrate resistant acid phosphatase (TRAP) and cathepsin K (CK) and
the levels of calcium (Ca) and hydroxyproline (Hyp) in the proximal
tibia (the quarter from the aspect of the knee of the tibia) were
determined as described previously (20,21).
Histomorphometry
Tibias were fixed in 4% paraformaldehyde,
decalcified in 10% EDTA and embedded in paraffin. Sections (4 μm)
were stained for TRAP activity using a leukocyte acid phosphatase
kit (387-A; Sigma-Aldrich, St. Louis, MO, USA) as described
previously (20). Morphometric
measurements of trabecular structure (trabecular bone volume, bone
surface, thickness and number) and the number of osteoblasts
(cuboidal cells on trabecular surfaces) and osteoclasts
(TRAP-stained cells with >3 nuclei) were performed at
standardized sites (300 × 300 μm) under the growth plate in the
metaphysis of the proximal tibia (22).
Fatty acid analysis
Serum total lipids were extracted as described
previously (23), with specific
modifications (24). Following
methylation, fatty acid methyl esters were separated using a gas
chromatograph (GC2014; Shimadzu, Kyoto, Japan) equipped with a 25 m
× 0.5 mm capillary column (HR-SS-10; Shimadzu) and were identified
by comparison of retention times with a fatty acid methyl ester
standard (68A; Nu-Chek Prep, Inc., Elysian, MN, USA).
Quantitative real-time RT-PCR
Total RNA from the proximal tibia was prepared using
a commercial kit (Sepasol RNA I Super G; Nacalai Tesque Inc.,
Kyoto, Japan) after bone marrow cells were washed and homogenized
in the presence of 0.1 M EDTA. Total RNA was reverse-transcribed
using a first-strand cDNA synthesis kit (Toyobo, Tokyo, Japan). PCR
was performed using cDNA or total RNA (negative control) with
Thunderbird SYBR qPCR mix (Toyobo) and specific primers, as
described previously (21,25). Levels of gene expression were
determined relative to an internal standard (actin) and expressed
relative to the ShamC values.
Western blot analysis
Bone extracts of the proximal tibia were prepared as
described previously (20) for
western blot analysis. Protein concentrations were measured using
the BCA protein assay kit (Thermo Fisher Scientific Inc., Rockford,
IL, USA). Equal amounts of protein were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
membranes. Western blotting and reprobing were performed and the
chemiluminescent signals were quantified using a densitometer, as
described previously (26).
Antibodies recognizing actin, M-CSF, RANK, RANKL, osteoprotegerin
(OPG), microphthalmia-associated transcription factor (MITF), PU.1,
NFκB p65 and phosphorylated (p)-NFκB p65 (Ser 276) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Statistical analysis
Data are presented as the mean ± SEM. All
statistical analyses were performed by one-way analysis of variance
with pairwise comparison by the Bonferroni method using the
Microsoft Excel data analysis program. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of fish oil on clinical
characteristics and bone biochemical markers
Food intake and final body weight were identified to
be significantly higher and serum concentrations of estradiol were
significantly lower in the Ovx groups compared with the Sham groups
(Table III). Fish oil did not
affect these values. The weights of the femur and tibia were
significantly lower in OvxC rats than in the Sham groups, and were
restored to Sham levels in OvxF rats; however, bone lengths were
similar in the four groups (Table
III).
 | Table IIIEffects of fish oil on clinical
characteristics and bone biochemical markers. |
Table III
Effects of fish oil on clinical
characteristics and bone biochemical markers.
