Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer. It is the third-leading cause of
cancer-related mortality and the fifth most common type of cancer
worldwide, with >600,000 cases diagnosed annually (1–3). HCC
is normally diagnosed at an advanced stage and typically has a poor
prognosis even following surgical resection and liver
transplantation (4). Due to
various etiologies, the prevention and treatment of HCC remains a
challenge worldwide (5). HCC is
more prevalent in developing countries with ~80% of the total
incidence occurring in Asia and sub-Saharan Africa (6,7). The
prevalence of HCC is increasing in western countries due to the
increasing prevalence of hepatitis C virus (HCV) infection
(8–10). Thus, with the global incidence of
HCC on the rise, there have been increasing calls for the
development of new and improved approaches for the treatment of
HCC.
Decorin, a functional component of the extracellular
matrix (ECM), has multiple biological functions, which include
regulating matrix assembly and fibrillogenesis, and controlling
cell proliferation (11–13). Recently, an in vivo
investigation demonstrated that decorin-null mice developed severe
liver fibrosis with a significantly delayed healing process
(14). Decorin is often
downregulated in various types of cancer of epithelial origin
(15). In addition to its
important biological functions, decorin inhibits cancer growth
in vitro and in vivo. The mechanism for the
suppression of tumor growth is independent of the functional p53
tumor suppressor gene; however, requires p21 to be functional
(16). Decorin causes a rapid
phosphorylation of the epidermal growth factor receptor (EGFR),
leading to the activation of mitogen-activated protein kinase and
the upregulation of p21, a cyclin-dependent kinase (CDK) inhibitor,
and ultimately growth arrest (17). In an in vivo experiment,
mice that were decorin and p53 null (DCN−/−
and p53−/−) developed a more aggressive form
of lymphoma than those that were only p53 null (12), suggesting that decorin deficiency
is permissive for tumorigenesis. Another study by Bi et al,
using a decorin-deficient mouse model, demonstrated that the
intestinal tumorigenesis in DCN−/− mice was
linked to the downregulation of p21 and p27 (18), implying that the role of decorin in
repressing tumorigenesis requires the upregulation of
cyclin-dependent kinase inhibitors (CKIs).
Cyclin-CDK inhibitors include three proteins:
p21CIP1, p27KIP1 and p57KIP2. CDK
inhibitors regulate the cell cycle of mammalian cells by binding to
cyclin-CDK complexes. In addition to cell cycle regulation, CKIs
have CDK-independent functions, including regulating transcription,
apoptosis, cell migration and the cytoskeleton (19). Unlike p21CIP1 and
p27KIP1, p57KIP2 has a unique role in
embryogenesis, in which the genetic deletion of p57KIP2
has been demonstrated to be lethal in p57KIP2 null mice
(20,21). Relative to p21CIP1 and
p27KIP1, p57KIP2 is the newest and least
studied CIP/KIP member.
The p57KIP2 human gene is located on
chromosome 11 at the 11p15.5 locus and encodes a protein 316 amino
acids long. Structurally, p57KIP2 is almost identical to
p27KIP1 and functionally, it leads to cell cycle arrest
in the G1 phase. In addition, it has been reported that
p27KIP1 and p57KIP2 play conducive roles in
neuronal migration and may, when working together, coordinate the
timing of neuronal differentiation, migration and, potentially,
cell cycle arrest in neocortical development. p57KIP2
may serve as an important domain for protein interactions
implicated in functions other than the CDK-inhibitory role
(21). Additionally,
p57KIP2, but not p21CIP1/WAF1 or
p27KIP1, interacts in vivo and in vitro
through its amino-terminal domain with transcription factor B-Myb.
Mutations of p57KIP2 occur in Beckwith-Wiedemann
syndrome and its reduced protein expression in breast, lung, liver,
prostate, colorectal and bladder cancer is suggestive of its tumor
suppressive properties (22–26).
There is mounting evidence that the p57KIP2 protein
level is normally downregulated in these types of cancer through
several mechanisms, including maternal-specific loss of
heterozygosity (LOH), loss of imprinting and promoter methylation
(19). A recent study revealed
that the downregulation of p57KIP2 accelerates the
growth and invasion of HCC (27),
indicating that the upregulation of this tumor suppressor is
essential for the prevention and therapy of HCC. Activating the
cyclin-CDK inhibitors using a small molecule inhibitor to restore
their regulatory role in the cell cycle, proliferation and
differentiation is an attractive therapeutic strategy for cancer
treatment. As the expression of p57KIP2 is downregulated
in several types of cancer, it may have therapeutic and prognostic
uses. Hence, an investigation into upregulating its expression is
essential. In this study, we demonstrated that recombinant human
decorin upregulated the expression of p57KIP2, a CDK
inhibitor, in HepG2 cell lines.
Materials and methods
Cell culture
HepG2 cell lines were purchased from the American
Type Culture Collection (Manassas, VA, USA); cells were grown in
DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) plus 10%
fetal bovine serum, and supplemented with 100 μ/ml penicillin and
100 mg/ml streptomycin (Sigma, St. Louis, MO, USA). Cells were
maintained at 37°C in a humidified atmosphere containing 95% air
and 5% CO2. Recombinant human decorin was synthesized in
our laboratory (28).
