Introduction
Breast cancer is the leading cause of cancer-related
mortality in females worldwide and is the second leading cause of
cancer-related mortality (10.9%), resulting in 14% of total
fatalities due to cancer (1,2).
Traditional therapies for breast cancer (including surgery,
radiotherapy and chemical treatment) may cause various adverse
effects and have a high risk of relapse (3,4).
Therefore, improved technologies and treatment methods for breast
cancer are required. The field of cancer vaccination is currently
being investigated in clinical practice (5), and various antigens have been used in
vaccine formulation. Several breast cancer antigens, such as human
epidermal growth factor receptor 2 (HER2), mucin 1 (MUC1), human
telomerase reverse transcriptase (hTERT), tumor protein 53 (p53)
and cancer embryonic antigen (CEA), have been utilized in the
preparation of these vaccines (6–9). The
vaccines have either been tested alone, with antigenic epitopes or
in combination with immune stimulants, including
granulocyte-macrophage colony-stimulating factor (GM-CSF) and
Bacillus Calmette-Gúerin (BCG). Presently, the vaccines have yet to
demonstrate a protective outcome in clinical trials. Hence, novel
vaccines capable of stimulating effective antitumor immune
responses are required.
Epidermal growth factor receptor pathway substrate 8
(Eps8) is a 97-KDa protein, measuring 821 amino acids in length,
which was originally identified as a substrate for epidermal growth
factor receptor (EGFR) kinase (which mediates mitogenic signaling).
The human Eps8 gene locus was mapped to chromosome 12q13.2.
Computer-assisted analysis of the predicted amino acid sequence of
Eps8 revealed a structural organization similar to that of a
typical signaling molecule, including a putative N-terminal PTB
domain, a central SH3 domain and a C-terminal effector region. Two
functional regions of Eps8 have been identified by
structural-functional studies. The EGFR binding region is a binding
surface for the juxtamembrane region of the EGFR, at amino acids
298–362. The other region is the C-terminal effector region,
located at amino acids 648–821, which is sufficient to activate Rac
and result in remodeling of the actin cytoskeleton (10,11).
Previous studies have demonstrated that Eps8 is
overexpressed in the majority of cancer types, including oral
squamous cell carcinoma and breast, cervical, colon, esophageal,
pancreatic, thyroid and pituitary cancer, but is not expressed (or
is poorly expressed) in normal tissues (12–19).
Overexpressed Eps8 has also been demonstrated to be involved in
numerous signaling pathways associated with the genesis,
proliferation, migration, metastasis and chemoresistance of cancer,
and is a marker of poor prognosis in cancer patients (12,14–21).
The present study focused on whether Eps8 was able to block T
regulatory (Treg) cells and induce antitumor immunity in a murine
breast cancer model, as a novel protein vaccine.
Materials and methods
Mice and cell lines
Female BALB/c mice (age, 6–8 weeks) were purchased
from the Laboratory Animal Center of Southern Medical University
(Guangzhou, Guangdong, China). The experiments were conducted
according to the guidelines of the China Council on Animal Care.
The mice were allowed free access to food and water, and were
maintained in specific pathogen-free conditions. The murine breast
cancer cell line 4T1, was purchased from the Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China. The
murine breast cancer cell line, MA782, was provided by Dr. Yanqing
Ding (Southern Medical University). All cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Inc., Logan, UT, USA), penicillin (100 units/ml;
Sigma-Aldrich, St. Louis, MO, USA) and streptomycin (100 μg/ml;
Sigma-Aldrich), at 37°C in a humidified atmosphere containing 5%
CO2. The study was approved by the ethics committee of
Southern Medical University, Guangzhou, China.
Bacterial strains and vectors
BL21 E. coli cells and the pET28a+
expression vector were purchased from Novagen, Inc. (Madison, WI,
USA). The bacterial strains were grown at 37°C in Luria-Bertani
(LB) broth or on LB agar plates, and kanamycin was added to the
culture medium at a concentration of 50 μg/ml when required.
Isopropyl-β-D-thiogalactopyranoside (IPTG, 1 mM) was added to the
media as indicated.
PCR amplification and cloning of the Eps8
gene
Total RNA was extracted from MA782 cells using
TRIzol reagent (Life Technologies, Inc., Gaithersburg, MD, USA).
Based on the murine Eps8 gene sequence in the Genbank, a pair of
PCR primers were designed (amino acids, 440–710). The primer
sequences used were as follows: Forward: 5′-GCG GAA TTC CCG ATG CTG
AAC TTC ATG-3′, containing an EcoRI site; and reverse:
5′-ATA CTC GAG CCC GAT GGT CAG CCT CTG-3′, containing a XhoI
site. The reaction conditions were as follows: 94°C for 5 min, 94°C
for 30 sec, 60°C for 45 sec and 72°C for 30 sec (for a total of 35
cycles), and then 72°C for 10 min. Following PCR amplification, the
desired fragment was identified by agarose gel electrophoresis.
Subsequent to purification, the PCR product and the pET28a vector
were digested with EcoRI and XhoI double enzymes, and
linked by T4 DNA ligase, at 16°C for 16 h. The sequence was
analyzed by the sequencing laboratory of Invitrogen Corporation
(Guangzhou, Guangdong, China). Following verification, the
recombinant plasmid (pET28a-Eps8) was transformed into BL21 E.
coli cells.
Eps8 protein expression, purification and
western blot analysis
BL21 E. coli cells containing pET28a-Eps8
were inoculated in 5 ml LB broth (with 50 μg/ml kanamycin sulfate)
and cultured overnight at 37°C. The bacterial culture was grown in
100 ml LB broth to an OD600 of 0.8, and was induced by 1 mM IPTG
for 4–5 h at 37°C, harvested by centrifugation (5 min, 8,228 × g at
4°C), and then dispersed into a lysis buffer and sonicated.
Nickel-nitrilotriacetic acid (Ni-NTA) agarose was used for 6X
His-tagged protein purification, according to the manufacturer's
instructions (Qiagen, Hilden, Germany). Endotoxin was removed from
the protein using Pierce High Capacity Endotoxin Removal Resin
(Thermo Fisher Scientific, Rockford, IL, USA), according to the
manufacturer's instructions. The purified protein was boiled for 10
min, and then loaded onto a 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and
electrophoresed. The samples were transferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). The membrane
was blocked by 5% skimmed milk for 1 h, washed three times with
phosphate-buffered saline (PBS)-Tween 20 (10 mM Tris-HCl, pH 7.5;
150 mM NaCl; 0.05% Tween 20), and incubated with mouse anti-EPS8
monoclonal antibodies (BD Biosciences, San Diego, CA, USA)
overnight at 4°C. The membrane was then washed three times with
PBS-Tween 20, and incubated with goat anti-mouse immunoglobulin G
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 4 h at 37°C.
