Introduction
Condyloma acuminatum (CA) is caused by human
papillomavirus (HPV) infection and has become one of the most
common sexually transmitted diseases worldwide. Since the early
1980s, the clinical identification of HPV infections have sharply
increased. In addition, relapse rates following the administration
of existing treatments have continued to rise and to date, attempts
to eradicate HPV infection have failed (1). Studies have reported that the relapse
rate of HPV-induced CA has increased to 60–70% (2). Furthermore, recurrent HPV infection
has been reported to be closely associated with cervical, vulvar
and anal carcinomas, and other types of genital tumor (3). Therefore, the control of HPV
infection has become an important issue and of interest to
scientists in the fields of medicine, pharmacology and biology.
Toll-like receptors (TLRs) are one of the most
important types of pathogen recognition receptors in natural
immunity, functioning as bridges in the innate and specific
immunity systems (4). TLRs
selectively recognize pathogen-associated molecular patterns
(PAMPs) carried by pathogenic microorganisms. Recognition of PAMPs
leads to the initiation of signal transduction and induction of the
activation and maturation of macrophages and dendritic cells (DCs),
promoting the secretion of cytokines by these cells which activates
the immune response and the acquired immune system. TLR family
members, TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9, are the main
receptors involved in viral recognition. TLR2 and TLR4 recognize
viral envelope glycoproteins, TLR3 identifies double-stranded RNA
viruses, TLR7/8 largely identifies single-stranded RNA viruses and
TLR9 discerns the CpG DNA sequence in viral chromosome genes
(5). Myeloid differentiation
factor 88 (MyD88), a key adapter molecule in TLR signal
transduction, is responsible for the initiation of downstream
signaling pathways (6). However,
at present, few studies have aimed to determine the role of the
TLR/MyD88 signaling pathway in the resistance of HPV. Therefore,
the significance of TLR/MyD88 in the anti-HPV process must be
investigated.
Previous studies have demonstrated that the
occurrence, remission, relapse and cancerization of CA is
associated with immune imbalance caused by immune hypofunction or
immunoregulatory disorders in patients (7). At present, studies on the infection
process of HPV in humans have indicated that hosts are capable of
generating a certain degree of humoral and cell-mediated immunity;
however, this immunity is far from sufficient to clear the virus.
In addition, immune status studies of local lesions revealed that
HPV-infected patients often suffer from systemic or local immune
dysfunction or defects. Studies have reported that the
downregulation of major histocompatibility complex I and II in
local lesions, alternation of the ratio of CD4+ and
CD8+ T lymphocytes, decrease in expression of tumor
necrosis factor (TNF)-α, GM-CSF, interleukin (IL)-1α and IL-1β,
increased expression of IL-10, the dysfunction and decreased number
of langerhans cells and expression defects of costimulatory
molecules, result in the inhibition of HPV antigen presentation
followed by inhibition of T-lymphocyte activation (8,9).
Therefore, specific immunity may not be induced to clear HPV. The
correlation between HPV infection and hypoergia of the immune
system to viral antigens remains unclear, making it difficult to
investigate the pathological mechanisms and therapeutic strategies
of HPV infection. In recent years, further exploration of the
biological characteristics and functions of regulatory T cells
(Tregs) have revealed that the presence of abnormal Tregs may be
involved in HPV infection. For instance, overexpression of Tregs
may contribute to immunosuppression in local lesions of HPV and
lead to the overexpression of membrane molecules, IL-10 and
transforming growth factor (TGF)-β, without causing abnormal
changes in the number of CD4+ T lymphocytes (10). Therefore, the present study aimed
to investigate the possible mechanisms of immunosuppression in CA
patients by analyzing the expression levels of TLRs, MyD88 and
Treg-related factors and changes in cytokine levels in CA
patients.
Materials and methods
Clinical data
CA wart lesions and blood specimens were obtained
from dermatology outpatients at the Second Affiliated Hospital of
Guangzhou Medical College (Guangdong, China) and were consistent
with diagnostic criteria of CA (11) and confirmed by histopathological
examination. Patients had no medical history of systemic antiviral
drugs or immunomodulators two weeks prior to treatment. Patients
with autoimmune, severe systemic or other infectious diseases were
excluded from the study. Subjects included 26 males and 18 females
with an average age of 33.9±11.0 (20–56) years and average disease
duration of 97.24±152.1 (4–320) days, among which 28 were newly
diagnosed and 16 cases were relapsed. Lesions of 40 specimens were
obtained from male foreskin, corona of glans penis, perianal
region, female pudendum and perianal region, and stored in liquid
nitrogen. In addition, 5 ml venous blood was sampled routinely and
stored at −20°C following isolation of mononuclear cells. Among 40
specimens of the control group, 20 specimens were normal foreskin
from circumcision procedures performed in our hospital (The Second
Affiliated Hospital of Guangzhou Medical University) and another 20
were blood samples obtained from healthy subjects. The gender and
age differences between the groups were of no statistical
significance. This study was approved by the hospital ethics
committee and informed consent was obtained from all patients.