Transient transfection of HepG2 cell
lines
HepG2 cells were divided into three groups: The
pcDNA3.1-DCN group, the pcDNA3.1 group and the untransfected group
(control group). Transient transfection was performed using Lipotap
liposomal reagent according to the manufacturer's instructions
(Beyotime Institute of Biotechnology, Haimen, Jiangsu, China).
Cell viability assay
Cells were seeded in 96-well plates (104
cells per well). The cell proliferation assay was performed in all
three HepG2 groups using the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide]
method (Sigma, 5 mg/ml). After 72 h of transfection, 20 μl (5
mg/ml) of MTT was added to each well for a 4-h incubation at 37°C.
The supernatant was removed and 150 μl DMSO (Sigma) was added
following 10 min of oscillation. The optical density (OD) value was
determined with an ELISA machine (Biotek Synergy HT, Winooski, VT,
USA) at 490 nm and the assays were performed in triplicate. In each
group, the cells were analyzed and the data are presented as the
means ± SD.
Cell cycle detection by flow
cytometry
Cells were plated (3×105 cells per well)
in 6-well plates, treated with pcDNA3.1-DCN, pcDNA3.1 and/or with
neither pcDNA3.1 nor pcDNA3.1-DCN for 72 h and then trypsinized,
washed using 1X buffer A, fixed with 70% ice-cold ethanol and
incubated overnight. RNase A (up to 0.25 mg/ml) was added and the
DNA was labeled with propidium iodide (PI; 5 μl; Becton-Dickinson,
Franklin Lakes, NJ, USA). The cells were analyzed using flow
cytometry (FC500, Beckman Coulter, Miami, FL, USA). The experiment
was performed in triplicate.
Analysis of apoptosis by annexin
V-FITC/PI assay
Analysis was conducted by cultivating cells in
6-well plates (3×105 cells per well) treated with
pcDNA3.1-DCN, pcDNA3.1 and/or with neither pcDNA3.1 nor
pcDNA3.1-DCN for 72 h and then trypsinized. Apoptosis was
determined using the Annexin V-FITC Apoptosis kit
(Becton-Dickinson). The cells were analyzed using flow cytometry in
triplicate. The experiment was performed in triplicate.
RNA extraction
RNA was extracted using TRIzol RNA reagent (Sangon
Biotech Co. Ltd., Shanghai, China) from three groups of HepG2 cells
after 72 h transient transfection according to the manufacturer's
instructions. Briefly, adhering to protocol, cells
(3×105 cells) from each group were washed three times
using 0.1M PBS, trypsinized, and then transferred to an RNase-free
Eppendorf tube and centrifuged at a low speed. The supernatant was
discarded, 0.5 ml of TRIzol reagent was added to lyse the cells and
they were thoroughly mixed. Samples were allowed to sit at room
temperature for 5 min and 0.2 ml of chloroform was added. The
samples were mixed by hand for 15 sec and allowed to stand for 5–10
min at room temperature. The resulting mixture was centrifuged at
12,000 × g for 15 min at 4°C. The uppermost supernatant aqueous
phase was transferred to a fresh RNase-free microcentrifuge tube,
an equal amount of 70% ethanol was added, mixed and then
transferred to a column. RPE solution (0.5 ml) was then added,
incubated at room temperature and centrifuged. The purity and
concentration of the RNA was checked using NanoDrop 1000 (Thermo
Scientific, West Palm Beach, FL, USA) spectrophotometry and the OD
260/280 nm ratio was between 1.85–1.95 for each RNA sample group.
The quality of the total RNA was verified by running an agarose gel
and the total RNA was stored at −70°C.
Complementary DNA (cDNA) synthesis
Total RNA (1 μg) from each HepG2 cell group was
reverse transcribed into complementary DNA (cDNA) using the First
Strand cDNA Synthesis kit (GeneCopoeia, Rockville, MD, USA).
Briefly, 1 μl random primer was added, and ddH2O
(RNase/DNase free) was added up to 13 μl volume and the mixture was
incubated for 10 min at 65°C, cooled and centrifuged. The final
volume of 25 μl was kept at 37°C for 1 h followed by enzyme
deactivation at 85°C for 5 min. The final volume was stored at
−20°C.
Real time-PCR
The relative expression levels of mRNA
p57KIP2 and caspase-3 from each group of cells were
determined by quantitative PCR using the SYBR All-in-One qPCRMix
(GeneCopoeia) with GADPH as a reference (Takara Bio, Inc., Shiga,
Japan). Samples were run in separate tubes on an ABI Prism 7500
according to the manufacturer's instructions. In brief, the 25 μl
samples were treated at 95°C for 10 min followed by 40 cycles of
95°C for 20 sec and 60°C for 30 sec, and a final extension of 5 min
at 72°C. The real time-PCR (RT-PCR) primers were synthesized by
Sangon Biotech Co. Ltd., and the sequences (5′ to 3′) were as
follows: p57KIP2, forward: 5′-CAGAACCGCTGGGATTACGA-3′,
reverse: 5′-CACCGAGTCGCTGTCCACTT-3′ and caspase-3, forward:
5′-GAGTGCTCGCAGCTCATACCT-3′, reverse: 5′-CCTCACGGCCTGGGATTT-3′.