| A, Clinical
characteristics. |
|
| Parameters | ShamC | ShamF | OvxC | OvxF |
|
| Body weight, g |
| Start (prior to
fasting) | 208.4±1.3 | 206.2±1.7 | 208.5±1.4 | 208.0±1.0 |
| Final | 222.6±1.9 | 230.2±2.7 | 257.1±2.7a | 253.3±3.9a |
| Serum estradiol,
pg/ml | 25.0±2.4 | 27.7±3.4 | 14.0±0.4a | 18.3±1.8a |
| Bone length,
cm |
| Femur | 3.38±0.03 | 3.36±0.01 | 3.38±0.01 | 3.37±0.02 |
| Tibia | 3.70±0.01 | 3.71±0.02 | 3.71±0.02 | 3.72±0.02 |
| Bone weight, g |
| Femur | 0.666±0.005 | 0.664±0.008 | 0.632±0.011a | 0.669±0.008b |
| Tibia | 0.501±0.003 | 0.497±0.007 | 0.481±0.004a | 0.505±0.006b |
| Proximal
tibia | 0.223±0.002 | 0.224±0.003 | 0.212±0.002a | 0.224±0.002b |
|
| B, Bone biochemical
markers. |
|
| Parameters | ShamC | ShamF | OvxC | OvxF |
|
| Proximal tibia |
| ALP activity,
U/g | 20.93±0.81 | 18.88±0.62 | 25.58±0.62a | 25.87±0.89a |
| TRAP activity,
U/g | 0.764±0.039 | 0.717±0.035 | 1.263±0.035a | 1.049±0.034a,b |
| CK activity,
U/g | 397.4±34.0 | 325.7±23.1 | 518.7±28.6a | 415.7±34.6b |
| Ca, mg/g | 119.8±3.2 | 118.9±2.8 | 97.7±1.3a | 113.6±1.1b |
| Hyp, μmol/g | 102.4±1.9 | 104.0±2.4 | 91.9±1.3a | 99.5±1.2b |
| Serum osteocalcin,
ng/ml | 145.6±5.2 | 154.1±6.1 | 191.7±7.4a | 177.4±4.1a |
ALP activity in the proximal tibia and serum
osteocalcin levels were significantly higher in the Ovx groups
compared with Sham groups (Table
III). The activities of TRAP and CK in OvxC rats increased
significantly to 1.6- and 1.3-fold of ShamC levels, respectively.
Ca and Hyp levels in OvxC rats decreased to ~80 and 90% of the
ShamC value, respectively. Fish oil did not affect the increased
levels of ALP activity and osteocalcin in OVX rats. However, the
OVX-increased activities of TRAP and CK were suppressed and the
decreased levels of Ca and Hyp were recovered to the Sham values by
fish oil. Fish oil did not affect bone biochemical markers in Sham
rats.
Histological analysis
Morphometric measurements revealed that the number
of osteoclasts and osteoblasts in the OvxC rats increased to 1.7-
and 1.5-fold the ShamC value (Table
IV). In OvxC, trabecular bone volume and thickness were
decreased and trabecular bone surface was increased, although
trabecular numbers were not significantly altered by OVX. These
changes in the number of osteoclasts and trabecular bone volume,
thickness, and surface were reversed by fish oil, however,
osteoblast number was not recovered.
 | Table IVBone histomorphometry. |
Table IV
Bone histomorphometry.
| Parameters | ShamC | ShamF | OvxC | OvxF |
|---|
| Trabecular number,
no./mm | 13.4±0.6 | 13.7±0.3 | 13.8±0.6 | 14.8±1.2 |
| Trabecular bone
volume, % | 60.0±1.4 | 61.7±1.8 | 46.1±1.6a | 59.2±0.8b |
| Trabecular bone
surface, mm/mm2 | 26.4±1.1 | 27.5±0.6 | 33.7±1.7a | 27.7±1.9b |
| Trabecular
thickness, μm | 48.4±3.8 | 45.2±2.0 | 27.3±2.1a | 42.0±2.8b |
| Osteoblast index
(no. Ob/mm trabecular bone length) | 16.32±0.59 | 16.45±0.78 | 23.72±0.96a | 23.80±1.12a |
| Osteoclast index
(no. Oc/mm trabecular bone length) | 2.93±0.12 | 2.75±0.26 | 5.08±0.26a | 3.45±0.24b |
Expression of genes and proteins involved
in osteoclastic differentiation in the proximal tibia
Gene expression levels of the
osteoclastogenesis-related factors, M-CSF, RANKL, OPG, c-fms, PU.1,
MITF, RANK, c-fos, c-jun and osteoclast-specific proteins, TRAP and
cathepsin K, relative to the internal control, actin, are
demonstrated in Fig. 1A. mRNA
levels of M-CSF and RANKL in OvxC rats were ~1.6-fold the ShamC
value, although a significant difference was not observed in OPG
levels. The expression of c-fms in OvxC rats did not differ from
ShamC values. However, levels of RANK, PU.1, MITF, c-fos and c-jun
in the OvxC rats were 2-, 2-, 2-, 3- and 4-fold the ShamC values,
respectively. The expression of TRAP and CK mRNA also increased to
3.7- and 2.5-fold the ShamC value, respectively. These increases
induced by OVX were suppressed to Sham levels by fish oil, although
the expression of TRAP in OvxF rats was significantly higher than
the ShamC value but lower than the OvxC level. Fish oil did not
affect expression levels in Sham rats.
