Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most common liver disease worldwide, ranging from simple steatosis
to cirrhosis and hepatocellular carcinoma (1). Hepatic steatosis [mainly
triglycerides (TGs)] is believed to contribute to liver
inflammation and nonalcoholic steatohepatitis progression (2). Therefore, it is important to identify
factors that regulate hepatic lipid metabolism involved in the
hepatic steatosis.
It has been reported that insulin resistance (IR) is
a primary factor for the development of NAFLD (3). IR is associated with increased
lipolysis and the reduced utilisation of free fatty acids (FFA) in
adipose tissues, which leads to an elevated influx of FFA to the
liver (4). Fatty acids are
cytotoxic, and there are two metabolic methods for the influx of
FFAs, one is by reesterification to TGs and the second is to
participate in β-oxidation. These two methods are important in
protecting the liver against the lipotoxicity of FFAs. Thus, if
hepatic import and the synthesis of TGs exceeds hepatic TG
oxidation and export, TGs accumulate in hepatocytes (5,6)
Therefore, a reduction in IR, the inhibition of FFA liberation, the
promotion of FFA β-oxidation and export as very low density
lipoproteins (VLDLs) may be effective strategies to decrease liver
TG deposits.
A growing body of evidence indicates that peroxisome
proliferator activated receptor-α (PPARα) is important in the
catabolism of fatty acids. PPARα participates in numerous aspects
of lipid metabolism, including fatty acid uptake through membranes,
fatty acid binding in cells, fatty acid oxidation and lipoprotein
assembly and transport (7), by
regulating the expression of pivotal enzymes, such as carnitine
palmitoyltransferase 1A (CPT1A) and microsomal triglyceride
transfer protein (MTTP) (8). PPARα
agonists, such as fibrates, have been shown to be effective in the
treatment of obesity and IR (9),
and have been used to correct hypertriglyceridaemia and NAFLD in
rats (10); thus, we used
fenofibrate (FF) as a positive control.
Oxymatrine (OMT) is one of the major components
extracted from Sophora flavescens Ait. (kushen), a
traditional Chinese medicine, and has been reported to possess
various pharmacological effects, including anti-hepatitis virus
infection, anti-hepatic fibrosis, anti-inflammation and
anti-anaphylaxis activities and immune regulation (11–13).
However, little is known about the effect of OMT on lipid
metabolism, with the exception of a recent study, which reported
that the oral administration of OMT for one week eliminated hepatic
steatosis in fatty liver mice (14). New studies regarding matrine,
another quinolizidine alkaloid isolated from Sophora
flavescens Ait. (kushen) that has a chemical structure and
pharmacological effects similar to those of OMT (Fig. 1), have demonstrated its beneficial
effects on serum lipids and hepatic steatosis in
vivo(15,16). Additionally, matrine treatment
decreases fat accumulation in 3T3-L1 preadipocytes in
vitro(17). Based on the
aforementioned studies, we hypothesise that OMT may have similar
beneficial effects on the abnormal lipid metabolism in the
high-fructose diet (HFD)-induced NAFLD rats.
The main aim of the present study was to investigate
the effects of OMT on serum lipids, insulin sensitivity and hepatic
steatosis in NAFLD rats. An additional goal was to explore whether
these effects were associated with PPARα activation.
Materials and methods
Drugs and reagents
OMT was obtained from Chia-Tai Tianqing Pharmacy
Co., Ltd. (Jiangsu, China). FF (Lipanthyl; Laboratories Fournier,
Diax, France) was purchased from The Third Hospital of Shijiazhuang
City affiliated with Hebei Medical University (Shijiazhuang,
China). The drugs were suspended in a 0.5% sodium carboxymethyl
cellulose solution, respectively. Crystallised fructose was
purchased from Hebei Huaxu Pharmaceutical Co., Ltd. (Shijiazhuang,
China). Rabbit anti-rat PPARα (ab8934) polyclonal antibody was
purchased from Abcam Inc. (Cambridge, MA, USA). Rabbit anti-rat
CPT1A (bs-2047R) and rabbit anti-rat MTTP (bs-5083R) polyclonal
antibodies were purchased from Beijing Biosynthesis Biotechnology
Co., Ltd. (Beijing, China). Liver tissue TG assays (E1013) and
protein (BCA) assays (P1511) were obtained from Applygen
Technologies Inc. (Beijing, China). Serum FFA diagnostic kits were
purchased from Jiancheng Bioengineering Institute (Nanjing, China)
and rat insulin radioimmunoassays were purchased from Millipore
Corporation (Billerica, MA, USA). All other chemicals used in these
experiments were purchased from Shanghai Sangon Biotechnology Co.,
Ltd. (Shanghai, China).
Animals
Thirty-six male Wistar rats aged 5–6 weeks (176–190
g) were provided by the Experimental Animal Center of Hebei Medical
University (Shijiazhuang, China), housed individually in an
air-conditioned room under controlled illumination (12-h light/dark
cycle), temperature (22–28°C) and humidity (40–60%). The rats had
free access to rodent chow and tap water throughout the
experimental period and were allowed to acclimatise for 7 days
prior to the experiment. The ethics committee of Hebei Medical
University approved the experimental protocol and all animals
received humane care in compliance with institutional animal care
guidelines.