GAPDH was purchased from Takara Bio, Inc., and was used as an
endogenous reference and its primer sequence was as follows:
forward: 5′-TGCACCACCAACTGCTTAGC-3′ and reverse:
5′-GGCATGGACTGTGGTCATGAG-3′. The mRNA expression of
p57KIP2 and caspase-3 was determined from each group of
HepG2 cell cultures and performed in triplicate. Relative
quantitation using the comparative CT method was performed for each
sample group.
Statistical analysis
The Student's t-test was used to identify
statistically significant differences among the samples for cell
proliferation, cell cycle, apoptosis and quantitative PCR assays.
The experiments were performed with three replicates and repeated
three times. P<0.05 was considered to indicate a statistically
significant result.
Results
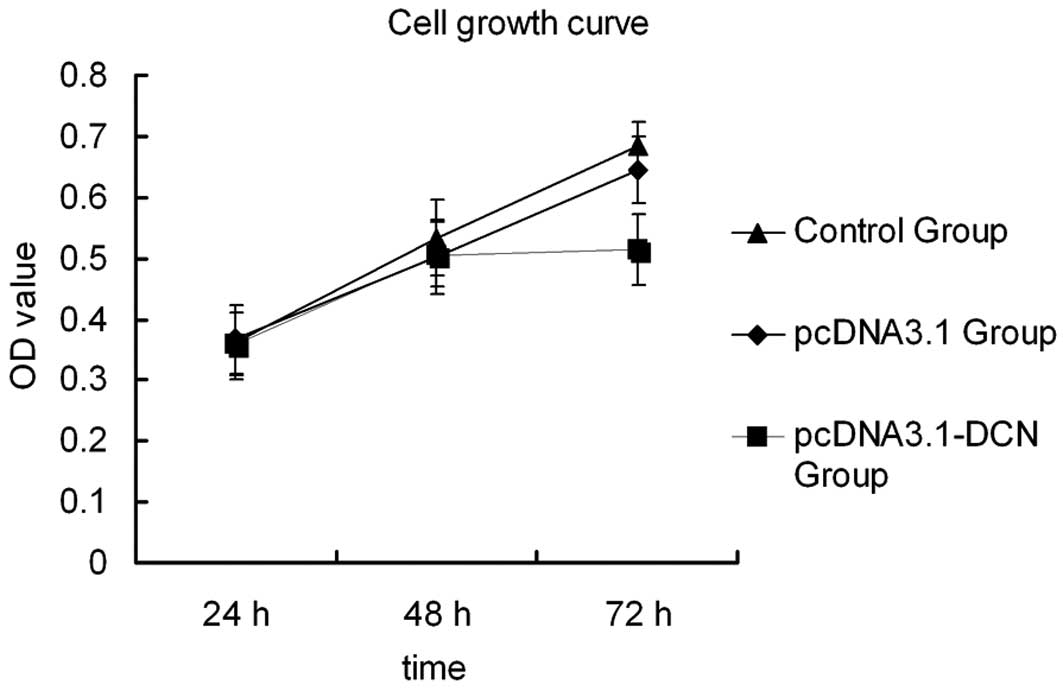
Recombinant human decorin inhibits cell
growth in HepG2 hepatoma cells
To investigate the cell proliferation inhibitory
role of recombinant human decorin, an MTT assay was performed. As
shown in Fig. 1, the control group
cells and pcDNA3.1 group cells exhibited a higher OD after 72 h
transient transfection, whereas in the pcDNA3.1-DCN group, cell
proliferation was markedly inhibited at a statistically significant
level. This result revealed that recombinant human decorin
represses cell growth in HepG2 cells after 72 h of transient
transfection and so all of the following experiments were conducted
after culturing for 72 h.