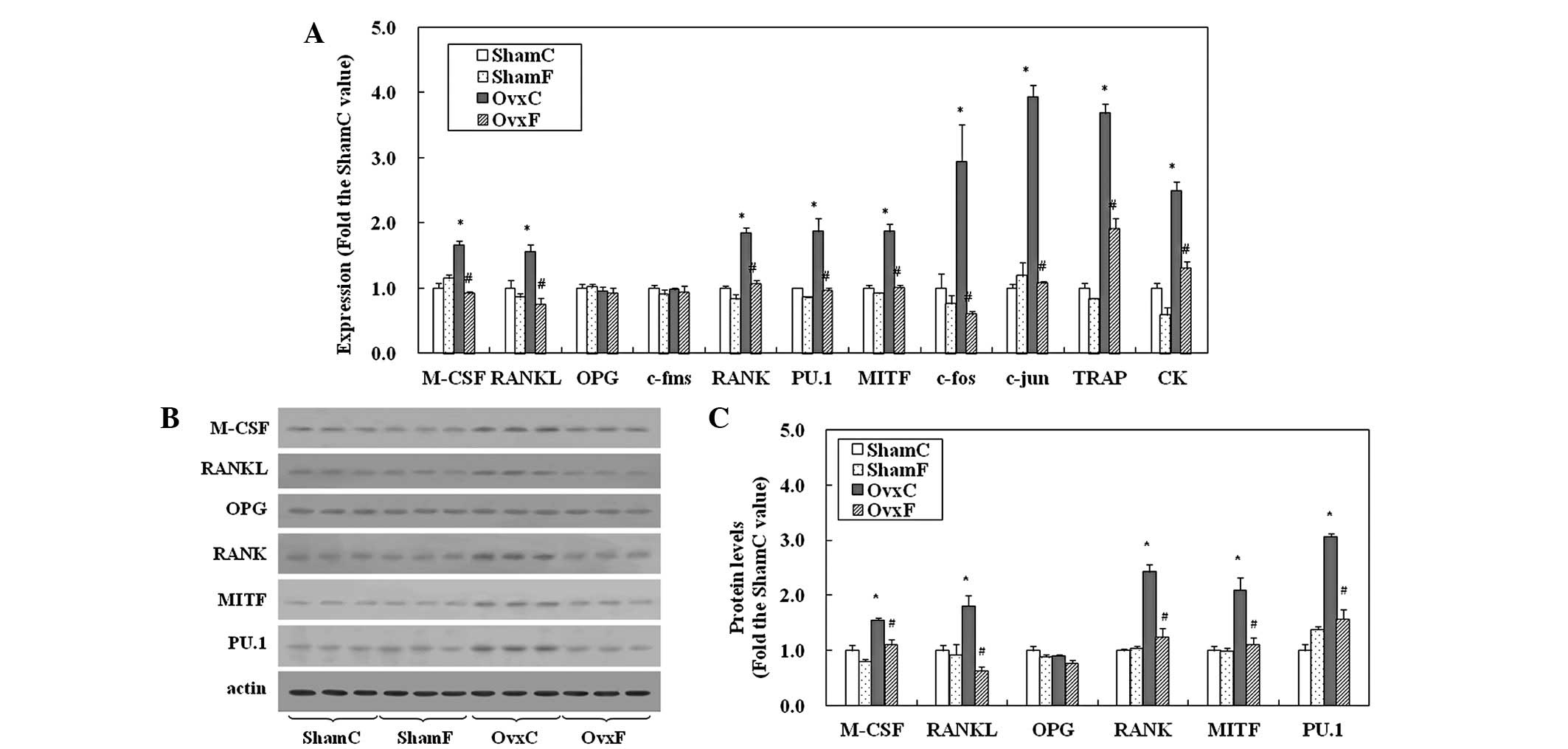 | Figure 1mRNA and protein expression of genes
involved in osteoclastic differentiation in the proximal tibia,
including M-CSF, RANKL, OPG, c-fms, RANK, PU.1, MITF, c-fos, c-jun,
TRAP and CK. (A) RT-PCR and (B) western blot analysis of bone
extracts from the proximal tibia. (C) Quantification of protein
levels. Data are presented as the mean ± SEM (n=8).
*P<0.05 vs. ShamC; #P<0.05 vs. OvxC.
M-CSF, macrophage colony-stimulating factor; RANKL, receptor for
activation of NFκB ligand; OPG, osteoprotegerin; c-fms, colony
stimulating factor 1 receptor; MITF, microphthalmia-associated
transcription factor; TRAP, tartrate-resistant acid phosphatase,
CK, cathepsin K; C, corn oil-containing; F, fish oil-containing;
Ovx, ovariectomy; Sham, sham-operation. |
The results of the western blot analysis are
presented in Fig. 1B. Protein
levels of M-CSF, RANKL, RANK, MITF and PU.1 in the OvxC group
increased to ~2-fold of the ShamC values, while OPG levels were
largely unchanged (Fig. 1B and C).
Fish oil was observed to suppress these increases to Sham values in
OVX rats, but had no effect in Sham animals.