Establishment of the NAFLD rat model and
drug intervention
The rats were randomly separated into two groups,
the standard diet (SD) group and the HFD group. The HFD (35%
fructose, 35% starch, 10% fat and 20% protein by energy) was
prepared according to the literature (18,19).
At the end of the eighth week, four rats were selected from the HFD
group and sacrificed, and their liver tissues were assessed for
fatty liver development. Subsequently, the HFD rats were randomly
subdivided into three groups (n=8): the model (HFD), OMT (80
mg/kg/day) and FF (30 mg/kg/day) groups. Drugs were administered
intragastrically once a day for four weeks, while the SD and HFD
groups were gavaged with an equivalent volume of the vehicle (0.5%
sodium carboxymethyl cellulose solution). Food intake and body
weights were monitored twice a week.
Following 4 weeks of treatment, rats were selected
(three animals per group) for euglycemic hyperinsulinaemic
clamping, then all rats were anaesthetised and sacrificed following
an overnight fast. Blood samples were collected via abdominal
aortic punctures and plasma was stored at −20°C. Visceral adipose
tissue (VAT) from various anatomical locations (perirenal,
epididymal and mesenteric) was dissected and weighed. The liver was
quickly excised, frozen in liquid nitrogen and stored at −80°C or
fixed for further analysis.
Evaluation of insulin sensitivity with
the euglycemic hyperinsulinaemic clamp
Rats were anaesthetised with pentobarbital (35
mg/kg, i.p.) and cannulas were implanted into the right jugular
vein and the left carotid artery under aseptic conditions for
infusion and sampling, respectively. Catheters were exteriorised at
the back of the neck. Six days after cannulation surgery, a
clamping test was performed as previously described (20) while rats achieved presurgery
weight. The rats were continuously infused with regular human
insulin (Novo Nordisk, Bagsvaerd, Denmark) at a rate of 10
mU/kg/min. During the insulin infusion, blood glucose was measured
at 5 min intervals to maintain euglycaemia (at the fasting level)
by adjusting the glucose infusion rate (GIR) using 30% glucose. The
average GIR (mg/kg/min) during the final 30 min was used as an
index of insulin sensitivity. Following testing, the animals were
euthanised and the liver tissues were rapidly removed,
freeze-clamped and stored at −80°C for subsequent analysis.
Biochemical analysis
Serum biochemistry, including serum TG, total
cholesterol (TC) and alanine aminotransferase (ALT) levels were
measured using standard laboratory procedures. Liver TGs were
extracted from liver tissue homogenates and assessed using an assay
kit and the values were normalised to the total protein
concentration in the liver homogenate using a bicinchoninic acid
(BCA) assay. Insulin levels were measured using a radioimmunoassay.
Serum FFA levels were determined spectrophotometrically, according
to the manufacturer’s instructions.
Histology examination
Freshly isolated liver tissues were fixed in 10%
formaldehyde PBS (pH 7.4) overnight, embedded in paraffin,
sectioned and H&E-stained for general histology. Lipid staining
was performed by cryostat sectioning of the liver, followed by Oil
Red O staining.
Quantitative PCR (qPCR) analysis
Total RNA was extracted from homogenised rat liver
tissue with TRIzol reagent (~20 mg of liver sample in 1 ml TRIzol)
and isolated by phenol/chloroform extraction. First-strand cDNA was
synthesised from 5 μg of total RNA using EasyScript First-Strand
cDNA Synthesis SuperMix (Beijing Transgen Biotechnology Co., Ltd.,
Beijing, China), according to the manufacturer’s instructions. qPCR
reactions were performed on an ABI 7300 instrument (Applied
Biosystems Inc., Foster City, CA, USA), using Ultra SYBR Mixture
[with 6-carboxy-x-rhodamine (ROX); Beijing CoWin Bioscience Co.,
Ltd., Beijing, China]. The total reaction volume was 20 μl and
contained 8 μl of cDNA, 1 μl of each primer and 10 μl of 2X Ultra
SYBR Mixture (with ROX). The amplification reactions were performed
as follows: 10 min at 95°C, 40 cycles of 95°C for 15 sec, 60°C for
1 min and 72°C for 35 sec. GAPDH was used as an internal control
and tests were performed in triplicate for each sample. The
relative quantity was calculated using the 2−ΔΔCt
method. The primers used for PPARα, CPT1A and GAPDH are listed in
Table I.
 | Table IPrimers sequences used for
quantitative PCR. |
Table I
Primers sequences used for
quantitative PCR.