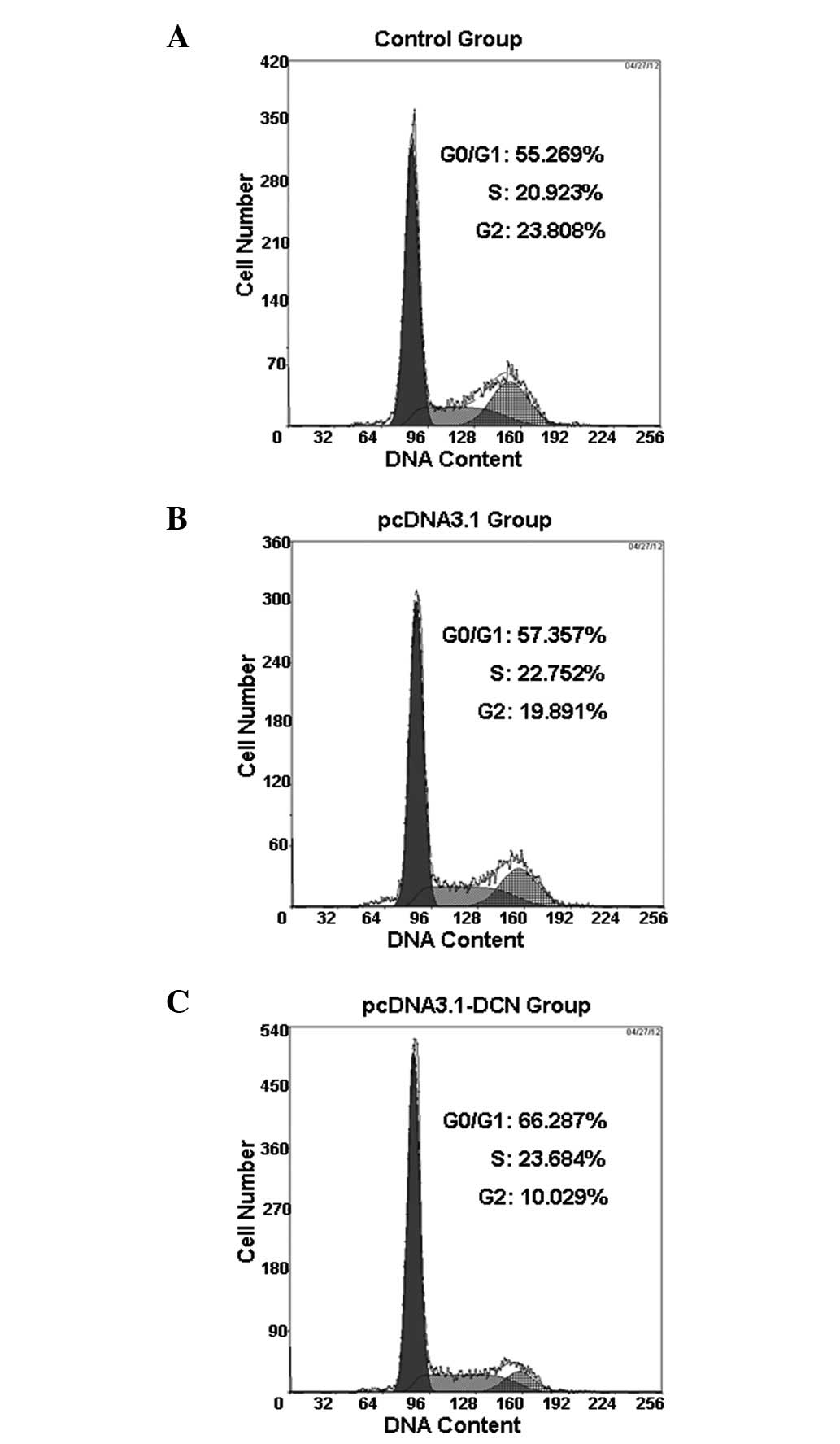
Recombinant human decorin induces
G0/G1 cell cycle arrest in HepG2 cells
Flow cytometry was used to investigate the cell
cycle regulatory role of recombinant human decorin in HepG2 cells.
Our result revealed that recombinant human decorin caused cell
cycle arrest at the G0/G1 phase in HepG2
cells at 72 h following transient transfection (Fig. 2). More HepG2 cell cultures that
were treated with pcDNA3.1-DCN accumulated in the
G0/G1 phase compared with the control and
pcDNA3.1 groups. The percentage of cells in the
G0/G1 phase was 66.126±2.701, 57.116±1.421
and 55.323±1.641% in the pcDNA3.1-DCN, pcDNA3.1 and control groups,
respectively (Table I).
 | Table ICell cycle distribution of
pcDNA3.1-DCN, pcDNA3.1 and control group cells (n=3). |
Table I
Cell cycle distribution of
pcDNA3.1-DCN, pcDNA3.1 and control group cells (n=3).