Plasma fatty acid composition and serum
concentrations of TNFα, IL-6 and PGE2
OVX significantly increased levels of arachidonic
acid (AA, 20:4 n-6), as revealed in Table V. Fish oil reduced this increase.
In the fish oil-fed rats, ShamF and OvxF, levels of AA and linoleic
acid (18:2 n-6) significantly decreased and those of palmitoleic
(16:1 n-7), eicosapentaenoic (EPA, 20:5 n-3), docosapentaeic (DPA;
22:5 n-3) and docosahexaenoic (DHA, 22:6 n-3) acid increased
compared with the corresponding levels in corn oil-fed rats.
 | Table VEffects of fish oil on plasma fatty
acid composition (mol %). |
Table V
Effects of fish oil on plasma fatty
acid composition (mol %).
| Fatty acid | (C:D) | ShamC | ShamF | OvxC | OvxF |
|---|
| Myristic acid | 14:0 | 0.91±0.18 | 1.68±0.38a | 1.09±0.39 | 1.39±0.37 |
| Palmitic acid | 16:0 | 16.89±5.83 | 19.00±1.23 | 17.80±1.62 | 18.82±7.96 |
| Palmitoleic
acid | 16:1 n-7 | 2.25±0.30 | 4.49±0.78a | 2.25±0.12 | 4.26±1.23b |
| Stearic acid | 18:0 | 19.71±1.88 | 21.49±1.31 | 20.59±1.81 | 21.13±3.31 |
| Oleic acid | 18:1 n-9 | 11.14±2.05 | 8.12±1.49 | 9.21±1.13 | 8.56±1.68 |
| Vaccenic acid | 18:1 n-7 | 2.04±0.30 | 2.10±0.30 | 1.87±0.25 | 2.30±0.26 |
| Linoleic acid | 18:2 n-6 | 15.88±0.74 | 5.38±0.43a | 14.12±1.11 | 5.63±0.25b |
| γ–Linolenic
acid | 18:3 n-6 | 0.30±0.06 | 0.20±0.02 | 0.34±0.06 | 0.38±0.15 |
| α–Linolenic
acid | 18:3 n-3 | 0.16±0.05 | 0.20±0.01 | 0.08±0.05 | 0.25±0.14b |
| Arachidic acid | 20:0 | 0.13±0.01 | 0.17±0.02 | 0.17±0.07 | 0.29±0.13 |
| Eicosanoic
acid | 20:1 | 0.38±0.20 | 0.54±0.14 | 0.36±0.27 | 0.58±0.21 |
| Eicosadienoic
acid | 20:2 n-6 | 0.20±0.02 | 0.25±0.18 | 0.20±0.14 | 0.21±0.16 |
| Dihomo-γ-linolenic
acid | 20:3 n-6 | 0.49±0.07 | 0.64±0.23 | 0.57±0.09 | 0.64±0.08 |
| Arachidonic
acid | 20:4 n-6 | 20.95±1.45 | 11.81±1.75a | 25.68±1.82a | 11.53±1.23b |
| Eicosatetraenoic
acid | 20:4 n-3 | ND | 0.31±0.04 | ND | 0.31±0.04 |
| Eicosapentaenoic
acid | 20:5 n-3 | 0.12±0.02 | 13.51±0.72a | 0.09±0.02 | 14.89±2.04b |
| Docosapentaenoic
acid | 22:5 n-3 | 0.29±0.08 | 1.30±0.35a | 0.24±0.08 | 0.88±0.21b |
| Docosahexaenoic
acid | 22:6 n-3 | 2.55±0.25 | 5.77±0.85a | 2.26±0.20 | 4.95±0.74b |
The concentrations of TNFα, IL-6 and PGE2 were
significantly increased by 1.4-, 1.4- and 1.2-fold of Sham levels
in OvxC rats, respectively (Table
VI). The increases were suppressed to Sham levels by fish oil.
Fish oil did not affect these concentrations in Sham rats.
 | Table VIEffects of fish oil on plasma
concentrations of TNFα, IL-6 and PGE2. |
Table VI
Effects of fish oil on plasma
concentrations of TNFα, IL-6 and PGE2.
| Protein | ShamC | ShamF | OvxC | OvxF |
|---|
| TNFα (pg/ml) | 19.8±2.0 | 20.7±1.9 | 28.7±1.6a | 19.6±2.7b |
| IL-6 (pg/ml) | 84.7±7.2 | 87.0±3.0 | 116.2±4.0a | 81.6±11.04b |
| PGE2 (ng/ml) | 1.30±0.06 | 1.06±0.1 | 1.61±0.05a | 1.11±0.05b |
mRNA levels of TNFα, IL-6, cyclooxygenase
2 (COX2) and phospholipase A2 (PLA2) and NFκB activation in the
proximal tibia
mRNA levels of TNFα, IL-6, COX2 and PLA2 in the OvxC
rats increased to ~2.4-, 1.8-, 1.7- and 2.5-fold the ShamC values,
respectively (Fig. 2A). These
increases were restored to Sham levels by fish oil; however,
significant effects of fish oil were not observed in the Sham
rats.