Fluorescence quantitative PCR
Wart tissue was ground into a fine powder in liquid
nitrogen and peripheral blood mononuclear cells were separated by
lymphocyte separation medium. Total RNA was extracted using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RNA integrity was detected by
electrophoresis and quantitative determination was performed,
followed by reverse transcription using the One Step SYBR
PrimeScript RT-PCR kit (Takara Bio, Inc., Shiga, Japan). The
reaction was performed using the ABI PRISM 7500 Real-Time PCR
System (Life Technologies, China) at 42°C for 5 min, 95°C for 10
sec; then 40 cycles of 95°C for 5 sec, 55°C for 30 sec and 72°C for
30 sec. The primers used for quantitative determination are
presented in Table I, among which
TLR primer sequences were obtained from Daud et al(12). Each specimen was analyzed in
triplicate and quantification was derived using the
2−ΔΔCt method.
 | Table IPrimers for quantitative PCR. |
Table I
Primers for quantitative PCR.
| Primer name | Direction | Primer sequence
(5′-3′) | Primer size (bp) | GenBank accession
number |
|---|
| MyD88 | F |
CAGAGCAAGGAATGTGACTTC | 140 | NM_001172567 |
| R |
TCGCAGACAGTGATGAACC | | |
| TLR2 | F |
AACCGGAGAGACTTTGCTCA | 91 | NM_003264 |
| R |
CCACTGACAAGTTTCAGGCA | | |
| TLR3 | F |
CCTGGTTTGTTAATTGGATTAACGA | 82 | NM_003265 |
| R |
TGAGGTGGAGTGTTGCAAAGG | | |
| TLR4 | F |
GGACTGGGTAAGGAATGAGCTAGTA | 94 | NM_138554 |
| R |
CACACCGGGAATAAAGTCTCTGT | | |
| TLR7 | F |
AAGCCCTTTCAGAAGTCCAAGTT | 91 | NM_016562 |
| R |
GGTGAGCTTGCGGGTTTGT | | |
| TLR8 | F |
GCTGCTGCAAGTTACGGAAT | 118 | NM_016610 |
| R |
CGCATAACTCACAGGAACCA | | |
| TLR9 | F |
TGAAGACTTCAGGCCCAACTG | 75 | NM_017442 |
| R |
TGCACGGTCACCAGGTTGT | | |
| FOXP3 | F |
GAAGCAGCGGACACTCAATG | 106 | NM_014009 |
| R |
ACTCAGGTTGTGGCGGATG | | |
| TGFB1 | F |
CTGAACCCGTGTTGCTCTC | 112 | NM_000660 |
| R |
AGGTATCGCCAGGAATTGTTG | | |
| IL-10 | F |
AACCAAGACCCAGACATC | 135 | NM_000572 |
| R |
ATTCTTCACCTGCTCCAC | | |
| CTLA4 | F |
TTCTCTTCATCCCTGTCTTCTG | 127 | NM_005214 |
| R |
CGGACCTCAGTGGCTTTG | | |
| GITR | F |
AATTCCACTGCGGAGACC | 120 | NM_004195 |
| R |
CCGAGGCACAGTCGATAC | | |
| PD-1 | F |
CCAGGATGGTTCTTAGACTC | 128 | NM_005018 |
| R |
AAGCTCTCCGATGTGTTG | | |
| NKG2D | F |
TCCCTCTCTGAGCAGGAATCC | 107 | NM_007360 |
| R |
AGACCTCCGACCACGAATCC | | |
| NKp46 | F |
CCGAGGGACATACCGATG | 106 | NM_004829 |
| R |
AAGGCTGGTGTTCTCAATG | | |
| GAPDH | F |
GGTATCGTGGAAGGACTC | 128 | NM_002046 |
| R |
GTAGAGGCAGGGATGATG | | |
Western blot analysis
Total cellular proteins were extracted by incubating
100 mg ground tissue specimens in lysis buffer. Protein
concentrations in the lysates were determined by Quick Start
Bradford Protein assay (Bio-Rad, Hercules, CA, USA). SDS-PAGE was
performed in 12% glycine gels (Bio-Rad), loading equal amounts of
proteins per lane. Following electrophoresis, separated proteins
were transferred onto a PVDF membrane and blocked with 5% non-fat
milk. Next, membranes were incubated with antibodies against MyD88
(1:500), TLR2 (1:400), TLR3 (1:500), TLR4 (1:500), TLR7 (1:200)
TLR8 (1:500), TLR9 (1:250), forkhead box P3 (FOXP3; 1:250), TGFβ1
(1: 500), IL-10 (1:800), cytotoxic T-lymphocyte antigen 4 (CTLA4;
1:1,000), GITR (1:200), programmed cell death protein 1 (PD1;
1:50), NKG2D (1:200), NCR1 (1:200) and GAPDH (1:1,000; all
antibodies were purchased from Abcam, Cambridge, UK) in 5% non-fat
milk overnight at 4°C and then goat anti-rabbit IgG monoclonal
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
conjugated with horseradish peroxidase. Protein bands were detected
using the West Femto system (Pierce Biotechnology, Inc., Rockford,
IL, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Peripheral blood mononuclear cells were separated by
lymphocyte separation medium. IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α
and interferon (IFN)-γ levels were detected according to the
manufacturer’s instructions by ELISA (Insight Genomics, Falls
Church, VA, USA).