| Cell cycle
distribution (%) |
|---|
|
|
|---|
| Group |
G0/G1 | S |
G2/M |
|---|
| Control | 55.323±1.641 | 21.045±1.442 | 23.632±1.815 |
| pcDNA3.1 | 57.116±1.421 | 21.284±1.735 | 21.602±1.912 |
| pcDNA3.1-DCN |
66.126±2.701a | 22.108±1.915 |
11.776±1.043a |
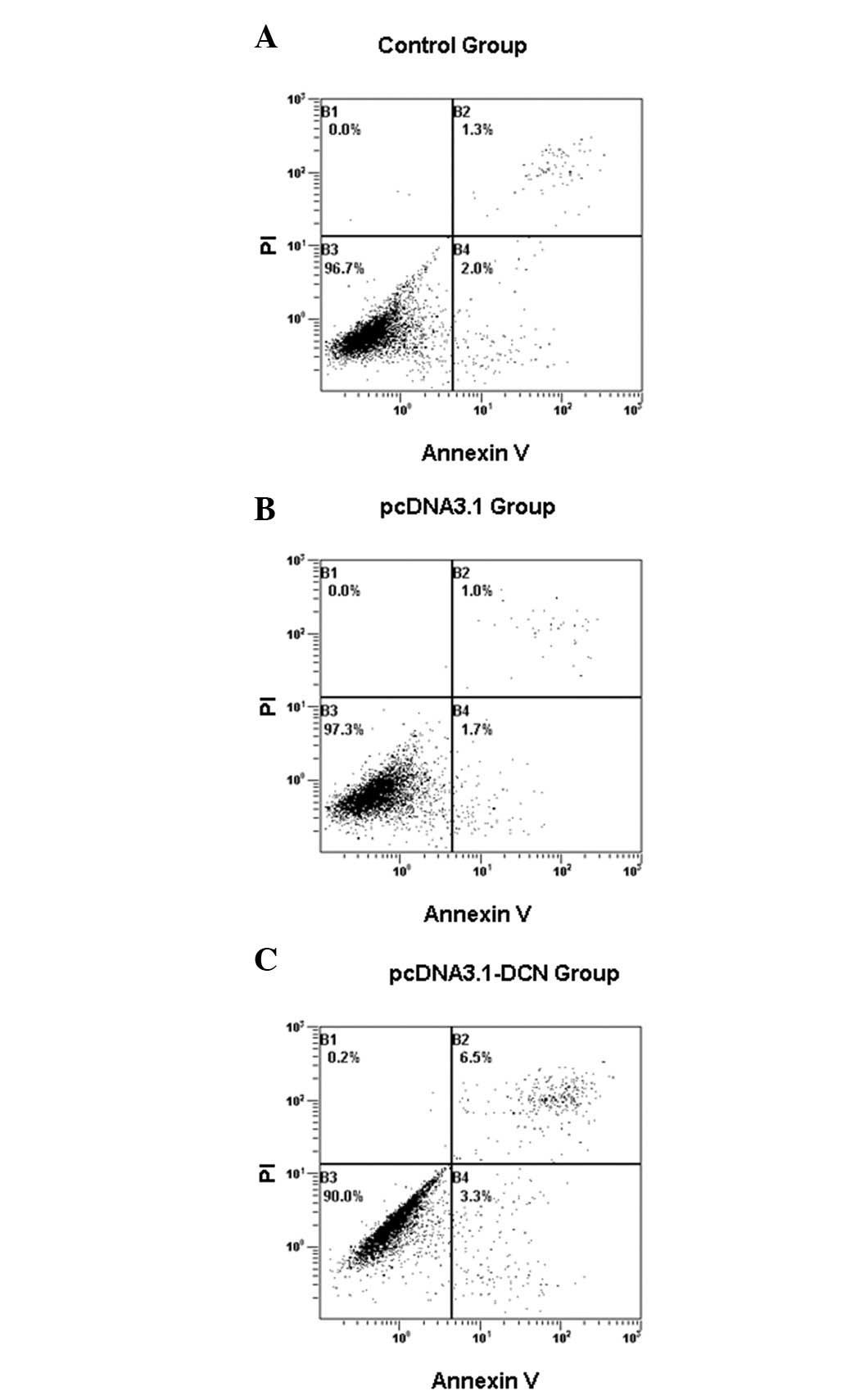
Recombinant human decorin induces
apoptosis in HepG2 cells
We examined the effect of recombinant human decorin
on the induction of apoptosis and, as shown in Fig. 3, recombinant decorin induced
apoptosis. The proportion of cells stained with annexin V and PI
was higher in the pcDNA3.1-DCN group compared with the control and
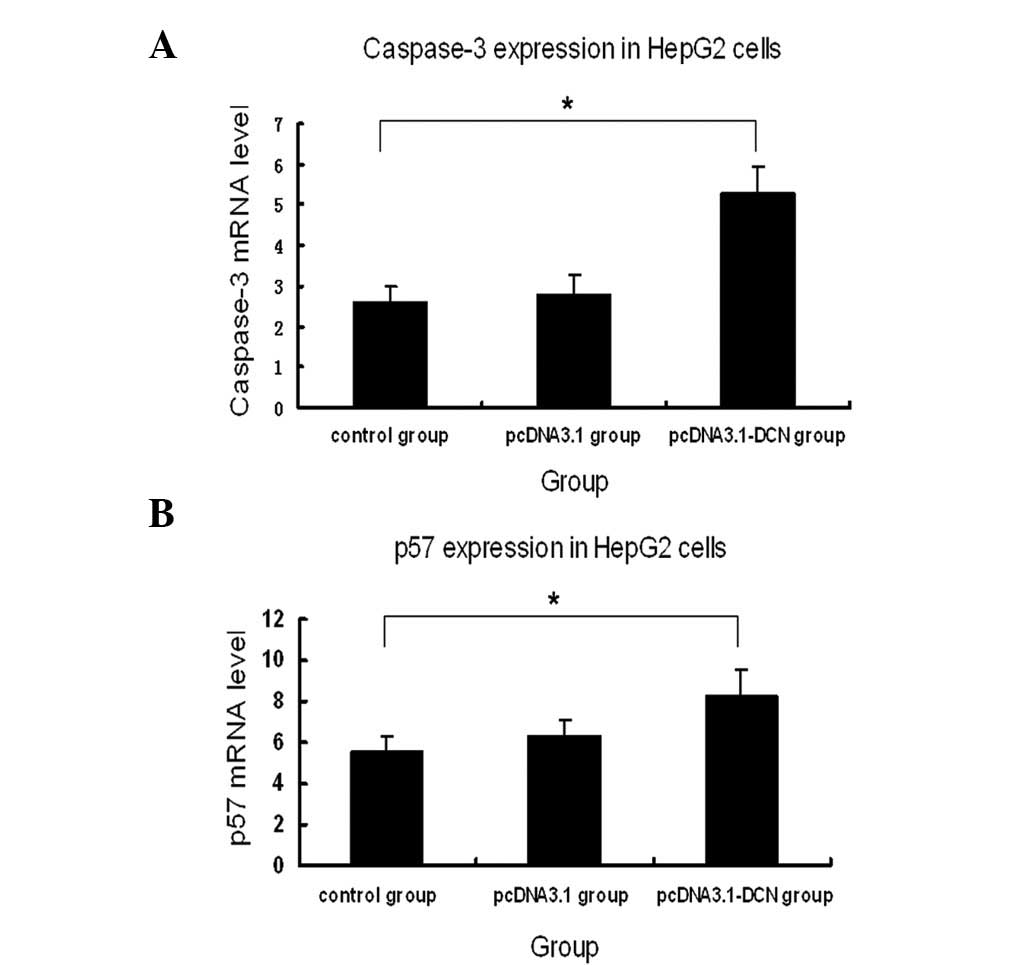
pcDNA3.1 groups (Table II). We
measured caspase-3 expression by quantitative RT-PCR in the three
human HepG2 groups. As illustrated in Fig. 4, caspase-3 expression was markedly
increased in the pcDNA3.1-DCN group compared with the pcDNA-3.1 and
control groups. There was no statistically significant difference
in the expression of caspase-3 between the control group and
pcDNA3.1 group (P>0.05).
 | Table IIRole of recombinant human decorin in
inducing apoptosis in HepG2 group cells (n=3). |
Table II
Role of recombinant human decorin in
inducing apoptosis in HepG2 group cells (n=3).
| Group | Apoptosis (%) |
|---|
| Control | 3.1±0.3 |
| pcDNA3.1 | 2.8±0.2 |
| pcDNA3.1-DCN | 10.2±0.6a |
p57KIP2 expression in the
three HepG2 cell groups
To determine the effect of human recombinant decorin
on the relative expression of p57KIP2 mRNA between each
cell group, quantitative RT-PCR was used. GAPDH was used as an
endogenous reference for normalization. The p57KIP2 mRNA
level was more highly expressed in the pcDNA3.1-DCN group than in
the control and pcDNA3.1 groups.
Discussion
To the best of our knowledge, our findings
demonstrate for the first time that recombinant human decorin
upregulates p57KIP2 mRNA levels in HepG2 cells. By using
quantitative RT-PCR, we examined the expression of
p57KIP2 transcriptional mRNA levels and identified that