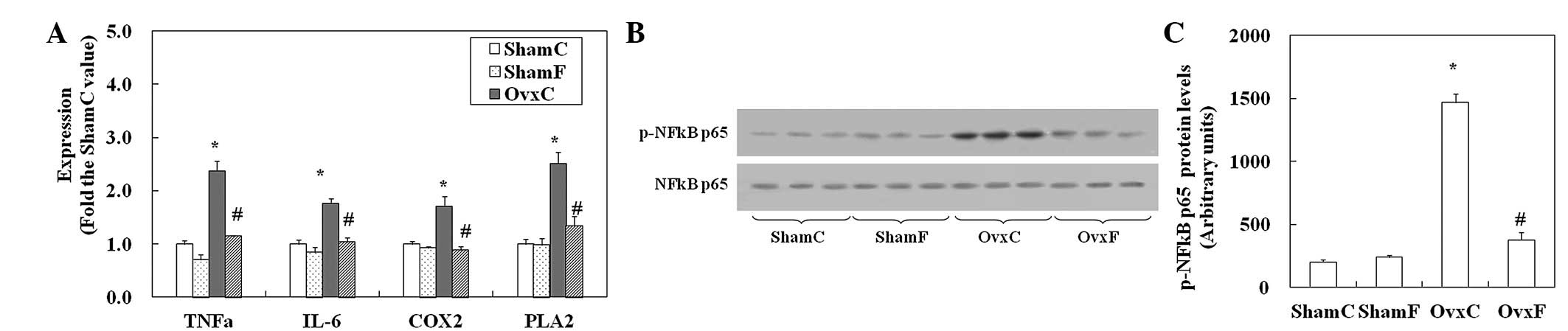 | Figure 2mRNA levels of TNFα, IL-6, COX2 and
PLA2 and the activation of NFκB in the proximal tibia. (A) Total
RNA was extracted from the proximal tibia and the mRNA levels of
TNFα, IL-6, COX2 and PLA2 were assessed by RT-PCR. (B) Western blot
analysis of bone extracts from the proximal tibia using p-NFκB p65
(Ser276) or p65 antibodies. (C) Quantification of protein levels.
Data are presented as the mean ± SEM (n=8). *P<0.05
vs. ShamC; #P<0.05 vs. OvxC. TNFα, tumor necrosis
factor α; IL-6, interleukin-6; COX2, cyclooxygenase 2; PLA2,
phospholipase A2; C, corn oil-containing; F, fish oil-containing;
Ovx, ovariectomy; Sham, sham-operation. |
The phosphorylation of NFκB p65 (p-NFκB p65) is
crucial for NFκB transcriptional activity. The p-NFκB p65 protein
in OvxC rats increased to 7-fold the ShamC level (Fig. 2B and C). Stimulation of
phosphorylation was reduced to Sham level by fish oil. Fish oil did
not affect the phosphorylation of NFκB p65 in Sham rats.
Discussion
Results of the present study confirm that fish oil
suppresses increased bone resorption induced by OVX. OVX resulted
in substantial decreases in Ca and Hyp and increases in
osteoclastic and osteoblastic activities. Fish oil suppressed the
increase in osteoclastic activity and osteoclast number. However,
it did not affect the activity or number of osteoblasts. These
results indicate that fish oil suppresses the decrease in Ca and
Hyp levels in bone by reducing the increase in bone resorption
associated with decreases in osteoclastogenesis. It should be noted
that n-3 PUFAs had no effect on bone formation in vivo in
ovariectomized rats, although previous studies reported a role in
increasing osteoblastic activity in growing rats (17,27)
or osteoblastogenesis in osteoblast-like cells (19).
Osteoclast precursors are derived from hematopoietic
stem cells in bone marrow. Differentiation into osteoclasts,
however, occurs on the bone surface in vivo(1,28).
Therefore, in the current study, gene expression of
osteoclastogenesis-related factors was examined in the bone. mRNA
and protein levels of M-CSF significantly increased in OvxC rats
compared with Sham rats, and fish oil suppressed these increases to
ShamC and ShamF levels. For the first time, this study revealed the
suppression of M-CSF expression by fish oil in the bone of
ovariectomized rats. M-CSF induces the proliferation of osteoclast
precursor cells, supports their survival and upregulates expression
of the receptor of RANKL, RANK, which is a prerequisite for
osteoclast precursor cells (5).