The immunoreactive bands were detected using the Amersham enhanced
chemiluminescence (ECL) detection system and ECL hyperfilm (GE
Healthcare, Waukesha, WI, USA), according to the manufacturer's
instructions. The FluorChem FC2 imaging system was used to analyze
the chemiluminescent blot (Alpha InnoTech Corp., San Leandro, CA,
USA).
Generation of dendritic cells (DCs)
Bone marrow cells were isolated from the femurs and
tibias of 6- to 8-week-old BALB/c mice, and incubated in hypotonic
lysis buffer (9.84 g/l NH4Cl, 1 g/l KHCO3,
0.1 mM EDTA) for 5 min at 37°C in a water bath, to remove the red
blood cells. The cells were washed twice with PBS buffer, and then
plated in 6-well plates at a density of 1×106 cells/ml
(5×105 cells/well) for 1 h at 37°C, in RPMI-1640 medium
with 10% FBS. Non-adherent single cells were gently removed, and
the adherent cells were cultured in 2 ml RPMI-1640 with 10% FBS, 50
ng/ml murine rGM-CSF (PeproTech, Inc., Rocky Hill, NJ, USA) and 20
ng/ml murine recombinant interleukin-4 (rIL-4; PeproTech, Inc.) per
well for six days. For the induction of maturation, cultures of
bone marrow-derived DCs (BM-DCs) were supplemented with 50 ng/ml
murine tumor necrosis factor-α (TNF-α; PeproTech, Inc.) for 24 h on
the sixth day of the culturing process, prior to use (22,23).
The DCs were pulsed on day 7 with either Eps8 protein (50 μg/ml) or
chicken egg ovalbumin (OVA; 50 μg/ml; Alpha Diagnostic Intl Inc.,
San Antonio, TX, USA) for 24 h, and the pulsing was terminated by
medium replacement. Different groups of DCs were collected and
washed three times with PBS buffer. The DCs were then incubated
with the corresponding antibodies on ice for 30 min. Flow
cytometric analysis was performed on a fluorescence-activated cell
sorter (FACSCalibur flow cytometer; Becton-Dickinson, Mountain
View, CA, USA). The DCs were phenotyped with antibodies against
complement component 3 receptor 4 subunit (CD11c), cluster of
differentiation 80 (CD80), CD86 and major histocompatibility
complex class II (MHC-II; BioLegend, San Diego, CA, USA). The
negative controls consisted of DCs labeled with mouse Ig.
Proliferation and cytotoxicity of
splenocytes in vitro
The spleens were removed from 6- to 8-week-old
BALB/c mice under sterile conditions. Subsequently, single-cell
suspensions were prepared by pressing the spleens through a sterile
100-mesh metal sieve and filtrated using a 80-μm nylon mesh. The
lymphocytes from the suspension of the spleen cells (6 ml) were
isolated by Ficoll density gradient centrifugation at 514 × g for
30 min at room temperature. The cells of the middle layer were
carefully removed and washed twice with serum-free RPMI-1640
medium. The splenocytes were counted with a hemacytometer and
plated in 6-well plates (5×106 cells/well), in 2-ml
RPMI-1640 medium with heat-inactivated FBS and 20 ng/ml recombinant
mouse IL-2 (24).
Proliferation of the splenocytes was determined by
an MTT assay as previously described (25). Splenocytes were harvested and
co-cultured with autologous DCs at a ratio of 5:1 in 96-well plates
for 72 h, and this ratio had been identified to be optimal (data
not shown). The OD570 was read and the stimulation index was
calculated by dividing the background mean absorbance from cultures
with unstimulated splenocytes by the experimental mean absorbance
from cultures with DC-stimulated splenocytes. All experiments were
performed in triplicate and repeated three times.
The cytotoxic activity of the splenocytes was
assessed by the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit
(Promega, Madison, WI, USA), according to the manufacturer's
instructions. The splenocytes that were co-cultured with autologous
DCs served as effector cells, while the 4T1 cells served as target
cells. The target cell suspension was adjusted to 5×105
cells/ml and the effector cell suspension was adjusted to
1×107 cells/ml, with RPMI-1640 medium (containing 5%
FBS), and the cells were plated in 96-well plates. The effector to
target (E/T) cell ratio was 20:1. Wells containing only target
cells or only effector cells with culture medium or 0.5% Triton
X-100 served as spontaneous or maximal release controls. Following
4 h of incubation at 37°C and 5% CO2, 50 μl supernatant
was analyzed by the Model 680 microplate reader at a reference
wavelength of 650 nm and a test wavelength of 450 nm (Bio-Rad,
Hercules, CA, USA). The percentage of specific lysis was calculated
as follows: Specific lysis (%) = 100 × (experimental release -
effector spontaneous release - target spontaneous release) /
(target maximum release - target spontaneous release).
Enzyme-linked immunosorbent assay (ELISA)
for evaluating interferon-γ (IFN-γ) and IL-12
The supernatant of the splenocytes was collected at
different time points (days 1, 3 and 5) and tested for the presence
of IFN-γ by ELISA, according to the manufacturer's instructions
(Endogen, Inc., Cambridge, MA, USA). In order to detect the level
of IL-12 that was secreted by the DCs, supernatants were collected
at the aforementioned time points and analyzed for IL-12 p70, using
the ELISA (Biosource, Camarillo, CA, USA).
Vaccination and inoculation protocol
The experiment was performed on 4- to 6-week-old
female BALB/c mice. The BABL/c mice were randomly divided into two
groups: The Eps8 group and the PBS group, with 8 healthy mice
included in each experimental group. The animals were immunized
subcutaneously once a week for three weeks; the mice received 100
μg of either Eps8 protein or PBS mixed with complete Freund's
adjuvant for the first immunization, and for the second and third
immunizations, the same quantity of either Eps8 protein or PBS was
mixed with incomplete Freund's adjuvant. Seven days subsequent to
the third immunization, the mice were inoculated subcutaneously
with 7×106 4T1 tumor cells in the right armpit. The mice
were monitored each day and the day of the tumor cell inoculation
was recorded as day 0.