Statistical analysis
Experiments were performed at least in triplicate
and repeated three times. All data are expressed as the mean ± SD.
Statistical analysis was performed using SPSS statistical package
(SPSS 13.0 for Windows; SPSS, Inc., Chicago, IL, USA). The
difference between two groups was analyzed by two-tailed Student’s
t-test. The difference between three or more groups was analyzed by
one-way analysis of variance multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
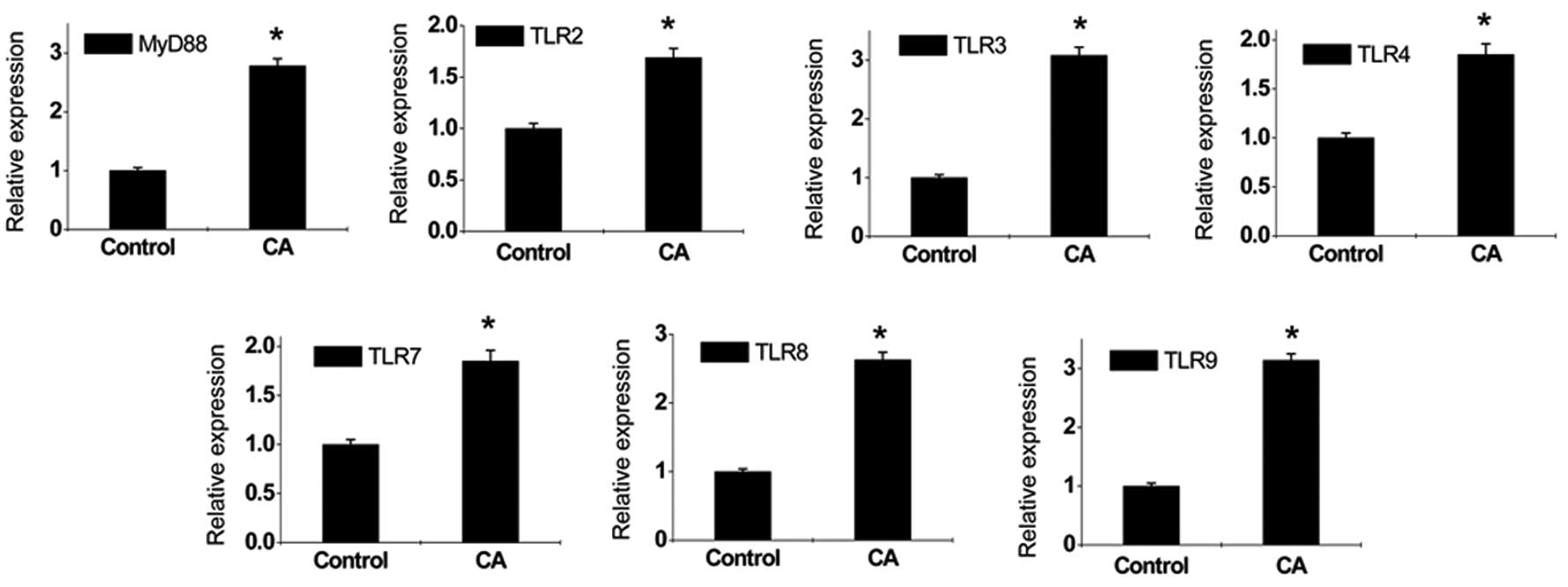
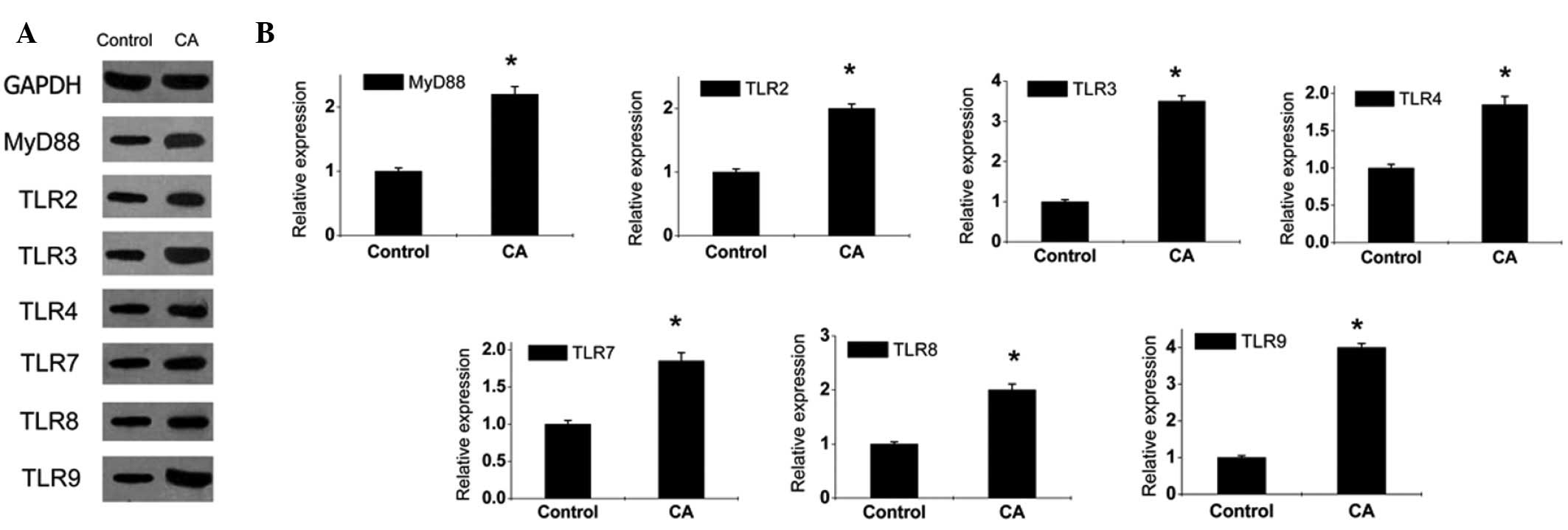
Expression of MyD88 and TLRs increases in
the lesion tissues of CA patients
Changes in the expression levels of MyD88 and TLRs
were detected by quantitative PCR and western blot analysis. The
results revealed that the expression of MyD88 and TLRs increased
markedly in the lesion tissues of CA patients compared with those
of normal specimens (Figs. 1 and
2); however, changes in peripheral
blood were not detected, indicating that HPV infection activated
the local immune system only and the immune response was confined
to viral infection sites. We have previously showed that, by
comparing with normal epidermis, stratum spinosum cells in CA
lesions were significantly thickened and MyD88 expression was
increased (13). In addition,
MyD88 was expressed in the whole epidermal layer, and particularly
overexpressed in the basal layer (13). Increased expression of MyD88 and
TLRs contributed to enhanced downstream signaling effects and
cellular immune function. However, the current study demonstrated
that, in addition to expression in effector cells of the innate
immune system which mediates the anti-pathogen reaction, TLRs are
also expressed in T- and B-lymphocytes of the adaptive immune
system. CD4+ and CD25+ Tregs mainly exerted
negative regulatory roles in the immune response to inhibit the