Gene expression of RANK is regulated by the transcription factors
PU.1 and MITF (29). In
ovariectomized rats, mRNA and protein levels of PU.1 and MITF
increased compared with ShamC rats, and fish oil suppressed this
increase. Suppression of M-CSF expression may lead to the reduced
expression of PU.1 and MITF and a subsequent decrease in RANK
expression in OvxF rats. This study demonstrated that fish oil
suppressed the expression of M-CSF, followed by PU.1, MITF and
RANK, in the early stages of osteoclastogenesis, including the
differentiation of hematopoietic stem cells into osteoclast
precursor cells upstream of RANKL signaling.
M-CSF expression is upregulated by a variety of
inflammatory cytokines, including TNFα (30). TNFα expression is induced by NFκB
(31). In the present study,
increased NFκB activation (p-NFκB p65) was observed in the bones of
OvxC rats compared with ShamC rats. Increased NFκB activation was
suppressed in the proximal tibia of OvxF rats. Simultaneously, a
decrease in the levels of n-6 PUFAs (AA and linoleic acid) and an
increase in n-3 PUFAs (EPA, DPA and DHA) was observed in the plasma
of OvxF rats. A number of previous in vitro studies have
also reported that n-3 PUFAs, which are major fatty acids of fish
oil, downregulate NFκB activity (13,32–35).
Consistent with these in vitro results, the present study
suggested that an increase in the n-3/n-6 PUFA ratio induced by
dietary fish oil led to the suppression of NFκB activation in the
bones of ovariectomized rats in the present study. NFκB suppression
was found to be associated with a reduction in serum TNFα levels
and the mRNA levels of TNFα in bone. These results indicate that
inhibition of NFκB activation by increases in serum n-3 PUFAs
suppresses downstream events, including TNFα/M-CSF/PU.1/MITF/RANK
expression in the proximal tibia. Notably, the effects of increased
n-3 PUFAs were observed in ovariectomized rats only and not in
normal animals.
M-CSF stimulates the production of IL-6 and PGE2
(36,37), which is known to be upregulated by
estrogen deficiency (38,39). In the current study, serum
concentrations of IL-6 and PGE2 and mRNA levels of IL-6 and a PG
synthesis enzyme, COX2, in the proximal tibia increased in OvxC
rats compared with ShamC rats. Fish oil restored these levels to
Sham values, coinciding with the suppression of M-CSF, in OvxF
rats. In addition, increased expression of PLA2, which plays a key
role in PGE2 synthesis (40) and
is induced by TNFα (41), was
suppressed by fish oil. TNFα, IL-6 and PGE2 induced the expression
of RANKL (38,42), an additional essential factor for
osteoclastogenesis. Downregulation of RANKL, as well as M-CSF
expression, by fish oil was observed in bone in the current study.
Previous studies have also reported a decrease in RANKL expression
in RAW264.7 (16) or activated
splenic CD4 cells (13). In the
present study, downregulation of RANKL expression by fish oil was
confirmed at the mRNA and protein level in vivo in bone.
These results indicate that the suppression of RANKL expression
resulted from decreased production of IL-6 and PGE2, caused by the
suppression of M-CSF, as well as a decrease in TNFα, in the bone of
ovariectomized rats.
Results of the present study indicate that fish oil
reduces ovariectomy-stimulated osteoclastogenesis by suppressing
the expression of M-CSF, PU.1, MITF and RANK in the early stages of
osteoclastogenesis and RANKL signaling in later stages.
Abbreviations:
|
AA
|
arachidonic acid
|
|
ALP
|
alkaline phosphatase
|
|
COX2
|
cyclooxygenase 2
|
|
DHA
|
docosahexaenoic acid
|
|
EPA
|
eicosapentaenoic acid
|
|
IL-6
|
interleukin-6
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
MITF
|
microphthalmia-associated
transcription factor
|
|
OPG
|
osteoprotegerin
|
|
OVX
|
ovariectomy
|
|
PGE2
|
prostagrandin E2
|
|
PLA2
|
phospholipase A2
|
|
PUFAs
|
polyunsaturated fatty acids
|
|
RANK
|
receptor for activation of NFκB
|
|
RANKL
|
receptor for activation of NFκB
ligand
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
TNFα
|
tumor necrosis factor α
|
References
|
1
|
Manolagas SC: Birth and death of bone
cells: basic regulatory mechanisms and implications for the
pathogenesis and treatment of osteoporosis. Endocr Rev. 21:115–137.