In vivo antitumor experiments
The animals were vaccinated and then challenged with
tumor cells. The antitumor effects were evaluated by measuring the
tumor volume, tumor growth inhibition rate and overall survival
time. In the tumor protection experiments, the tumor size was
determined by the measurement of the shortest (a) and longest (b)
diameter using vernier calipers, every 3 days. The volume (V) of
the tumors was calculated using the formula: V = (a2b/2)
(26). For the comparison of tumor
growth inhibition rates, mice were sacrificed by neck dislocation
28 days following inoculation. The tumors were surgically removed
and weighed. The inhibition rate was calculated as follows:
Inhibition rate (%) = (1 - tumor weight of Eps8 group/tumor weight
of PBS group) × 100. For survival analysis, an observation period
of 60 days was adopted following the inoculation of the mice with
4T1 tumor cells.
Flow cytometric analysis
BALB/c mice were grouped and treated as described
previously, and sacrificed 28 days following inoculation with tumor
cells. The splenocytes were collected as described previously, and
incubated with the corresponding antibodies: Fluorescein
isothiocyanate (FITC) anti-mouse CD4 mAb and PerCP anti-mouse CD8
mAb (BioLegend). The negative control consisted of splenocytes
labeled with the corresponding isotype control antibodies (27). The Mouse Regulatory T cell Staining
kit (eBiosciences, Inc., San Diego, CA, USA) was used for detecting
the CD4+CD25+ FoxP3+ Treg cells
among the splenocytes. Briefly, the splenocytes of the BALB/c mice
were surface-stained with FITC anti-mouse CD4 mAb and APC
anti-mouse CD25 mAb. Subsequently, the cells were permeabilized
using the FoxP3 Staining Buffer set, and stained with rat IgG2a
kappa isotype control PE or anti-mouse/rat Foxp3 PE. All samples
were run on a FACSCalibur flow cytometer (Becton-Dickinson) and
data were analyzed using WinMDI 2.9 software (Joseph Trotter, The
Scripps Institute, La Jolla, CA, USA).
Cytotoxic T lymphocyte (CTL) assay
The splenocytes were harvested from the
tumor-bearing mice that had been immunized with vaccines and
cultured as described previously. The viable splenocytes were
regarded as the effector cells and the 4T1 cells were used as the
target cells. The cytotoxic effect of the splenocytes against the
4T1 cells was measured by the lactate dehydrogenase (LDH) assay and
calculated as described previously.
Statistical analysis
Data are presented as the mean ± standard deviation.
The significance of the statistical comparisons was calculated by
an analysis of variance (ANOVA) or the Student's t-test using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). The
comparison of the survival data of the two groups was evaluated
with a log-rank test of the Kaplan-Meier survival curves. The tumor
volumes were analyzed using a repeated measures ANOVA. P<0.05
was considered to indicate a statistically significant
difference.
Results
Preparation and identification of
Eps8
The PCR products were electrophoresed on 1% agarose
gels and visualized by ethidium bromide staining under UV light.
The results revealed that the fragment was ~801 bp, as expected
(Fig. 1A, lanes 1 and 2).
Following purification, the Eps8 protein was expressed on the gel
with a purity of 90% in the total proteins (Fig. 1B, lanes 4 and 5). In addition, a
western blot assay confirmed that the expression product had the
antigenicity of Eps8 (Fig. 1C,
lanes 1–3).
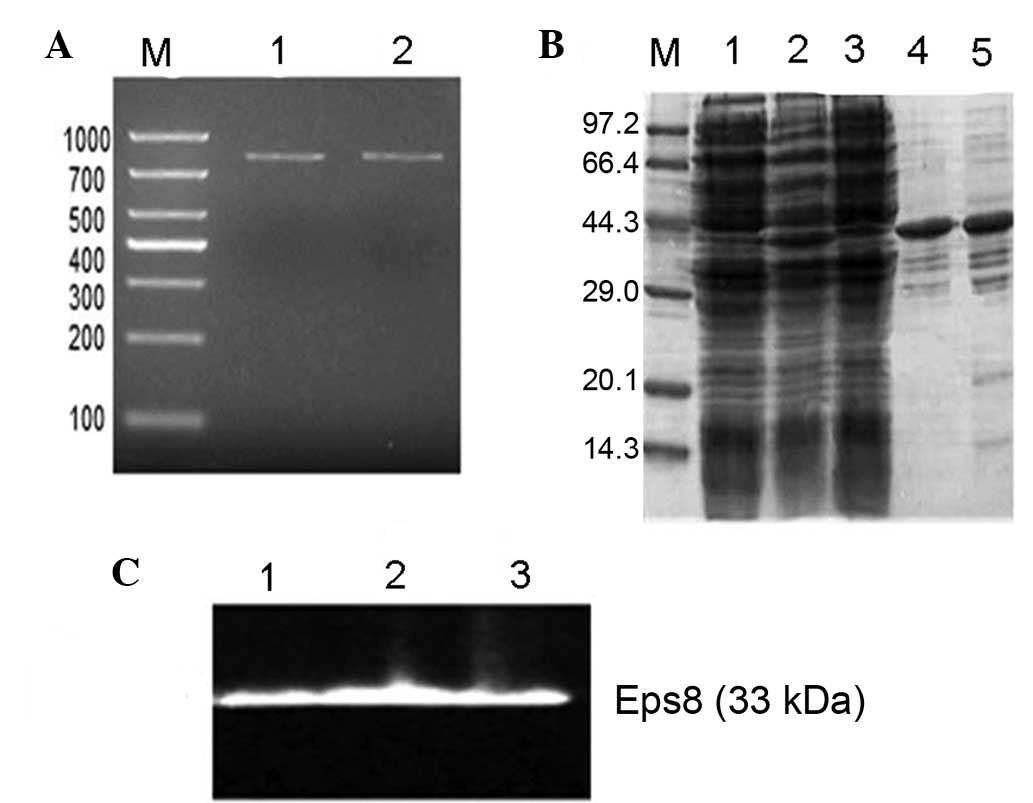 | Figure 1Agarose electrophoresis, SDS-PAGE and
western blot analysis of the expression product of the recombinant
plasmid pET28a-Eps8. (A) Agarose electrophoresis of the RT-PCR
products of mouse Eps8 cDNA. Lanes 1 and 2, RT-PCR product of Eps8;
lane M, DNA ladder marker. (B) SDS-PAGE of the different samples
followed by Coomassie blue staining of the gel. Lane 1, eluent of
the BL21 strain carrying pET28a-Eps8; lane 2, supernatants of the
BL21 strain carrying Eps8 following induction by IPTG; lane 3,
supernatants of the BL21 strain carrying Eps8 prior to induction by
IPTG; lanes 4 and 5, proteins purified from the BL21 strain
carrying pET28a-Eps8; lane M, molecular weight protein markers. (C)
Western blot analysis with an anti-Eps8 monoclonal antibody. Lanes
1–3, expression product of the recombinant plasmid pET28a-Eps8 in
BL21 E.coli cells. Eps8, epidermal growth factor receptor
pathway substrate 8. |
Changes in the phenotype of DCs following
pulsation with Eps8
The DCs were generated from mouse bone marrow cells.