expression was higher in recombinant human decorin-treated HepG2
cells compared with the control and pcDNA3.1 groups.
Earlier studies have revealed that decorin, a member
of the family of small leucine-rich proteoglycans, inhibits the
growth of cancer cells. For instance, Hu et al demonstrated
that decorin suppressed prostate cancer cells through the EGFR and
androgen receptor pathways (29).
In addition, a study conducted by De Luca et al revealed
that the inhibitory effect of decorin was correlated with the
overexpression of p21, a CDK inhibitor (30).
Furthermore, previous studies have revealed that the
decorin protein core causes cell death in both in vivo and
in vitro experiments by activating the caspase-3 enzyme
(31). In agreement with this, our
study demonstrated that recombinant human decorin induced apoptosis
via the activation of caspase-3 in the HepG2 cell line. In this
study, the caspase-3 gene was significantly overexpressed in the
recombinant human decorin-transfected HepG2 cells compared with the
control and pcDNA3.1 groups. Caspase-3 is a crucial enzyme for
apoptosis (32). Thus, one of the
tumor suppressive properties of recombinant decorin in HepG2 cells
promotes cell death via activation of the caspase-3 enzyme.
Growing evidence indicates that CDK inhibitors,
including p57KIP2, are important in regulating cell
proliferation and differentiation, cell cycle and cell apoptosis
(19,20). Enhancing the expression of CKIs in
order to suppress the activity of CDK in cancer has become a focus
of cancer therapy research. Thus, reactivating the cyclin-CDK
inhibitors using a small tumor inhibitor molecule, such as
recombinant human decorin, provides an attractive therapeutic
strategy for cancer treatment. We recently demonstrated that
recombinant human decorin represses the growth of HepG2 cells by
upregulating p21 via the p53-independent pathway (28). Ma and Cress demonstrated that
p57KIP2 was significantly upregulated using small
molecule CDK inhibitors, for instance BMS-387032 (SNS-032), in a
breast cancer cell line (33).