proliferation and activation of effector cells (18). TLR1-9 were expressed in Tregs at
different degrees. Therefore, it is likely that increased
expression of TLRs enhances Treg function leading to
immunosuppression.
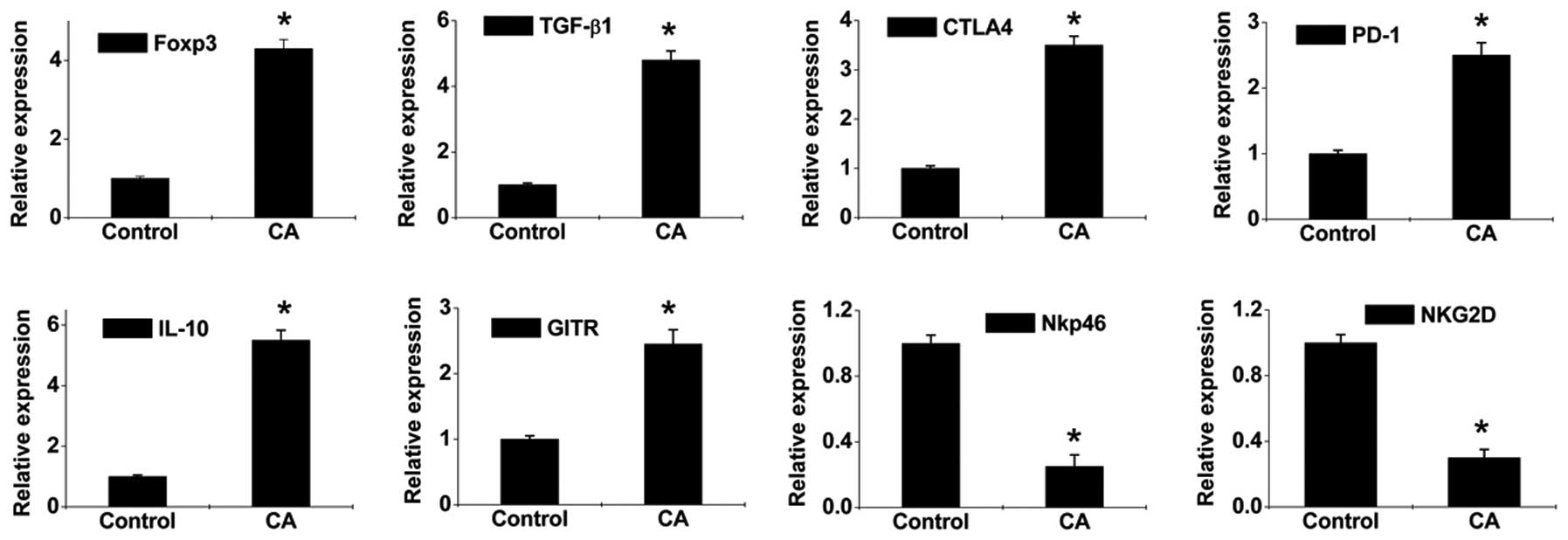
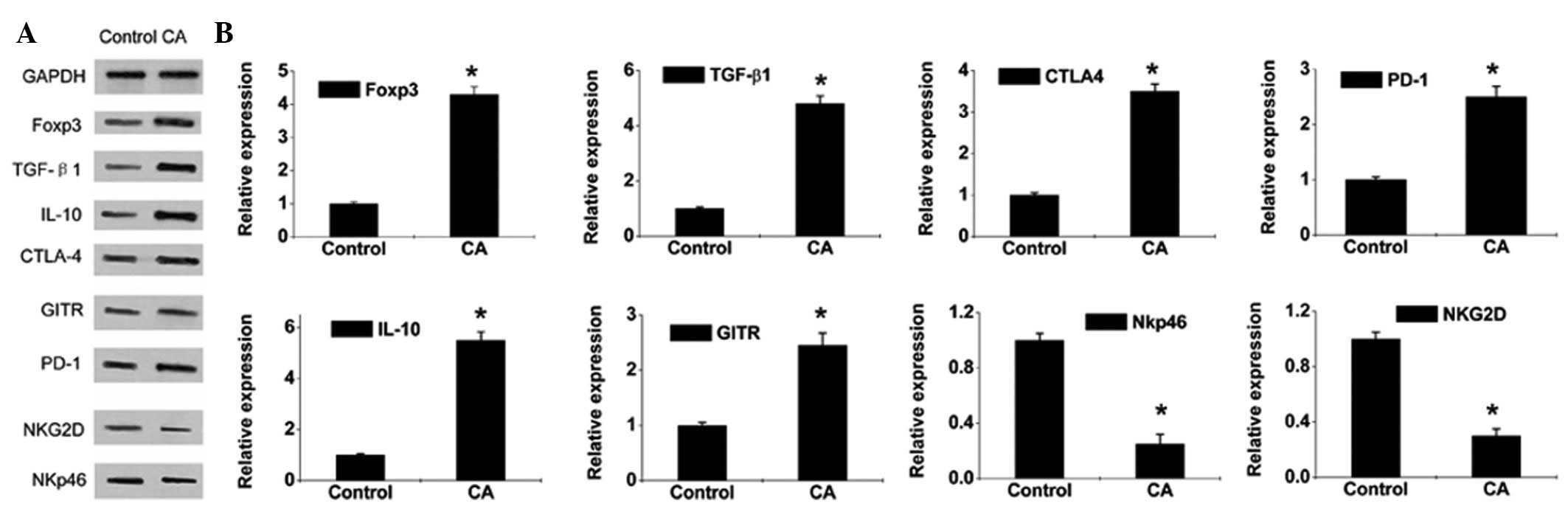
Expression levels of Treg-related factor,
Foxp3, NKG2D and NKp46 in CA lesion tissues
Results of quantitative PCR and western blot
analysis demonstrated that the expression of Foxp3, a
characteristic marker of Tregs and several inhibitory costimulatory
molecules, including CTLA-4, glucocorticoid-induced TNFR-related
protein (GITR) and PD-1, was markedly increased in the lesion
tissues of CA patients (Figs. 3
and 4) compared with controls,
indicative of a significantly enhanced immunosuppressive function
in Tregs. In addition,
Foxp3+CD4+CD25+ Tregs largely
mediated suppression via direct interaction between cells and
secretion of the inhibitory factors, TGF-β1 and IL-10. In addition,
results of quantitative PCR and western blot analysis revealed a
marked increase in TGF-β1 and IL-10 levels (Figs. 3 and 4). The expression of NKG2D and NKp46,
which activate specific natural killer (NK) cell surface receptors,
decreased markedly, indicating that HPV infection downregulates the
expression of activated receptors of NK cells and suppresses
activation of NK cells.
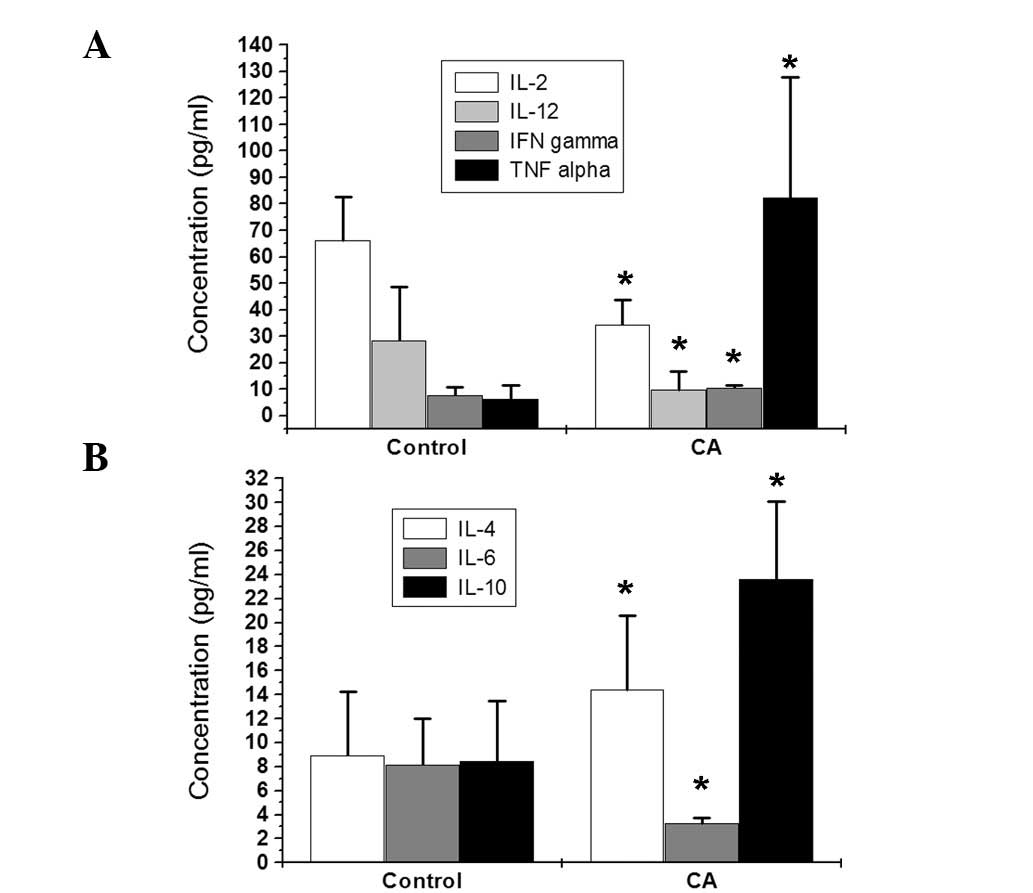
CA patients exhibit an imbalance of
Th1/Th2, Tc1/Tc2 and secreted cytokines
Helper T lymphocyte subsets (Th1/Th2) are important
for anti-HPV immunity, since the Th1 response, which is involved in
cellular immunity, is the main immune response following HPV
infection (14). CD8+ T
cells, known as cytotoxic T cells, are vital for the antiviral
cellular immune response which directly kills the target cells
infected by the virus. Similar to CD4+ helper T cells,