On day 7, either TNF-α, Eps8 or OVA were added to the DCs, to
induce maturation. Following 8 days of cultivation, unpulsed DCs
(the DC group), OVA-pulsed DCs (the DC + OVA group) and Eps8-pulsed
DCs (the DC + Eps8 group) were collected and analyzed by flow
cytometry (Fig. 2). Compared with
the DC and the DC + OVA groups, significant increases in the
expression of MHC-II, CD80 and CD86 were observed in the DC + Eps8
group (P<0.05); however, there was no significant difference in
CD11c expression among these three groups (P>0.05).
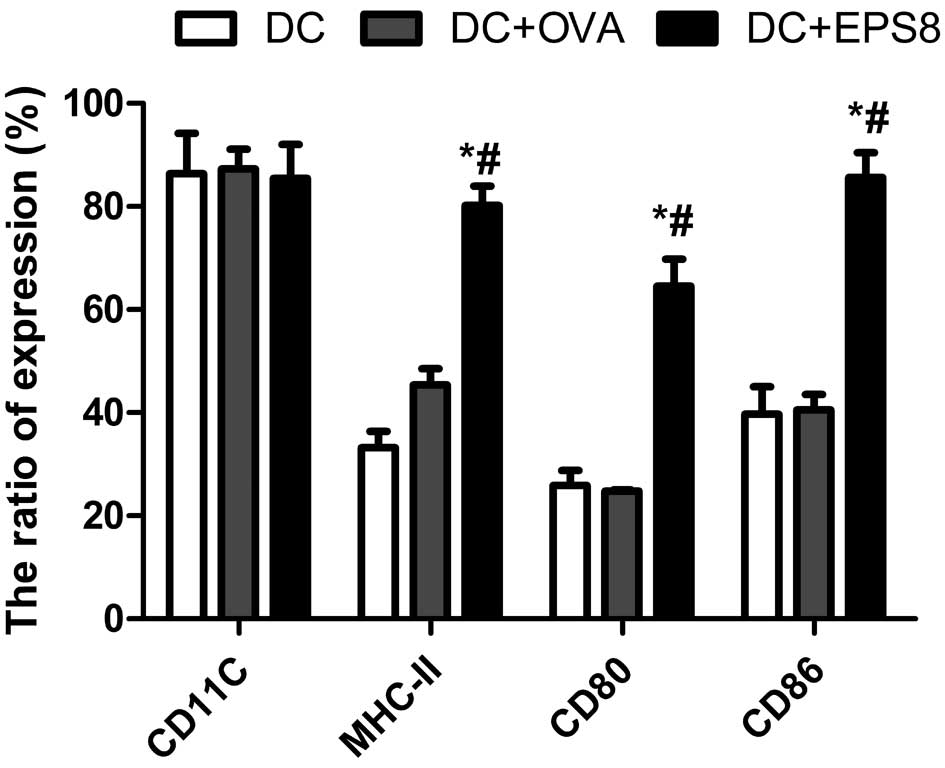 | Figure 2Flow cytometric analysis of DCs. In
the DC group, the expression rates of the antigen-presenting
molecule, MHC II, and the co-stimulatory molecules, CD80 and CD86,
were 33.22±3.18, 25.88±2.94 and 39.69±5.35%, respectively. In the
DC + OVA group, the expression rates were 45.38±3.17, 24.82±0.26
and 40.53±2.98%, respectively; in the DC + Eps8 group, the
expression rates were 80.19±3.78, 64.63±4.83 and 85.63±4.83%,
respectively. *P<0.05 vs. the DC group and
#P<0.05 vs. DC + OVA group. DC, dendritic cell; OVA,
ovalbumin; DC, unpulsed DCs; DC + OVA, DCs pulsed with OVA protein;
DC + Eps8, DCs pulsed with Eps8 protein; MHC II, major
histocompatibility complex class II. |
Eps8 promotes DC maturation, T-cell
proliferation and cytotoxicity in vitro
The results demonstrated that Eps8 stimulated the
maturation of DCs, and their secretion of IL-12 p70. The IL-12 p70
level peaked at day 3 in all groups of DCs; the highest level was
achieved by the DC + Eps8 group, and was significantly higher than
the levels observed in the remaining two (control) groups (Fig. 3C). IFN-γ is a significant effector
cytokine released by T cells. The ELISA results revealed that
Eps8-pulsed DCs enhanced the IFN-γ secretion by the splenocytes;
the concentration of IFN-γ was significantly greater in this group,
compared with that of the two control groups (Fig. 3D).
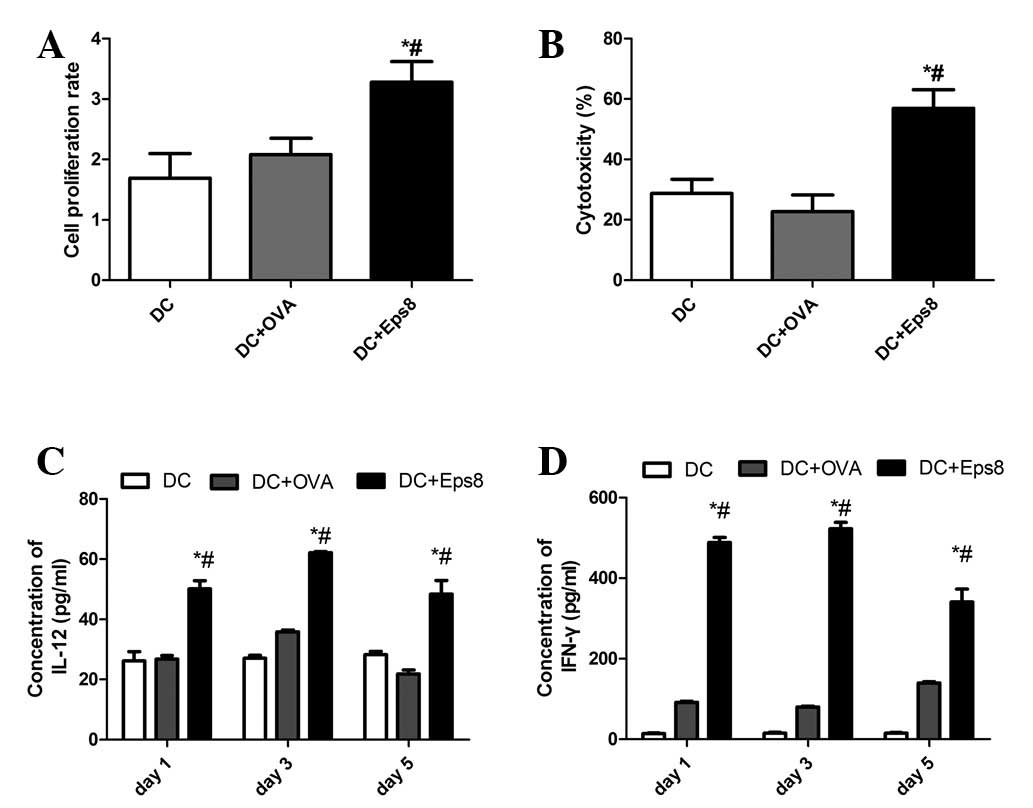 | Figure 3Eps8 protein stimulates DC and T-cell
activities in vitro. (A) The proliferation rate of
splenocytes co-cultured with DCs. The splenocytes were co-cultured
with different DCs for 3 days at a stimulator to responder ratio of
1:5. The proliferation rate was significantly higher in the DC +
Eps8 group compared with all other groups (P<0.01), while no
significant differences were observed between the DC + OVA group
and the DC group. The results are representative of three
independent experiments. (B) Cytotoxic activity of splenocytes
against 4T1 breast tumor cells. The splenocytes were co-cultured
with different DCs, and incubated with 4T1 cells for 24 h at an
effector to target ratio (E:T) of 20:1. The CTL activity of the
splenocytes was significantly increased in the DC + Eps8 group
compared with that of the OVA + DC and DC groups (P<0.01).