2000.PubMed/NCBI
|
|
2
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Udagawa N, Takahashi N, Akatsu T, et al:
Origin of osteoclasts: mature monocytes and macrophages are capable
of differentiating into osteoclasts under a suitable
microenvironment prepared by bone marrow-derived stromal cells.
Proc Natl Acad Sci USA. 87:7260–7264. 1990. View Article : Google Scholar
|
|
4
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arai F, Miyamoto T, Ohneda O, et al:
Commitment and differentiation of osteoclast precursor cells by the
sequential expression of c-Fms and receptor activator of nuclear
factor kappaB (RANK) receptors. J Exp Med. 190:1741–1754. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiss LA, Barrett-Connor E and von Mühlen
D: Ratio of n-6 to n-3 fatty acids and bone mineral density in
older adults: the Rancho Bernardo Study. Am J Clin Nutr.
81:934–938. 2005.PubMed/NCBI
|
|
8
|
Priante G, Bordin L, Musacchio E, Clari G
and Baggio B: Fatty acids and cytokine mRNA expression in human
osteoblastic cells: a specific effect of arachidonic acid. Clin Sci
(Lond). 102:403–409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watkins BA, Li Y and Seifert MF: Dietary
ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone
mineral and serum biomarkers in ovariectomized rats. J Nutr
Biochem. 17:282–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchida R, Chiba H, Ishimi Y, et al:
Combined effects of soy isoflavone and fish oil on
ovariectomy-induced bone loss in mice. J Bone Miner Metab.
29:404–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahman MM, Bhattacharya A, Banu J, Kang JX
and Fernandes G: Endogenous n-3 fatty acids protect ovariectomy
induced bone loss by attenuating osteoclastogenesis. J Cell Mol
Med. 13:1833–1844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banu J, Bhattacharya A, Rahman M, Kang JX
and Fernandes G: Endogenously produced n-3 fatty acids protect
against ovariectomy induced bone loss in fat-1 transgenic mice. J
Bone Miner Metab. 28:617–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Krishnan A, Zaman K, Lawrence R,
Bhattacharya A and Fernandes G: Dietary n-3 fatty acids decrease
osteoclastogenesis and loss of bone mass in ovariectomized mice. J
Bone Miner Res. 18:1206–1216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahman MM, Bhattacharya A and Fernandes G:
Docosahexaenoic acid is more potent inhibitor of osteoclast
differentiation in RAW 264.7 cells than eicosapentaenoic acid. J
Cell Physiol. 214:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zwart SR, Pierson D, Mehta S, Gonda S and
Smith SM: Capacity of omega-3 fatty acids or eicosapentaenoic acid
to counteract weightlessness-induced bone loss by inhibiting
NF-kappaB activation: from cells to bed rest to astronauts. J Bone
Miner Res. 25:1049–1057. 2010.PubMed/NCBI
|
|
16
|
Rahman MM, Bhattacharya A and Fernandes G:
Conjugated linoleic acid inhibits osteoclast differentiation of
RAW264.7 cells by modulating RANKL signaling. J Lipid Res.
47:1739–1748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watkins BA, Lippman HE, Le Bouteiller L,
Li Y and Seifert MF: Bioactive fatty acids: role in bone biology
and bone cell function. Prog Lipid Res. 40:125–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhattacharya A, Rahman M, Sun D and
Fernandes G: Effect of fish oil on bone mineral density in aging
C57BL/6 female mice. J Nutr Biochem. 18:372–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watkins BA, Li Y, Lippman HE and Feng S:
Modulatory effect of omega-3 polyunsaturated fatty acids on
osteoblast function and bone metabolism. Prostaglandins Leukot
Essent Fatty Acids. 68:387–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goto A and Tsukamoto I: Increase in
tartrate-resistant acid phosphatase of bone at the early stage of
ascorbic acid deficiency in the ascorbate-requiring Osteogenic
Disorder Shionogi (ODS) rat. Calcif Tissue Int. 73:180–185. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hie M, Shimono M, Fujii K and Tsukamoto I:
Increased cathepsin K and tartrate-resistant acid phosphatase
expression in bone of streptozotocin-induced diabetic rats. Bone.
41:1045–1050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parfitt AM, Drezner MK, Glorieux FH, et
al: Bone histomorphometry: standardization of nomenclature, symbols
and units. Report of the ASBMR Histomorphometry Nomenclature
Committee. J Bone Miner Res. 2:595–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okita M, Gaudette DC, Mills GB and Holub
BJ: Elevated levels and altered fatty acid composition of plasma
lysophosphatidylcholine(lysoPC) in ovarian cancer patients. Int J
Cancer. 71:31–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hie M, Iitsuka N, Otsuka T, Nakanishi A
and Tsukamoto I: Zinc deficiency decreases osteoblasts and
osteoclasts associated with the reduced expression of Runx2 and
RANK. Bone. 49:1152–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hie M, Yamazaki M and Tsukamoto I:
Curcumin suppresses increased bone resorption by inhibiting
osteoclastogenesis in rats with streptozotocin-induced diabetes.