Tc subsets of CD8+ T cells are divided into Tc1 and Tc2
subsets. Tc1 and Th1 secrete IFN-γ, IL-2, IL-12 and several other
cytokines which mediate cellular immunity. Similarly, Tc2 and Th2
secrete IL-4, IL-5, IL-6, IL-10 and several other cytokines which
mediate humoral immunity. In the present study, ELISA was performed
to detect the secretion levels of cytokines in the peripheral blood
of CA patients. IL-2, IL-12 and IFN-γ levels were markedly lower
than those of control subjects; however, TNF-α levels were
increased. In addition, levels of IL-4 and IL-10 were increased,
and IL-6 was decreased compared with control patients (Fig. 5). Decreased levels of Th1-type
cytokines and increased Th2-type cytokines are indicative of
cellular immune suppression in CA patients.
Discussion
In recent years, the morbidity of CA has risen and
the clinical relapse rate has increased. In addition, several types
of HPV infection have been closely associated with malignant cancer
of the cervix, vulva and vagina, as well as precancerous atypical
hyperplasia (15). Increasing
public and clinical concern has meant that the control and
understanding of HPV infection has become an important issue. A
number of previous studies have demonstrated that increased
expression of MyD88 and TLRs is associated with HPV infection,
which may activate MyD88-dependent signaling pathways in the lesion
tissues of CA patients. Upregulation of MyD88 and TLRs has been
identified to contribute to enhanced downstream signal transduction
and cellular immune function, which promote the recognition and
clearance of the virus by the body. Through RT-PCR and
immunohistochemistry, Ku et al(16) found that the expression of TLR3 and
TLR9 in verruca plana and molluscum contagiosum
lesions was enhanced compared with healthy epidermis. Yang et
al(17) found that HPV