Results are representative of three independent experiments. (C)
Analysis of the level of IL-12 secreted by the DCs. The
concentration of IL-12 in the supernatant of cultured DCs was
analyzed by ELISA assay on days 1, 3 and 5. Eps8 protein (50 ng/ml)
stimulated DCs secreted the highest level of IL-12 on day 3, and
the concentration of IL-12 in the DC + Eps8 group was significantly
higher than that of the DC + OVA and DC groups at all time points.
(D) Regulation of T-cell function. The splenocytes were co-cultured
with different DCs for 5 days. The supernatants were collected at
different time points and cytokine production of IFN-γ was measured
by ELISA assay. The levels of IFN-γ secreted by the DC + Eps8 group
were significantly higher than the other two groups on days 1, 3
and 5 (P<0.01). Results are representative of three independent
experiments. *P<0.05 vs. the DC group and
#P<0.05 vs. the DC + OVA group. Eps8, epidermal
growth factor receptor pathway substrate 8; OVA, ovalbumin; DC,
dendritic cell; DC, unpulsed DCs; DC + OVA, DCs pulsed with OVA
protein; DC + Eps8, DCs plused with Eps8 protein; CTL, cytotoxic T
lymphocyte; IL, interleukin; ELISA, enzyme-linked immunosorbent
assay. |
The proliferative ability and the cytotoxicity of
the splenocytes were also increased by Eps8. The data demonstrated
an enhanced proliferation rate (by 3.28±0.34-fold) of splenocytes
co-cultured with Eps8-pulsed DCs (Fig.
3A). As demonstrated in Fig.
3B, at an E/T cell ratio of 5:1, the splenocytes that were
co-cultured with Eps8-pulsed DCs were able to lyse 54.89±4.99% of
the target cells, while the other two (control) groups of
splenocytes only exhibited a background lysis of 4T1 cells. In
summary, Eps8 possessed the ability to stimulate DC and T-cell
activities in vitro.
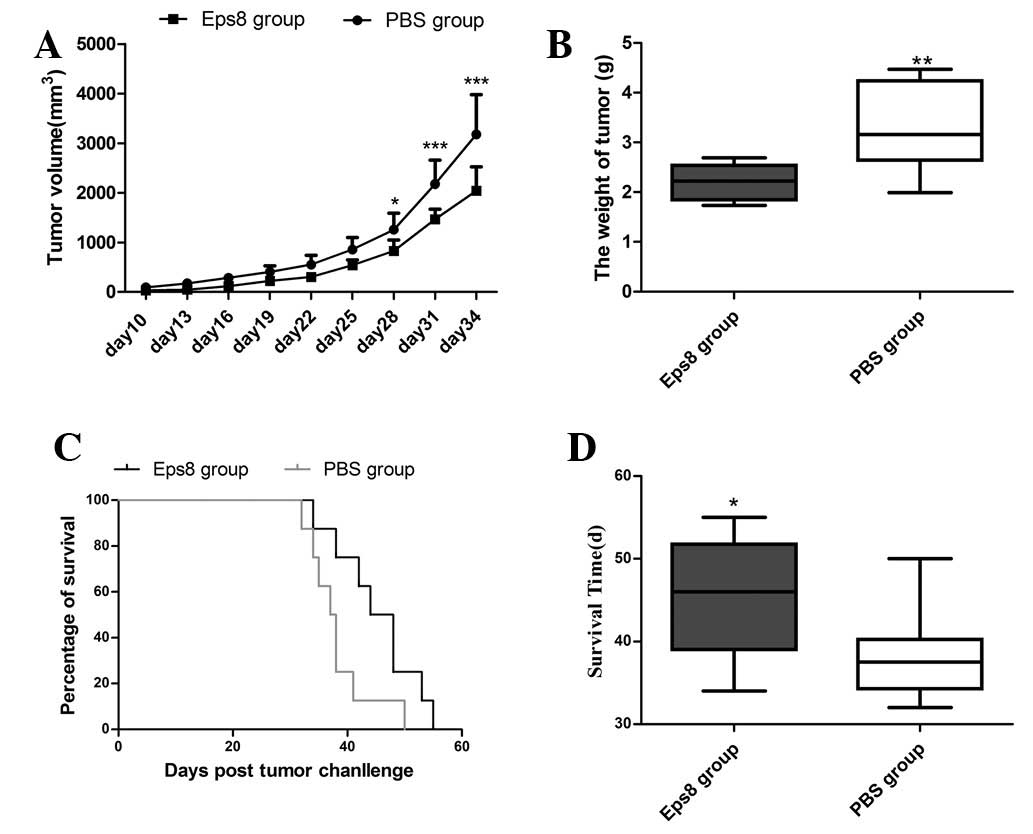
Prophylactic antitumor effect of the Eps8
vaccine in the murine 4T1 breast cancer model
The 4T1 breast cancer model was used to investigate
the effect of the Eps8 vaccine on tumor growth in vivo. The
results demonstrated that none (0%) of the mice remained tumor
free; however, the tumor growth in the Eps8-immunized mice was
inhibited significantly compared with that of the PBS control mice
(Fig. 4A). The tumor weights were
measured when the mice were sacrificed and the mean tumor weight of
the Eps8 group was significantly lower than that of the PBS group
(Fig. 4B). In addition, the
inhibition rate of tumor growth was calculated to be 33.23%. To
determine the immune protection effect of the Eps8 vaccine, mice
were inoculated subcutaneously with 4T1 cells following
immunization, and the survival time was measured. The results
revealed that all mice in the two groups succumbed within 55 days
of the inoculation, but the survival time of the tumor-bearing mice
that had been immunized with Eps8 was significantly longer than
that of the PBS group (Fig. 4C and
D). These results suggested that treatment with Eps8 prior to
tumor cell inoculation resulted in a significant prophylactic
antitumor effect in the 4T1 breast cancer model.