These findings led us to examine the role of recombinant decorin in
the reactivation of p57KIP2, a family member of the CDK
inhibitors in HepG2 culture cells. It is well documented that
p57KIP2 is a potential tumor suppressor gene (34). However, the expression of this
multifunctional CDK inhibitor is generally silenced in many types
of cancer. A recent study by Guo et al demonstrated that the
downregulation of p57KIP2 accelerates the growth and
invasion of HCC (27). It has also
been reported that p57KIP2 expression correlates with
the malignant transformation of hepatocytes (35). Furthermore, another recent study
revealed that the decreased expression of decorin and
p57KIP2 correlated with poor survival rates and
lymphatic metastasis in lung cancer patients (36). These studies suggest that
p57KIP2 has a role in tumor inhibition. Notably, besides
the possible function in tumorigenesis, the decreased expression of
p57KIP2 may provide important prognostic implications
for patients with ovarian, hepatocellular and colorectal cancer,
and acute lymphoblastic leukemia (23,37–39).
Previously, it had been reported that the marked loss of
p57KIP2 expression is a frequent event in HCC and so it
may be important in the differentiation of HCC (25). Taken together, these observations
imply that the reactivation of p57KIP2 suppresses the
growth of cancer cells. Thus, restoring the normal function of
p57KIP2 by increasing its expression using small
molecule inhibitors may have therapeutic value. The results from
our study demonstrated an increase in the expression of
p57KIP2 in the pcDNA3.1-DCN HepG2-infected group
compared with the control and pcDNA3.1 groups. Statistical analysis
of the changes in p57KIP2 mRNA levels revealed a
significant induction of p57KIP2 expression by
recombinant decorin in HepG2 culture cells.
In this study, our results demonstrate that
recombinant decorin induces the reactivation of p57KIP2
transcriptional mRNA in the HepG2 cell line. Therefore, it may
suppress cell growth in the HepG2 cell line by upregulating p21 and
also by reactivating p57KIP2, a cyclin-CDK inhibitor
identified in pcDNA3.1-DCN-infected HepG2 cells.
In conclusion, our results demonstrated that
recombinant human decorin increases the expression of
p57KIP2 mRNA in the HepG2 cell line. Since p57 protein
expression is silenced in various types of cancer, its reactivation
may have a therapeutic use in clinical practice, in addition to
other prognostic implications. In the present study, the
statistical analysis of the changes in p57KIP2 mRNA
levels revealed a significant induction of p57KIP2
expression by recombinant decorin in the HepG2 culture cells.
However, for a more detailed understanding, particularly regarding
the mechanism of recombinant decorin in the upregulation of
p57KIP2 in the HepG2 cell line, further studies are
required.
Acknowledgements
This study was supported by a grant from the
Department of Public Health (2009Z080) in the Jilin Province,
China.
References
|
1
|
Colak D, Chishti MA, Al-Bakheet AB, et al:
Integrative and comparative genomics analysis of early
hepatocellular carcinoma differentiated from liver regeneration in
young and old. Mol Cancer. 9:1462010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
3
|
El-Serag HB, Davila JA, Petersen NJ and
McGlynn KA: The continuing increase in the incidence of
hepatocellular carcinoma in the United States: an update. Ann
Intern Med. 139:817–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang MF, Zhang ZY, Fu J, Yang YF and Yun
JP: Correlation between expression of p53, p21/WAF1, and MDM2
proteins and their prognostic significance in primary
hepatocellular carcinoma. J Transl Med. 7:1102009. View Article : Google Scholar
|
|
5
|
Jain S, Singhal S, Lee P and Xu R:
Molecular genetics of hepatocellular neoplasia. Am J Transl Res.
2:105–118. 2010.
|
|
6
|
McGlynn KA and London WT: Epidemiology and
natural history of hepatocellular carcinoma. Best Pract Res Clin
Gastroenterol. 19:3–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherman M: Hepatocellular carcinoma:
epidemiology, risk factors, and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen MH, Whittemore AS, Garcia RT, et
al: Role of ethnicity in risk for hepatocellular carcinoma in
patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol
Hepatol. 2:820–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iozzo RV: Matrix proteoglycans: from
molecular design to cellular function. Annu Rev Biochem.
67:609–652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iozzo RV, Moscatello DK, McQuillan DJ and
Eichstetter I: Decorin is a biological ligand for the epidermal
growth factor receptor. J Biol Chem. 274:4489–4492. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reed CC and Iozzo RV: The role of decorin
in collagen fibrillogenesis and skin homeostasis. Glycoconj J.
19:249–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baghy K, Dezso K, László V, et al:
Ablation of the decorin gene enhances experimental hepatic fibrosis
and impairs hepatic healing in mice. Lab Invest. 91:439–451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu Y, Zhang S, Wu Q, et al: Differential
expression of decorin, EGFR and cyclin D1 during mammary gland
carcinogenesis in TA2 mice with spontaneous breast cancer. J Exp
Clin Cancer Res. 29:62010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moscatello DK, Santra M, Mann DM,
McQuillan DJ, Wong AJ and Iozzo RV: Decorin suppresses tumor cell
growth by activating the epidermal growth factor receptor. J Clin
Invest. 101:406–412. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Csordás G, Santra M, Reed CC, et al:
Sustained down-regulation of the epidermal growth factor receptor
by decorin. A mechanism for controlling tumor growth in vivo. J
Biol Chem. 275:32879–32887. 2000.PubMed/NCBI
|
|
18
|
Bi X, Tong C, Dockendorff A, et al:
Genetic deficiency of decorin causes intestinal tumor formation
through disruption of intestinal cell maturation. Carcinogenesis.