activated TLR9 via the MyD88 pathway to stimulate the generation of
the Th1-type immune response by DCs, which was crucial to
anti-HPV16 infection. In addition, Daud et al(12) reported that clearance of HPV16
infections was significantly associated with increased expression
of the four viral nucleic acid-sensing TLRs (TLR3, TLR7, TLR8 and
TLR9), as well as TLR2, upon viral acquisition. Based on these
observations, the authors concluded that, reduced TLR expression in
the cervical mucosa was caused by a type-specific mechanism in
which HPV16 interfered with the innate immune response and
contributed to viral persistence. In addition, the upregulation of
TLR was involved in subsequent viral clearance. Therefore, the
MyD88-dependent signaling pathway is vital for resistance to HPV
infection.
TLRs additionally directly modulate the adaptive
immune response. The activation of Tregs, a T-cell subset,
effectively inhibits the immune response of CD4+ and
CD8+ T effector cells, exerting negative
immunomodulatory effects (18).
Expression levels of TLRs in human and rat
CD4+CD25+ Tregs are higher than that of
CD4+ T cells. In the present study, specific TLRs,
including TLR2, TLR8 and TLR9, were observed to modulate the
function of CD4+CD25+ Tregs and eliminate or
reverse the immune suppression effect of
CD4+CD25+ Tregs, while TLR2, TLR4 and TLR5
mediated the opposite effect (19). Thus, in addition to activation of
antigen-presenting cells and costimulation of effector T cells,
TLRs affect the proliferation and function of Tregs (20), which may represent one of the
mechanisms of immune suppression in CA patients. Examination of the
expression of MyD88 and TLRs in peripheral blood samples of CA
patients revealed no significant difference in healthy subjects,
indicating that HPV infection is unable to activate the systemic
immune response.