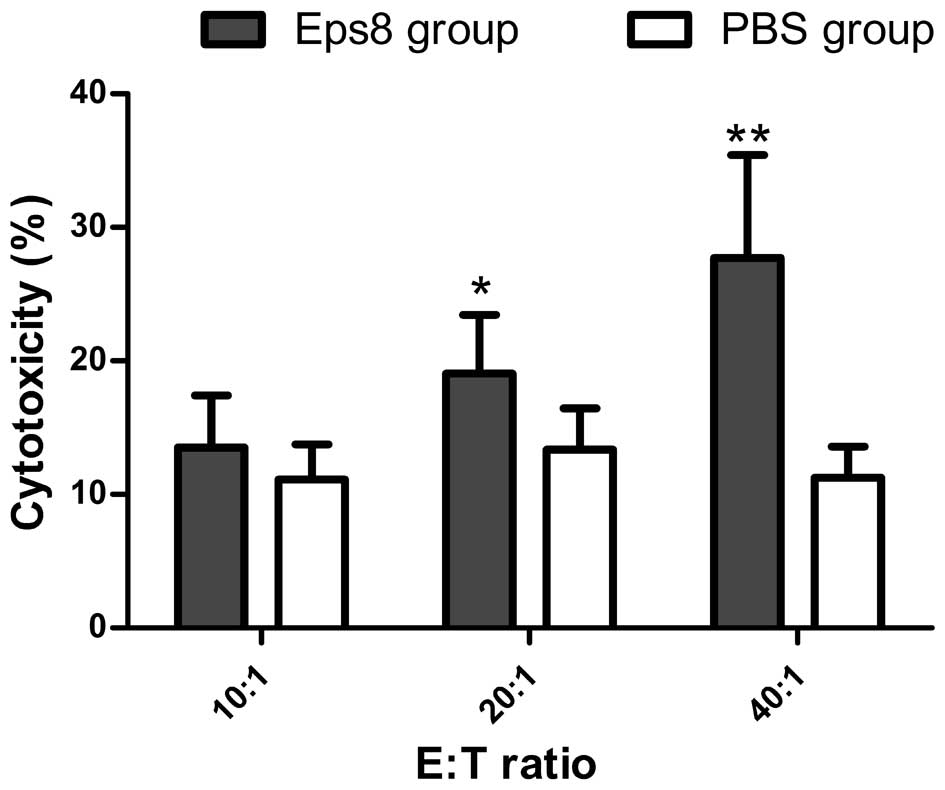
Eps8 vaccination increases the
cytotoxicity of splenocytes in tumor-bearing mice
CTL activity was induced by immunization with Eps8.
The LDH assay was performed at E/T cell ratios of 10:1, 20:1 and
40:1. T cells isolated from the spleens of the tumor-bearing mice
pre-treated with the Eps8 vaccine exhibited greater cytotoxicity
against 4T1 cells than those of the PBS group, when the E/T cell
ratios were 20:1 and 40:1 (Fig.
5). The results indicated that the killing activity observed
may have resulted from Eps8-specific cellular immune responses,
which contributed to the observed in vivo antitumor
immunity.
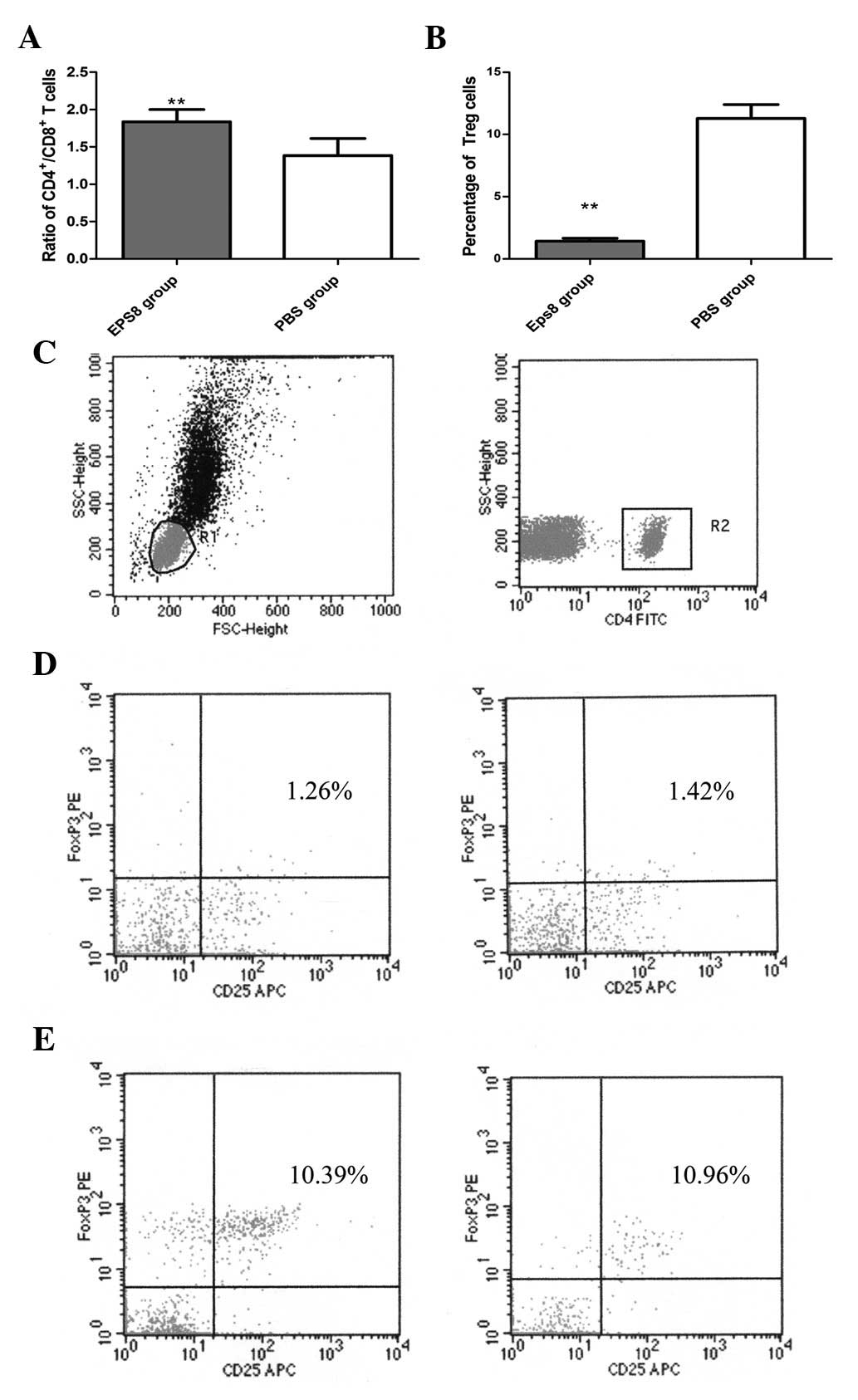
Changes in the immune cell population
with Eps8 vaccination
The proportions of splenocyte subsets were detected
by flow cytometry. Eps8 immunization increased the
CD4+/CD8+ T cell ratio compared with that of
the PBS control mice (Fig. 6A).