| Primer | Forward | Reverse |
|---|
| PPARα |
5′-GTACGGTGTGTATGAAGCCATCTT-3′ |
5′-GCCGTACGCGATCAGCAT-3′ |
| CPT1A |
5′-TGGTCAACAGCAACTACTACGC-3′ |
5′-GAAGACGAATGGGTTTGAGTTC-3′ |
| MTTP |
5′-CTTCTGCCTACACTGGCTACG-3′ |
5′-GTTCTCCTCTCCCTCATCTGG-3′ |
| GAPDH |
5′-TGAACGGGAAGCTCACTG-3′ |
5′-GCTTCACCACCTTCTTGATG-3′ |
Western blot analysis
Liver samples were homogenised in ice-cold RIPA
lysis buffer with protease inhibitors of phenylmethanesulphonyl
fluoride (PMSF) and incubated on an ice board for 30 min. Lysates
were clarified by centrifugation (12,000 × g for 10 min) and the
supernatants were collected and stored at −80°C. The protein
concentrations were determined and equal amounts of protein (40 μg)
were subjected to electrophoresis on 10% sodium dodecyl sulphate
polyacrylamide gels and then transferred to PVDF membranes using an
electroblotting apparatus. Non-specific protein binding sites were
blocked with PBS containing 0.1% Tween-20 and 5% fat-free milk for
1 h at room temperature. The samples were then incubated overnight
at 4°C with the following primary antibodies diluted in blocking
buffer: PPARα, CPT1A, MTTP and GAPDH. Subsequently, the membranes
were washed three times and incubated for 2 h at room temperature
with the appropriate HRP-conjugated secondary antibody in PBST
(Beijing CoWin Bioscience Co., Ltd.). Immunoreactive bands were
visualised using an enhanced chemiluminescence (ECL) detection
system. The membranes were exposed to Kodak films (Carestream
Health, Inc., Xiamen, China) for 5–10 min. Quantification of the
resulting images was performed by densitometry with Gel-Pros
Analyzer 4.0 software (Media Cybernetics, Inc., Bethesda, MD, USA)
and the final readings were normalised against GAPDH.
Statistical analysis
The data are expressed as the mean ± SEM.
Statistical analysis was performed using one-way analysis of
variance (ANOVA) with the SPSS 11.5 software package (SPSS, Inc.,
Chicago, IL, USA) to compare the experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight and VAT weight
The 12-week HFD caused a mild increase in body
weight that was not statistically significant, and a significantly
increased body weight gain (P<0.05) and VAT weight (P<0.01)
in the HFD group compared with the SD group. Four weeks of OMT
treatment led to a significantly lower body weight gain (P<0.01)
and VAT weight (P<0.01) compared with the HFD group, and FF also
reduced the body weight gain and VAT weight significantly
(P<0.05; Table II). During the
study period, the calorie intake did not significantly differ
amongst the groups, indicating that these effects occurred without
being affected by calorie intake, and no adverse effects of the
drugs, such as diarrhoea, were observed.
 | Table IIBody weight, visceral adipose tissue
weight and euglycemic clamping study of insulin sensitivity. |
Table II
Body weight, visceral adipose tissue
weight and euglycemic clamping study of insulin sensitivity.
| Parameter | SD | HFD | OMT | FF |
|---|
| Body weight prior to
treatment (g) | 381.63±19.19 | 354.87±23.73 | 352.63±50.42 | 356.12±38.63 |
| Body weight following
treatment (g) | 397.00±26.58 | 412.17±24.08 | 362.17±39.96 | 382.50±42.78 |
| Body weight gain
(g) | 16.60±11.59 | 58.10±16.60a | 10.11±8.09d | 26.10±12.64c |
| VAT weight (g) | 11.60±0.70 | 18.90±0.50b | 12.30±0.40d | 14.80±0.60c |
| Caloric intake
(kcal/rat/day) | 97.50±5.60 | 105.30±7.30 | 92.30±8.50 | 93.60±6.90 |
| GIR (mg/kg/min) | 21.69±3.11 | 14.72±2.13a | 19.55±3.29c | 18.32±2.76c |
Biochemical parameters
Rats fed a HFD for 12 weeks demonstrated markedly
increased serum TG, TC, FFA, ALT and liver TG levels (P<0.05 or
P<0.01) compared with the SD group. When compared with rats of
the HFD group, OMT and FF treatment significantly lowered the serum
TG, TC, FFA and liver TG levels (P<0.05). Moreover, OMT
treatment significantly reduced the serum ALT level (P<0.01),
which was elevated in the HFD group, whereas FF had only a slight
effect on the ALT level (Table
III).
 | Table IIISerum biochemical parameters and
liver triglyceride (TG) contents. |
Table III
Serum biochemical parameters and
liver triglyceride (TG) contents.
| Parameter | SD | HFD | OMT | FF |
|---|
| Serum TG
(mmol/l) | 0.44±0.26 | 1.62±0.45b | 0.53±0.27d | 0.98±0.23c |
| Serum TC
(mmol/l) | 1.01±0.21 | 1.99±0.19a | 0.95±0.16d | 1.33±0.15c |
| Serum ALT
(IU/l) | 29.41±7.89 | 45.28±6.52a | 30.19±11.69c | 41.55±9.75 |
| Serum FFA
(μmol/l) | 669.92±142.68 |
958.64±236.71b |
607.51±154.82d |
790.75±199.73c |
| FBG (mmol/l) | 5.56±0.21 | 6.03±0.27 | 5.90±0.32 | 6.00±0.52 |
| FinS (ng/ml) | 0.94±0.15 | 1.83±0.31b | 1.15±0.29c | 1.23±0.32c |
| Liver TG contents
(mmol/g) | 0.82±0.14 | 3.13±0.33b | 0.90±0.15d | 2.07±0.28c |
Insulin sensitivity
The HFD increased the fasting serum insulin (FinS)
level (P<0.01) and lowered the GIR (P<0.05) in the HFD group
compared with SD group, whereas 4-week treatment with OMT or FF
significantly decreased the FinS level and increased the GIR
(P<0.05) compared with the HFD group. The fasting blood glucose
(FBG) level was not altered by treatment with OMT or FF (Table II).