Foxp3, a specific marker of Tregs, is essential for
CD4+CD25+Treg differentiation and development
in the thymus (21). Foxp3
expression in peripheral T cells is required for preventing
autoimmunity and possibly for TR cell maintenance. Foxp3 determines
the inhibitory functions of Tregs and this function has been
hypothesized to be mediated at the level of translation (22). To regulate the cell-mediated immune
protective effect and form sustained infection, Tregs suppress the
activation and proliferation of T cells by inhibiting transcription
and expression of IL-2 in CD4+ and CD8+
cells, regulating the intensity levels of the antiviral immune
response by controlling the differentiation of Th1 and Th2 cells
induced by DCs. The mode of suppressive action of Tregs on immune
cells is divided into two categories, cytokine-secreting and cell
contact inhibition, both of which mainly target effector T cells.
Cytokine-secreting inhibition includes TGF-β1 and IL-10 signaling
pathway-mediated immunosuppression, while cell contact inhibition
is primarily mediated by CTLA-4 (23). Results of quantitative PCR and
western blot analysis demonstrated a marked increase in the
expression of Foxp3, TGF-β1, IL-10, CTLA4, GITR and PD-1,
indicative of an enhanced function of Tregs. In addition, TGF-β1
induces Foxp3 expression and transforms initial peripheral
CD4+CD25- T cells into
CD4+CD25+ Tregs, maintaining the number and
function of CD4+ CD25+ T cells (24). The expression levels of CTLA-4,
GITR and PD-l serve as negative co stimulatory molecules under high
expression levels of transduction inhibition signals. Although
without specificity, the change in the expression levels of CTLA-4,
GITR and PD-l may directly or indirectly reflect the function of
Tregs. Several studies have demonstrated that the number of Tregs
increase in the peripheral blood of persistently HPV16-infected
patients and CIN3 level patients (25). Similarly, Foxp3+ Tregs
in the peripheral blood of CA patients are enhanced compared with
healthy subjects and this observation is particularly evident in
relapsed patients (14).
Simultaneously, expression levels of NKG2D and NKp46, which
function as activated receptors on the surface of NK cells, reduce
markedly, revealing that reduced NK cell activity results in a
reduced ability to clear viral infections in vivo.
Sun et al(26) reported that TLR-mediated activation
of DCs may transmit negative regulatory signals to inhibit the Th2
response in a MyD88-dependent manner. By stimulating marrow-derived
DCs from wild-type and MyD88-deficient mice with LPS and
co-culturing with CD4+ T cells, Kaisho et
al(27) found that wild-type
DCs promoted the secretion of IFN-γ and IL-12; however, inhibited
the secretion of IL-4 by CD4+ T cells following
stimulation with LPS. By contrast, MyD88-deficient DCs did not
induce secretion of IFN-γ and IL-12 by CD4+ T cells, but
significantly promoted the secretion of IL-4. Therefore, increased
expression of MyD88 and TLRs was expected to promote the generation
of Th1-type cytokines; however, in the present study, opposite
results were obtained. Levels of secreted cytokines were detected
by ELISA in peripheral blood samples obtained from CA patients.
Levels of Th1-type cytokines, IL-2, IL-12 and IFN-γ, were markedly
reduced compared with healthy subjects; however, TNF-α levels were
increased. In addition, Th2-type cytokines, IL-4 and IL-10, were
increased in CA patients compared with the control, and IL-6 levels
were decreased. The overall decrease in Th1-type cytokines and
increase in Th2-type cytokines is indicative of cellular immune
suppression in CA patients. Bais et al(28) hypothesized that disequilibrium of
Th1/Th2 in HPV-infected individuals may affect correct activation
of Langerhans cells, leading to activation failure and subsequent
dysfunction of Tc cellular immunity. Cao et al(10) found that the immunosuppressive
environment in large warts was characterized by high expression of
IL-10 and TGF-β1, and low expression of IL-2 and IFN-γ. Similarly,
Xu et al(14) reported that
patients with CA were observed to exhibit a decreased proportion of
Th1 and Tc1 cells, and a decreased ratio of Th1/Th2 and Tc1/Tc2.
Consequently, the switch of the Th1-type immune response towards a
Th2 type may represent a mechanism by which HPV evades the immune
response.
In summary, enhanced expression of TLRs and MyD88 in
CA tissues may promote the immune suppressive function of Tregs,
leading to immunosuppression and sustained HPV infection.
References
|
1
|
O’Mahony C: Genital warts: current and
future management options. Am J Clin Dermatol. 6:239–243.
2005.PubMed/NCBI
|
|
2
|
McMurray HR, Nguyen D, Westbrook TF and
McAnce DJ: Biology of human papillomaviruses. Int J Exp Pathol.