Eur J Pharmacol. 621:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Seifert MF, Ney DM, et al: Dietary
conjugated linoleic acids alter serum IGF-I and IGF binding protein
concentrations and reduce bone formation in rats fed (n-6) or (n-3)
fatty acids. J Bone Miner Res. 14:1153–1162. 1999. View Article : Google Scholar
|
|
28
|
Hayashi S, Miyamoto A, Yamane T, et al:
Osteoclast precursors in bone marrow and peritoneal cavity. J Cell
Physiol. 170:241–247. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishii J, Kitazawa R, Mori K, et al:
Lipopolysaccharide suppresses RANK gene expression in macrophages
by down-regulating PU.1 and MITF. J Cell Biochem. 105:896–904.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oster W, Lindemann A, Horn S, Mertelsmann
R and Herrmann F: Tumor necrosis factor (TNF)-alpha but not
TNF-beta induces secretion of colony stimulating factor for
macrophages (CSF-1) by human monocytes. Blood. 70:1700–1703.
1987.PubMed/NCBI
|
|
31
|
Singer P, Shapiro H, Theilla M, Anbar R,
Singer J and Cohen J: Anti-inflammatory properties of omega-3 fatty
acids in critical illness: novel mechanisms and an integrative
perspective. Intensive Care Med. 34:1580–1592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xi S, Cohen D, Barve S and Chen LH: Fish
oil suppressed cytokines and nuclear factor-kappaB induced by
murine AIDS virus infection. Nutr Res. 21:865–878. 2001. View Article : Google Scholar
|
|
33
|
Novak TE, Babcock TA, Jho DH, Helton WS
and Espat NJ: NF-kappa B inhibition by omega -3 fatty acids
modulates LPS-stimulated macrophage TNF-alpha transcription. Am J
Physiol Lung Cell Mol Physiol. 284:L84–L89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Y, Joshi-Barve S, Barve S and Chen
LH: Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression
by preventing NF-kappaB activation. J Am Coll Nutr. 23:71–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weldon SM, Mullen AC, Loscher CE, Hurley
LA and Roche HM: Docosahexaenoic acid induces an anti-inflammatory
profile in lipopolysaccharide-stimulated human THP-1 macrophages
more effectively than eicosapentaenoic acid. J Nutr Biochem.
18:250–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurland JI, Pelus LM, Ralph P, Bockman RS
and Moore MA: Induction of prostaglandin E synthesis in normal and
neoplastic macrophages: role for colony-stimulating factor(s)
distinct from effects on myeloid progenitor cell proliferation.
Proc Natl Acad Sci USA. 76:2326–2330. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barreda DR, Hanington PC and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manolagas SC, Kousteni S and Jilka RL: Sex
steroids and bone. Recent Prog Horm Res. 57:385–409. 2002.
View Article : Google Scholar
|
|
39
|
Kawaguchi H, Pilbeam CC, Vargas SJ, Morse
EE, Lorenzo JA and Raisz LG: Ovariectomy enhances and estrogen
replacement inhibits the activity of bone marrow factors that
stimulate prostaglandin production in cultured mouse calvariae. J
Clin Invest. 96:539–548. 1995. View Article : Google Scholar
|
|
40
|
Dieter P, Kolada A, Kamionka S, Schadow A
and Kaszkin M: Lipopolysaccharide-induced release of arachidonic
acid and prostaglandins in liver macrophages: regulation by Group
IV cytosolic phospholipase A2, but not by Group V and Group IIA
secretory phospholipase A2. Cell Signal. 14:199–204. 2002.
View Article : Google Scholar
|
|
41
|
Lee CW, Lin CC, Lee IT, Lee HC and Yang
CM: Activation and induction of cytosolic phospholipase A2 by TNF-α
mediated through Nox2, MAPKs, NF-kB, and p300 in human tracheal
smooth muscle cells. J Cell Physiol. 226:2103–2114. 2011.
|
|
42
|
Fujita D, Yamashita N, Iita S, Amano H,
Yamada S and Sakamoto K: Prostaglandin E2 induced the
differentiation of osteoclasts in mouse osteoblast-depleted bone
marrow cells. Prostaglandins Leukot Essent Fatty Acids. 68:351–358.
2003. View Article : Google Scholar : PubMed/NCBI
|
