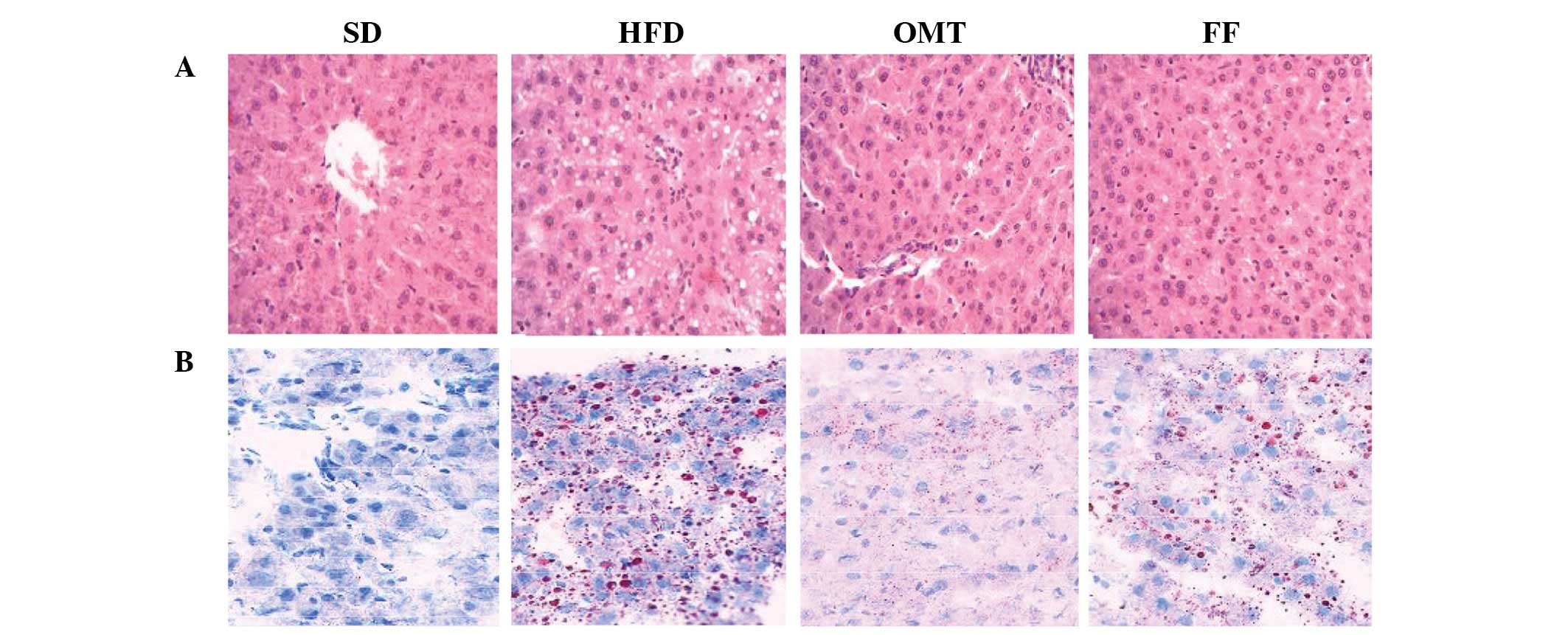
Histology examination
H&E staining of liver sections from the HFD
group revealed the derangement of liver cells and excessive
vacuoles in the hepatocytes, particularly surrounding the central
vein. These disorders were alleviated by treatment with OMT. Oil
Red O staining confirmed that the vacuoles visible with H&E
staining were lipid droplets. Similar improvements were also
observed in the FF group (Fig.
2).
qPCR and western blot analysis
The relative mRNA levels of PPARα, CPT1A and MTTP
were downregulated in the livers of HFD-fed rats compared with the
SD group, whereas OMT or FF treatment upregulated the expression of
PPARα, CPT1A and MTTP (P<0.05; Fig.
3). Consistent with alterations in the mRNA expression levels,
western blot analysis of liver protein extracts verified
corresponding changes in the protein levels of PPARα, CPT1A and
MTTP (P<0.05; Fig. 4).