29:1435–1440. 2008. View Article : Google Scholar
|
|
19
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kavanagh E and Joseph B: The hallmarks of
CDKN1C (p57, KIP2) in cancer. Biochim Biophys Acta. 1816:50–56.
2011.PubMed/NCBI
|
|
21
|
Pateras IS, Apostolopoulou K, Koutsami M,
et al: Downregulation of the KIP family members p27(KIP1) and
p57(KIP2) by SKP2 and the role of methylation in p57(KIP2)
inactivation in nonsmall cell lung cancer. Int J Cancer.
119:2546–2556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oya M and Schulz WA: Decreased expression
of p57(KIP2)mRNA in human bladder cancer. Br J Cancer. 83:626–631.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JQ, Wu F, Usuki H, et al: Loss of
p57KIP2 is associated with colorectal carcinogenesis. Int J Oncol.
23:1537–1543. 2003.PubMed/NCBI
|
|
24
|
Larson PS, Schlechter BL, King CL, et al:
CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast
cancer. BMC Cancer. 8:682008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito Y, Takeda T, Sakon M, Tsujimoto M,
Monden M and Matsuura N: Expression of p57/Kip2 protein in
hepatocellular carcinoma. Oncology. 61:221–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Atasoy P, Yilmaz E, Bozdogan O, Ayva S and
Batislam E: Expression profile and prognostic importance in
prostate lesions of the reverse transcriptase component of human
telomerase (hTERT) and of cyclin-dependent kinase inhibitor p57
(p57kip2a). Int Urol Nephrol. 41:55–60. 2009. View Article : Google Scholar
|
|
27
|
Guo H, Lv Y, Tian T, et al: Downregulation
of p57 accelerates the growth and invasion of hepatocellular
carcinoma. Carcinogenesis. 32:1897–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Wang Y, Du Z, et al: Recombinant
human decorin suppresses liver HepG2 carcinoma cells by p21
upregulation. Onco Targets Ther. 5:143–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Sun H, Owens RT, et al: Decorin
suppresses prostate tumor growth through inhibition of epidermal
growth factor and androgen receptor pathways. Neoplasia.
11:1042–1053. 2009.PubMed/NCBI
|
|
30
|
De Luca A, Santra M, Baldi A, Giordano A
and Iozzo RV: Decorin-induced growth suppression is associated with
up-regulation of p21, an inhibitor of cyclin-dependent kinases. J
Biol Chem. 271:18961–18965. 1996.PubMed/NCBI
|
|
31
|
Seidler DG, Goldoni S, Agnew C, et al:
Decorin protein core inhibits in vivo cancer growth and metabolism
by hindering epidermal growth factor receptor function and
triggering apoptosis via caspase-3 activation. J Biol Chem.
281:26408–26418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lakhani SA, Masud A, Kuida K, et al:
Caspases 3 and 7: key mediators of mitochondrial events of
apoptosis. Science. 311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Y and Cress WD: Transcriptional
upregulation of p57 (Kip2) by the cyclin-dependent kinase inhibitor
BMS-387032 is E2F dependent and serves as a negative feedback loop
limiting cytotoxicity. Oncogene. 26:3532–3540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scandura JM, Boccuni P, Massagué J and
Nimer SD: Transforming growth factor beta-induced cell cycle arrest
of human hematopoietic cells requires p57KIP2 up-regulation. Proc
Natl Acad Sci USA. 101:15231–15236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nan KJ, Guo H, Ruan ZP, Jing Z and Liu SX:
Expression of p57(kip2) and its relationship with clinicopathology,
PCNA and p53 in primary hepatocellular carcinoma. World J
Gastroenterol. 11:1237–1240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biaoxue R, Xiguang C, Hua L, et al:
Decreased expression of decorin and p57(KIP2) correlates with poor
survival and lymphatic metastasis in lung cancer patients. Int J
Biol Markers. 26:9–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen L, Toyota M, Kondo Y, et al: Aberrant
DNA methylation of p57KIP2 identifies a cell-cycle regulatory
pathway with prognostic impact in adult acute lymphocytic leukemia.
Blood. 101:4131–4136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakai S, Masaki T, Shiratori Y, et al:
Expression of p57(KIP2) in hepatocellular carcinoma: relationship
between tumor differentiation and patient survival. Int J Oncol.
20:769–775. 2002.PubMed/NCBI
|
|
39
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K and Tokuda M: Expression of p57kip2 and its clinical
relevance in epithelial ovarian tumors. Anticancer Res.
22:3191–3196. 2002.PubMed/NCBI
|