82:15–33. 2001. View Article : Google Scholar
|
|
3
|
Partridge JM and Koutsky LA: Genital human
papillomavirus infection in men. Lancet Infect Dis. 6:21–31. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar
|
|
5
|
Xagorari A and Chlichlia K: Toll-like
receptors and viruses: induction of innate antiviral immune
responses. Open Microbiol J. 2:49–59. 2008.PubMed/NCBI
|
|
6
|
Sin JI: MyD88 signal is required for more
efficient induction of Ag-specific adaptive immune responses and
antitumor resistance in a human papillomavirus E7 DNA vaccine
model. Vaccine. 29:4125–4131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmed AM, Madkan V and Tyring SK: Human
papillomaviruses and genital disease. Dermatol Clin. 24:157–165.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narayan S, Choyce A, Linedale R, et al:
Epithelial expression of human papillomavirus type 16 E7 protein
results in peripheral CD8 T-cell suppression mediated by
CD4+CD25+ T cells. Eur J Immunol. 39:481–490.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu XS, Leerberg J, MacDonald K, Leggatt
GR and Frazer IH: IFN-gamma promotes generation of IL-10 secreting
CD4+T cells that suppress generation of CD8 responses in
an antigen-experienced host. J Immunol. 183:51–58. 2009.PubMed/NCBI
|
|
10
|
Cao Y, Zhao J, Lei Z, et al: Local
accumulation of FOXP3+regulatory T cells: evidence for
an immune evasion mechanism in patients with large condylomata
acuminata. J Immunol. 180:7681–7686. 2008.PubMed/NCBI
|
|
11
|
Del Mistro A, Koss LG, Braunstein J,
Bennett B, Saccomano G and Simons KM: Condylomata acuminata of the
urinary bladder. Natural history, viral typing and DNA content. Am
J Surg Pathol. 12:205–215. 1988.PubMed/NCBI
|
|
12
|
Daud II, Scott ME, Ma Y, Shiboski S,
Farhat S and Moscicki AB: Association between toll-like receptor
expression and human papillomavirus type 16 persistence. Int J
Cancer. 128:879–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi YJ, Xiong F, Yang J, et al: The
expression and significance of MyD88 in condyloma acuminatum. Zhong
Guo PiFu Xing Bing Xue Za Zhi. 24:626–628. 2010.(In Chinese).
|
|
14
|
Xu Y, Zhu KJ, Zhu N, Jiang DH, Chen XZ and
Cheng H: Expression of
Foxp3+CD4+CD25+ regulatory T cells
and Th1/Th2, Tc1/Tc2 profiles in the peripheral blood of patients
with condyloma acuminatum. Clin Exp Dermatol. 34:229–235. 2009.
|
|
15
|
Villa LL, Costa RL, Petta CA, et al: High
sustained efficacy of a prophylactic quadrivalent human
papillomavirus types 6/11/16/18 L1 virus-like particle vaccine
through 5 years of follow-up. Br J Cancer. 95:1459–1466.
2006.PubMed/NCBI
|
|
16
|
Ku JK, Kwon HJ, Kim MY, et al: Expression
of Toll-like receptors in verruca and molluscum contagiosum. J
Korean Med Sci. 23:307–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang R, Murillo FM, Cui H, et al:
Papillomavirus-like particles stimulate murine bone marrow-derived
dendritic cells to produce alpha interferon and Th1 immune
responses via MyD88. J Virol. 78:11152–11160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochs HD, Gambineri E and Torgerson TR:
IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity.
Immunol Res. 38:112–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Maren WW, Jacobs JF, de Vries IJ,
Nierkens S and Adema GJ: Toll-like receptor signalling on Tregs: to
suppress or not to suppress? Immunology. 124:445–452.
2008.PubMed/NCBI
|
|
20
|
Caramalho I, Lopes-Carvalho T, Ostler D,
et al: Regulatory T cells selectively express toll-like receptors
and are activated by lipopolysaccharide. J Exp Med. 197:403–411.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khattri R, Cox T, Yasayko SA and Ramsdell
F: An essential role for Scurfin in CD4+CD25+ T regulatory cells.
Nat Immunol. 4:337–342. 2003.PubMed/NCBI
|
|
22
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakaguchi S, Wing K, Onishi Y,
Prieto-Martin P and Yamaguchi T: Regulatory T cells: how do they
suppress immune responses? Int Immunol. 21:1105–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huber S, Schramm C, Lehr HA, et al:
Cutting edge: TGF-beta signaling is required for the in vivo
expansion and immunosuppressive capacity of regulatory
CD4+CD25+ T cells. J Immunol. 173:6526–6531.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Molling JW, de Gruijl TD, Glim J, et al:
CD4(+)CD25hi regulatory T-cell frequency correlates with
persistence of human papillomavirus type 16 and T helper cell
responses in patients with cervical intraepithelial neoplasia. Int
J Cancer. 121:1749–1755. 2007.
|
|
26
|
Sun J, Walsh M, Villarino AV, et al: TLR
ligands can activate dendritic cells to provide a MyD88-dependent
negative signal for Th2 cell development. J Immunol. 174:742–751.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaisho T, Hoshino K, Iwabe T, Takeuchi O,
Yasui T and Akira S: Endotoxin can induce MyD88-deficient dendritic
cells to support T(h)2 cell differentiation. Int Immunol.
14:695–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bais AG, Beckmann I, Lindemans J, et al: A
shift to a peripheral Th2-type cytokine pattern during the
carcinogenesis of cervical cancer becomes manifest in CIN III
lesions. J Clin Pathol. 58:1096–1100. 2005. View Article : Google Scholar : PubMed/NCBI
|