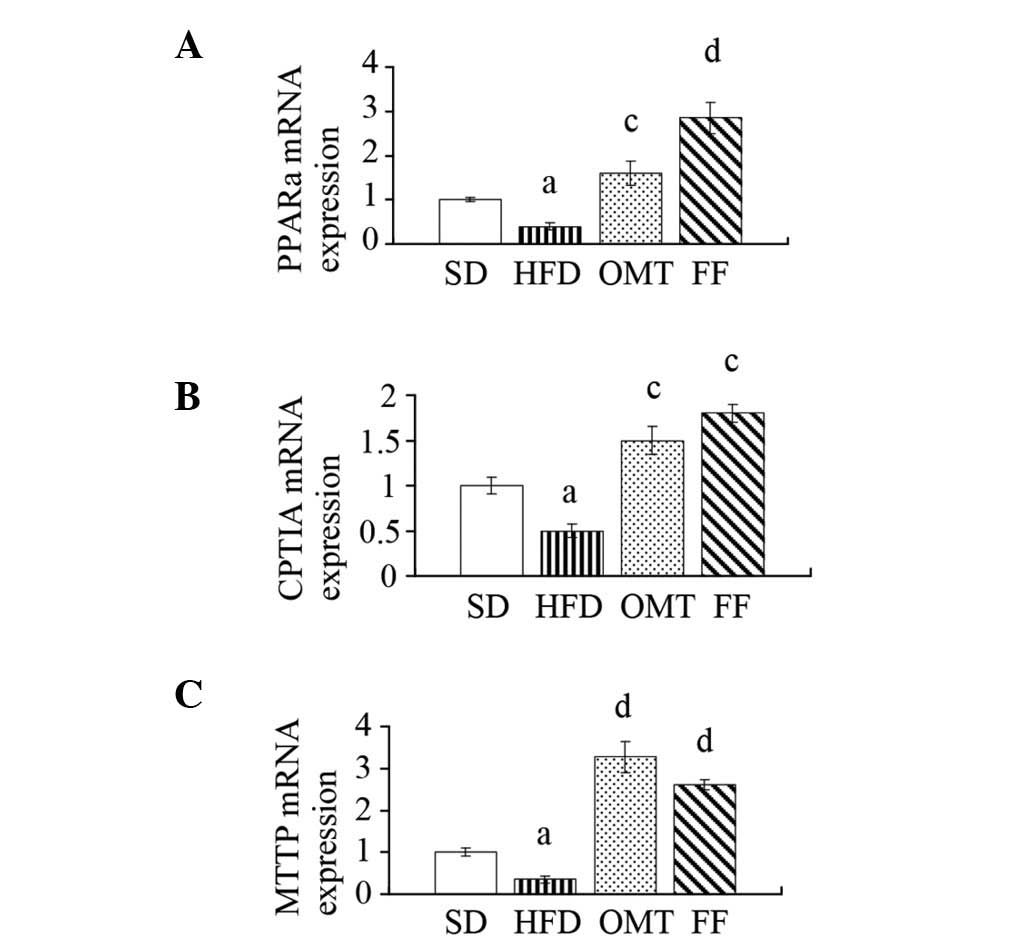 | Figure 3Effects of oxymatrine on (A) PPARα,
(B) CPT1A and (C) MTTP mRNA expression in hepatic tissue following
treatment for four weeks in NAFLD rats. Data are expressed as the
mean ± SEM. aP<0.05, bP<0.01 vs. the SD
group; cP<0.05, dP<0.01 vs. the HFD
group. PPARα, peroxisome proliferator activated receptor-α; CPT1A,
carnitine palmitoyltransferase 1A; MTTP, microsomal triglyceride
transfer protein; NAFLD, non-alcoholic fatty liver disease; SD,
standard diet; HFD, high-fructose diet; OMT, oxymatrine; FF,
fenofibrate. |
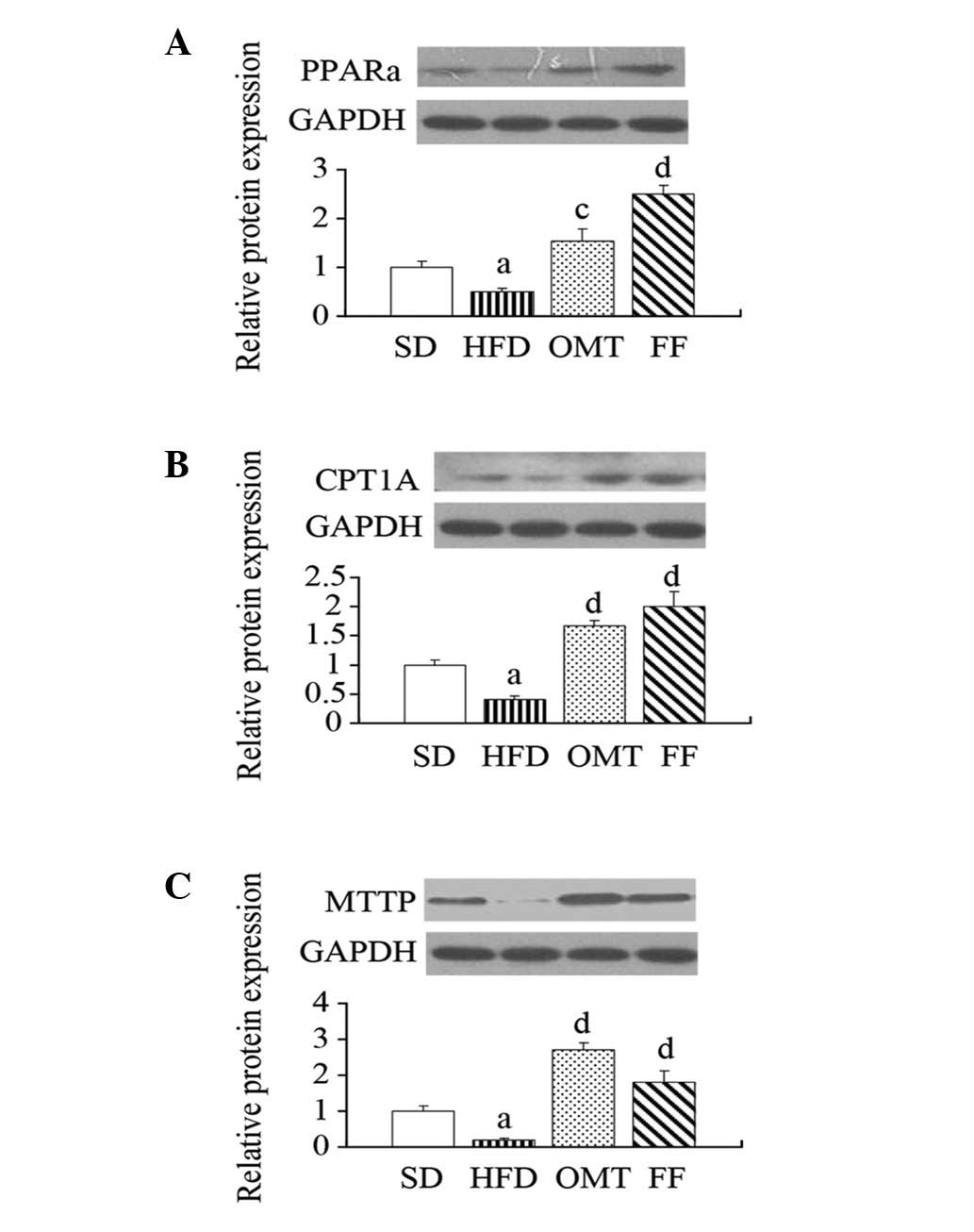 | Figure 4Effects of oxymatrine on (A) PPARα,
(B) CPT1A and (C) MTTP protein expression in hepatic tissue after
treatment for four weeks in NAFLD rats. Data are expressed as the
mean ± SEM. aP<0.05, bP<0.01 vs. the SD
group; cP<0.05, dP<0.01 vs. the HFD
group. PPARα, peroxisome proliferator activated receptor-α; CPT1A,
carnitine palmitoyltransferase 1A; MTTP, microsomal triglyceride
transfer protein; NAFLD, non-alcoholic fatty liver disease; SD,
standard diet; HFD, high-fructose diet; OMT, oxymatrine; FF,
fenofibrate. |
Discussion
The present study revealed that OMT improves the
biochemical and histological signatures of HFD-induced NAFLD rats
with problems such as hypertriglyceridaemia, IR and liver
steatosis. This result is consistent with a recent study by Zeng
et al(14), which
demonstrated that oral administration of OMT for one week
eliminated hepatic steatosis in fatty liver mice. We further
explored the mechanisms underlying the reduction in liver fat
accumulation.
As mentioned previously, hepatic steatosis is the
hallmark of NAFLD, and is also the product of lipid metabolism
imbalance. From this perspective, two possible mechanisms
responsible for the attenuation of NAFLD pathology in the OMT
treatment group may be considered: one is a decrease in the FFA
influx to the liver and the second is an increase in the lipid
outflux through the promotion of lipid discharge from the liver and
the β-oxidation of lipids in the liver.
With regard to the first pathway, it has been
demonstrated that ~60% of TGs deposited in the liver in NAFLD
originate from circulating non-esterified fatty acids (21). Currently, adipose tissue is
considered to be the main source of FFA and visceral fat is more