The proportion of CD4+CD25+ FoxP3+
Treg cells in the tumor-bearing mice immunized with the Eps8
vaccine or injected with PBS was also measured by FACS. Cells in
the lymphocyte gate were used for analysis and the CD4 gating
strategy was used as a secondary gating method (Fig. 6C). As demonstrated in Fig. 6B, the percentage of
CD4+CD25+ FoxP3+ Treg cells
significantly decreased in the splenocytes of the Eps8-vaccinated
mice compared with that of the PBS control mice. Representative
results are shown in Fig. 6D and
E.
Discussion
Eps8 is a significant tumor-associated antigen with
restricted expression and is the intracellular receptor of EGFR
(28). Certain studies have
suggested that the intracellular domain (ICD) of proteins may be
more immunogenic than the extracellular domain (ECD), as the ICD is
not usually exposed to the immune system. Antibodies binding to the
ICD presented in a tumor bed or shed by dying cells may contribute
to increases in the cellular immune response, by inducing
antibody-dependent cell-mediated cytotoxicity (29,30).
In the present study, Eps8 protein exerted a significant antibody
response and the antibody titer was increased with each
immunization (data not shown). Such unique characteristics make
Eps8 a candidate target antigen for antitumor vaccines.
In this study, the Eps8 protein vaccine induced
significant T-cell proliferation along with cytotoxic activity
towards the Eps8-expressing 4T1 tumor cells (Fig. 3). Treatment of the immature DCs
with Eps8 induced an increased expression of co-stimulatory
molecules, including CD80, CD86 and MHC-II (Fig. 2). DCs are able to process antigenic
material and present it on their surface to other cells of the
immune system; therefore, they play a significant role in the
antitumor effect by processing or carrying tumor-associated
antigens (31–33). A study by Banchereau et al
demonstrated that immature DCs are better equipped for capturing
antigen and migrating to the lymph nodes than mature DCs (19,31).
However, mature DCs are superior in representing antigens and
stimulating T cells compared with immature DCs (19). A suggested mechanism for the
antitumor effect of the Eps8 protein may include increased
maturation of the DCs and increased cytokine secretion, which
further activates the T cells.
Due to the results in vitro, we investigated
the potential use of Eps8 as a breast cancer vaccine in
vivo. The Eps8 protein vaccine promoted cytotoxic T-cell
activity towards Eps8-expressing targets. Immune responses induced
by the Eps8 protein inhibited tumor growth in the murine 4T1 breast
cancer model. In order to investigate the mechanism of action of
the prophylactic Eps8 vaccination, we measured the proportion of
CD4+CD25+ Treg cells in CD4+ T
cells, in Eps8 vaccinated mice. CD4+CD25+
Treg populations, originally demonstrated to suppress autoimmune
responses, are also crucial in controlling antitumor immune
responses (34). Naturally
occurring regulatory T cells (CD4+CD25+ T
cells), are important in tumor invasion and the downregulation of
immune responses against established tumors (35). Although tumors themselves are
potent inducers of Treg activity, the currently used cancer
vaccination schemes may also lead to enhanced frequencies of Treg
cells, characterized by a surface
CD4+CD25high phenotype and intracellular
expression of FOXP3 (36,37). Failure of host antitumor immunity
may be caused by exaggerated suppression of tumor-associated
antigen-reactive CTLs by Treg cells, which contributes to the
growth of human tumors in vivo. Treg cells are also
associated with a high risk of mortality and a reduced survival
rate. Thus, blocking Treg cells may be a therapeutic target in
numerous types of cancer (36).
Our results revealed that mice immunized with Eps8 exhibited
decreased ratios of CD4+CD25+ Treg cells to
CD4+ T cells, which may in turn assist in the prevention
of tumor outgrowth and prolong the survival of the challenged
mice.
To the best of our knowledge, our study is the first
to demonstrate that an Eps8 vaccination is able to provide
prophylaxis against breast cancer, in a 4T1 model system.
Therefore, our results may serve as a rationale for developing a
novel and effective vaccine against human breast cancer in the
future.
Acknowledgements
The authors would like to thank Dr Yanqing Ding for
providing the MA782 mouse breast cancer cell line, and Ms. Lin Lou
and Ms. Miaoxia Li for their expert administrative assistance. This
study was supported by the Key Program of Natural Science
Foundation of Guangdong Province, China (grant no.
9251051501000007) and the Program for New Century Excellent Talents
in University, Ministry of Education of China (grant no.
NCET-09-0087).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanari C, Wargon V, Rojas P and Molinolo
AA: Antiprogestins in breast cancer treatment: are we ready? Endocr
Relat Cancer. 19:R35–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher SW: Breast cancer screening: a
35-year perspective. Epidemiol Rev. 33:165–175. 2011.PubMed/NCBI
|
|
4
|
Esteva FJ: Monoclonal antibodies, small
molecules, and vaccines in the treatment of breast cancer.
Oncologist. 9(Suppl 3): S4–S9. 2004. View Article : Google Scholar
|
|
5
|
Fioretti D, Iurescia S, Fazio VM and
Rinaldi M: DNA vaccines: developing new strategies against cancer.