prone to lipolysis than subcutaneous fat. Furthermore, impaired
insulin action may increase lipolysis and reduce the utilisation of
FFA in adipose tissues (4).
Therefore, reducing body weight, specifically visceral fat, and
improving insulin sensitivity may be effective strategies for
decreasing FFA influx to the liver.
In this study, the HFD rats exhibited increased VAT
weights, marked IR and higher serum FFA levels compared with the SD
group, which signifies an enhanced influx of FFA to hepatocytes.
This increases hepatic TG production and lipid availability.
However, OMT decreased body weight gain and VAT weight, elevated
the GIR and reduced serum FFA levels, indicating a decreased FFA
influx to hepatocytes.
With regard to the second pathway, we examined the
PPARα, CPT1A and MTTP gene and protein expression levels, which are
important for the evaluation of FFA β-oxidation and the outflow of
lipids from the liver. PPARα belongs to a superfamily of nuclear,
ligand-activated transcription factors. PPARα is important in the
catabolism of fatty acids and is involved in the pathogenesis and
treatment of NAFLD (22).
Increasing evidence has suggested that PPARα activation normalises
serum TG and FFA levels, improves liver insulin sensitivity
(23) and prevents lipid
accumulation in the liver (24) by
regulating the expression of pivotal enzymes, including CPT1A and
MTTP. CPT1A is the key regulatory enzyme of mitochondrial fatty
acid oxidation and MTTP is the essential enzyme of VLDL assembly
and export from the liver.
In this study, the mRNA and protein levels of PPARα
and CPT1A were decreased in the HFD group compared with the SD
group (P<0.01), indicative of the impaired β-oxidation of FFA,
which may affect the imbalance of lipid metabolism toward lipid
accumulation. Similar changes were previously reported in patients
with NAFLD (25). In the treatment
groups, either OMT or FF administration for four weeks
significantly increased PPARα and CPT1A expression. A previous
study demonstrated that the overexpression of CPT1A was accompanied
by a 69% reduction in hepatic TG accumulation in obese
Sprague-Dawley rats (26). In this
study, we observed a similar change in CPT1A expression and a 71%
reduction in liver TG contents in the OMT treatment group,
consistent with the previous study.
MTTP, discovered by Wetterau et al(27) in 1984, is necessary for the
assembly and secretion of apoB100-containing lipoproteins (e.g.,
VLDL and LDL) to export lipids from the liver. The downregulation
of MTTP gene expression in ob/ob mice facilitates intracellular fat
accumulation in hepatocytes, which increases susceptibility to
hepatic steatosis (28). However,
the overexpression of MTTP decreases fat accumulation and increases
the secretion of apoB100-containing particles (29). In this study, the mRNA and protein
expression levels of MTTP in the rat liver decreased in the HFD
group compared with the SD group. After four weeks of treatment
with OMT, MTTP expression was upregulated. In the FF group, the
MTTP mRNA and protein levels were increased. These results
suggested that treatment with OMT or FF increases liver TG export
through the activation of liver MTTP and PPARα, thereby reducing
the hepatic TG accumulation and markedly attenuating the pathology
of NAFLD.
In this study, OMT and FF treatment decreased the
serum TG, TC and liver TG levels compared with the HFD group, but
the administration of OMT decreased the levels more efficiently
than FF treatment (−52 vs. −33% for serum TC, −67 vs. −40% for
serum TG and −71 vs. −34% for liver TG, respectively). Furthermore,
OMT reversed elevated ALT levels in the fatty liver, whereas FF
displayed only a mild decrease, which suggests that OMT is more
effective than FF for the modulation of liver function. Although
these results indicate that treatment with OMT is more advantageous
than with FF, their underlying mechanisms remain unknown.
A limitation of this study was that several details
with regard to lipid metabolism require clarification following
treatment with OMT, such as fatty acid absorption, fatty acid
uptake and fatty acid synthesis. Further studies are required to
evaluate the change and degree of the complex transcriptional
network in this process.
In conclusion, this study demonstrated that OMT had
a notable beneficial effect and may be useful in the treatment of
hepatic steatosis associated with visceral obesity, dyslipidaemia
and IR. The underlying mechanism was shown to be associated with
the modulation of abnormal lipid metabolism and reversed lipid
imbalance in the liver. Moreover, one molecular mechanism of OMT
treatment lies in its effect on PPARα activation and the
corresponding upregulation of PPARα target enzymes, including CPT1A
and MTTP. The present data indicate that these findings should be
extended to clinical trials in order to demonstrate the
effectiveness of OMT treatment in NAFLD.
Acknowledgements
This work was supported in part by grants from the
International Cooperation Project of Hebei province Department of
Science and Technology (11396406-D).
Abbreviations:
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
IR
|
insulin resistance
|
|
PPARα
|
peroxisome proliferator-activated
receptor-α
|
|
CPT1A
|
carnitine palmitoyltransferase 1A
|
|
MTTP
|
microsomal triglyceride transfer
protein
|
References
|
1
|
Tiniakos DG, Vos MB and Brunt EM:
Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu
Rev Pathol. 5:145–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheng L, Cho KW, Zhou Y, et al: Lipocalin
13 protein protects against hepatic steatosis by both inhibiting
lipogenesis and stimulating fatty acid β-oxidation. J Biol Chem.
286:38128–38135. 2011.PubMed/NCBI
|
|
3
|
Utzschneider KM and Kahn SE: Review: The
role of insulin resistance in nonalcoholic fatty liver disease. J
Clin Endocrinol Metab. 91:4753–4761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabbrini E, Mohammed BS, Magkos F,
Korenblat KM, Patterson BW and Klein S: Alterations in adipose
tissue and hepatic lipid kinetics in obese men and women with
nonalcoholic fatty liver disease. Gastroenterology. 134:424–431.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith BW and Adams LA: Non-alcoholic fatty
liver disease. Crit Rev Clin Lab Sci. 48:97–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaemers IC, Stallen JM, Kunne C, et al:
Lipotoxicity and steatohepatitis in an overfed mouse model for
non-alcoholic fatty liver disease. Biochim Biophys Acta.
1812:447–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 405:421–424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Améen C, Edvardsson U, Ljungberg A, et al:
Activation of peroxisome proliferator-activated receptor alpha
increases the expression and activity of microsomal triglyceride
transfer protein in the liver. J Biol Chem. 280:1224–1229.
2005.
|
|
9
|
Ye JM, Doyle PJ, Iglesias MA, Watson DG,
Cooney GJ and Kraegen EW: Peroxisome proliferator-activated
receptor (PPAR)-alpha activation lowers muscle lipids and improves
insulin sensitivity in high fat-fed rats: comparison with
PPAR-gamma activation. Diabetes. 50:411–417. 2001. View Article : Google Scholar
|
|
10
|
Seo YS, Kim JH, Jo NY, et al: PPAR
agonists treatment is effective in a nonalcoholic fatty liver
disease animal model by modulating fatty-acid metabolic enzymes. J
Gastroenterol Hepatol. 23:102–109. 2008.PubMed/NCBI
|
|
11
|
Cui X, Wang Y, Kokudo N, Fang D and Tang
W: Traditional Chinese medicine and related active compounds
against hepatitis B virus infection. Biosci Trends. 4:39–47.