J Biomed Biotechnol. 2010:1743782010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peoples GE, Gurney JM, Hueman MT, et al:
Clinical trial results of a HER2/neu (E75) vaccine to prevent
recurrence in high-risk breast cancer patients. J Clin Oncol.
23:7536–7545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohebtash M, Tsang KY, Madan RA, et al: A
pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients
with metastatic breast and ovarian cancer. Clin Cancer Res.
17:7164–7173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emens LA, Reilly RT and Jaffee EM: Breast
cancer vaccines: maximizing cancer treatment by tapping into host
immunity. Endocr Relat Cancer. 12:1–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curigliano G, Spitaleri G, Pietri E, et
al: Breast cancer vaccines: a clinical reality or fairy tale? Ann
Oncol. 17:750–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Offenhäeuser N, Borgonovo A, Disanza A,
Romano P, Ponzanelli I and Iannolo G: The eps8 family of proteins
links growth factor stimulation to actin reorganization generating
functional redundancy in the Ras/Rac pathway. Mol Biol Cell.
15:91–98. 2004.PubMed/NCBI
|
|
11
|
Fazioli F, Minichiello L, Matoska V,
Castagnino P, Miki T, Wong WT and Di Fiore PP: Eps8, a substrate
for the epidermal growth factor receptor kinase, enhances
EGF-dependent mitogenic signals. EMBO J. 12:3799–3808.
1993.PubMed/NCBI
|
|
12
|
Yap LF, Jenei V, Robinson CM, et al:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YJ, Shen MR, Chen YJ, Maa MC and Leu
TH: Eps8 decreases chemosensitivity and affects survival of
cervical cancer patients. Mol Cancer Ther. 7:1376–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maa MC, Lee JC, Chen YJ, et al: Eps8
facilitates cellular growth and motility of colon cancer cells by
increasing the expression and activity of focal adhesion kinase. J
Biol Chem. 282:19399–19409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Welsch T, Endlich K, Giese T, Büchler MW
and Schmidt J: Eps8 is increased in pancreatic cancer and required
for dynamic actin-based cell protrusions and intercellular
cytoskeletal organization. Cancer Lett. 255:205–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Patel V, Miyazaki H, Gutkind JS
and Yeudall WA: Role for EPS8 in squamous carcinogenesis.
Carcinogenesis. 30:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Shorts-Cary L, Knox AJ,
Kleinsmidt-DeMasters B, Lillehei K and Wierman ME: Epidermal growth
factor receptor pathway substrate 8 is overexpressed in human
pituitary tumors: role in proliferation and survival.
Endocrinology. 150:2064–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bashir M, Kirmani D, Bhat HF, et al:
P66shc and its downstream Eps8 and Rac1 proteins are upregulated in
esophageal cancers. Cell Commun Signal. 8:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matoskova B, Wong WT, Salcini AE, Pelicci
PG and Di Fiore PP: Constitutive phosphorylation of eps8 in tumor
cell lines: relevance to malignant transformation. Mol Cell Biol.
15:3805–3812. 1995.PubMed/NCBI
|
|
20
|
Funato Y, Terabayashi T, Suenaga N, Seiki
M, Takenawa T and Miki H: IRSp53/Eps8 complex is important for
positive regulation of Rac and cancer cell motility/invasiveness.
Cancer Res. 64:5237–5244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Teh MT, Ji Y, et al: EPS8
upregulates FOXM1 expression, enhancing cell growth and motility.
Carcinogenesis. 31:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terme M, Tomasello E, Maruyama K, et al:
IL-4 confers NK stimulatory capacity to murine dendritic cells: a
signaling pathway involving KARAP/DAP12-triggering receptor
expressed on myeloid cell 2 molecules. J Immunol. 172:5957–5966.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matheu MP, Sen D, Cahalan MD and Parker I:
Generation of bone marrow derived murine dendritic cells for use in
2-photon imaging. J Vis Exp. 17:773–776. 2008.PubMed/NCBI
|
|
24
|
Liu J, Schmidt CS, Zhao F, et al:
LIGHT-deficiency impairs CD8+ T cell expansion, but not effector
function. Int Immunol. 5:861–870. 2003.PubMed/NCBI
|
|
25
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HJ, Ahn KJ, Ahn SD, et al:
Susceptibility of cancer cells to beta-lapachone is enhanced by
ionizing radiation. Int J Radiat Oncol Biol Phys. 61:212–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Märten A, Renoth S, von Lilienfeld-Toal M,
et al: Enhanced lytic activity of cytokine-induced killer cells
against multiple myeloma cells after co-culture with
idiotype-pulsed dendritic cells. Haematologica. 86:1029–1037.
2001.
|
|
28
|
Burke P, Schooler K and Wiley HS:
Regulation of epidermal growth factor receptor signaling by
endocytosis and intracellular trafficking. Mol Biol Cell.
12:1897–1910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Disis ML, Schiffman K, Guthrie K, et al:
Effect of dose on immune response in patients vaccinated with an
her-2/neu intracellular domain protein-based vaccine. J Clin Oncol.
22:1916–1925. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foy TM, Bannink J, Sutherland RA, et al:
Vaccination with Her-2/neu DNA or protein subunits protects against
growth of a Her-2/neu expressing murine tumor. Vaccine.
19:2598–2606. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
32
|
Santegoets SJ, van den Eertwegh AJ, van de
Loosdrecht AA, Scheper RJ and de Gruijl TD: Human dendritic cell
line models for DC differentiation and clinical DC vaccination
studies. J Leukoc Biol. 84:1364–1373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morse MA, Chui S, Hobeika A, Lyerly HK and
Clay T: Recent developments in therapeutic cancer vaccines. Nat
Clin Pract Oncol. 2:108–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishikawa H, Kato T, Hirayama M, et al:
Regulatory T Cell-resistant CD8+ T cells induced by
glucocorticoid-induced tumor necrosis factor receptor signaling.
Cancer Res. 68:5948–5954. 2008.PubMed/NCBI
|
|
35
|
Akbari A and Rezaei A: In vitro selective
depletion of CD4(+)CD25(+) regulatory T-cells from PBMC using
anti-tac-SAP. J Immunotoxicol. 9:368–373. 2012.
|
|
36
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banerjee DK, Dhodapkar MV, Matayeva E,
Steinman RM and Dhodapkar KM: Expansion of FOXP3high regulatory T
cells by human dendritic cells (DCs) in vitro and after injection
of cytokine-matured DCs in myeloma patients. Blood. 108:2655–2661.
2006. View Article : Google Scholar : PubMed/NCBI
|