2010.PubMed/NCBI
|
|
12
|
Cao YG, Jing S, Li L, et al:
Antiarrhythmic effects and ionic mechanisms of oxymatrine from
Sophora flavescens. Phytother Res. 24:1844–1849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng ZY, Li J, Jin Y, Chen XL and Lü XW:
Effect of oxymatrine on the p38 mitogen-activated protein kinases
signalling pathway in rats with CCl4 induced hepatic fibrosis. Chin
Med J (Engl). 122:1449–1454. 2009.PubMed/NCBI
|
|
14
|
Zeng XY, Zhou X, Xu J, et al: Screening
for the efficacy on lipid accumulation in 3T3-L1 cells is an
effective tool for the identification of new anti-diabetic
compounds. Biochem Pharmacol. 84:830–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan LJ, Lu X, Wang J, Zheng TZ, Qu SY and
Zhang XY: Effects of matrine on weight, serum lipids and
anti-oxidative capacity in high-fatted rats. Lishizhen Medicine and
Materia Medica Research. 9:2062–2064. 2008.
|
|
16
|
Zhang HF, Shi LJ, Song GY, et al:
Protective effects of matrine against progression of high-fructose
diet-induced steatohepatitis by enhancing antioxidant and
anti-inflammatory defences involving Nrf2 translocation. Food Chem
Toxicol. 55:70–77. 2013. View Article : Google Scholar
|
|
17
|
Xing Y, Yan F, Liu Y, Liu Y and Zhao Y:
Matrine inhibits 3T3-L1 preadipocyte differentiation associated
with suppression of ERK1/2 phosphorylation. Biochem Biophys Res
Commun. 396:691–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thorburn AW, Storlien LH, Jenkins AB,
Khouri S and Kraegen EW: Fructose-induced in vivo insulin
resistance and elevated plasma triglyceride levels in rats. Am J
Clin Nutr. 49:1155–1163. 1989.PubMed/NCBI
|
|
19
|
Ren LP, Chan SM, Zeng XY, et al: Differing
endoplasmic reticulum stress response to excess lipogenesis versus
lipid oversupply in relation to hepatic steatosis and insulin
resistance. PLoS One. 7:e308162012. View Article : Google Scholar
|
|
20
|
Kraegen EW, James DE, Bennett SP and
Chisholm DJ: In vivo insulin sensitivity in the rat determined by
euglycemic clamp. Am J Physiol. 245:E1–E7. 1983.PubMed/NCBI
|
|
21
|
Donnelly KL, Smith CI, Schwarzenberg SJ,
Jessurun J, Boldt MD and Parks EJ: Sources of fatty acids stored in
liver and secreted via lipoproteins in patients with nonalcoholic
fatty liver disease. J Clin Invest. 115:1343–1351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Postic C and Girard J: Contribution of de
novo fatty acid synthesis to hepatic steatosis and insulin
resistance: lessons from genetically engineered mice. J Clin
Invest. 118:829–838. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou CJ, Haluzik M, Gregory C, et al:
WY14, 643, a peroxisome proliferator-activated receptor alpha
(PPARalpha) agonist, improves hepatic and muscle steatosis and
reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J Biol
Chem. 277:24484–24489. 2002. View Article : Google Scholar
|
|
24
|
Ip E, Farrell GC, Robertson G, Hall P,
Kirsch R and Leclercq I: Central role of PPARalpha-dependent
hepatic lipid turnover in dietary steatohepatitis in mice.
Hepatology. 38:123–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kohjima M, Enjoji M, Higuchi N, et al:
Re-evaluation of fatty acid metabolism-related gene expression in
nonalcoholic fatty liver disease. Int J Mol Med. 20:351–358.
2007.PubMed/NCBI
|
|
26
|
Stefanovic-Racic M, Perdomo G, Mantell BS,
Sipula IJ, Brown NF and O’Doherty RM: A moderate increase in
carnitine palmitoyltransferase 1a activity is sufficient to
substantially reduce hepatic triglyceride levels. Am J Physiol
Endocrinol Metab. 294:E969–E977. 2008. View Article : Google Scholar
|
|
27
|
Wetterau JR and Zilversmit DB: A
triglyceride and cholesteryl ester transfer protein associated with
liver microsomes. J Biol Chem. 259:10863–10866. 1984.PubMed/NCBI
|
|
28
|
Stefano JT, de Oliveira CP,
Corrêa-Giannella ML, et al: Nonalcoholic steatohepatitis (NASH) in
ob/ob mice treated with yo jyo hen shi ko (YHK): effects on
peroxisome proliferator-activated receptors (PPARs) and microsomal
triglyceride transfer protein (MTP). Dig Dis Sci. 52:3448–3454.
2007. View Article : Google Scholar
|
|
29
|
Chen Z, Newberry EP, Norris JY, et al:
ApoB100 is required for increased VLDL-triglyceride secretion by
microsomal triglyceride transfer protein in ob/ob mice. J Lipid
Res. 49:2013–2022. 2008. View Article : Google Scholar : PubMed/NCBI
|