Introduction
Intestinal ischemia-reperfusion injury (IIRI) plays
a critical role in the pathophysiology of numerous conditions,
including mesenteric arterial occlusion, shock, cardiopulmonary
bypass, trauma, liver transplantation and small bowel
transplantation. IIRI is one of the major causes leading to
systemic inflammatory response syndrome (SIRS) and multiple organ
failure (MOF), which are correlated with high mortality rates of
32.1–90% (1–4). A number of factors may contribute to
IIRI and the mechanisms by which intestinal ischemia induces
reperfusion injury are extremely complicated (5–7).
Mast cells are widely distributed in intestinal mucosa and are
known as intestinal mucosal mast cells (IMMCs). Andoh et
al(8) used Ws/Ws rats to
investigate the role of mucosal mast cells (MMCs) in the
development of IIRI, and results of those authors revealed that the
damage was greatly attenuated in mast cell-deficient rats. Kalia
et al(9) also reported that
ketotifen (mast cell stabilizer) inhibits
ischemia-reperfusion-induced leukocyte adhesion and prevents local
and remote organs from damage in rats subjected to intestinal
ischemia-reperfusion (IIR). Moreover, results of our previous study
(10) demonstrated that cromolyn
sodium (mast cell stabilizer) and ketotifen markedly increase the
survival rates at 3 days after IIR in rats, and also alleviate
local and remote organ injury. The findings strongly suggest that
mast cells play a key role in IIRI.
Previous studies (10,11)
have shown that IIR leads to changes in IMMC counts. Boros et
al(11) investigated the
changes of intestinal mast cells in rats following 15, 30, or 60
min ischemia and 30 min reperfusion, respectively, and the results
clearly showed that mast cell counts are in part implicated in the
severity of intestinal injury. However, those studies (10,11)
only focused on one end point in rats subjected to small intestinal
ischemia, and the changes of IMMC counts and their function at
various time points during reperfusion after intestinal ischemia
are poorly understood. Furthermore, the correlation between IIRI
and mast cell counts is also not well documented. It is well known
that the histological changes of the intestine induced by IIRI
begin to recover at 6 h after reperfusion in rats subjected to 60
min ischemia that were suffering from severe damage at 3 h
(12,13). Nevertheless, those studies did not
investigate the changes of mast cell counts and mast cell
activation in the process of IIRI. Therefore, it is imperative to
elucidate the correlations and changes in mast cell counts and
activation and intestinal injury in order to improve treatment of
IIRI. In the present study, we observed the number and activation
of IMMCs within 12 h of IIR.
Materials and methods
Animals and experimental groups
Thirty-five male Kunming mice weighing 18–24 g
(provided by Experimental Animal Center of Guangdong Province,
China) were used in this study. The experiments were approved by
the Institutional Animal Care and Use Committee of Sun Yat-Sen
University. The animals were housed with standard chow and free
access to water and were subjected to a 12-h light-dark cycle (8:00
a.m.–8:00 p.m. light). Mice were randomly divided into five groups:
baseline, IIR 1 h (intestinal ischemia for 30 min followed by 1 h
reperfusion), IIR 3 h (intestinal ischemia for 30 min followed by 3
h reperfusion), IIR 6 h (intestinal ischemia for 30 min followed by
6 h reperfusion) and IIR 12 h (intestinal ischemia for 30 min
followed by 12 h reperfusion) groups.
Experimental model of IRRI
Mice were fasted for 12 h and were anesthetized by
an intraperitoneal injection of 10% chloral hydrate (3.5 ml/kg).
Following anesthesia, the mice were fixed in a supine position, the
abdomen was incised and the superior mesenteric artery (SMA) was
confirmed and isolated. Mice in the IIR 1, 3, 6 and 12 h groups
suffered ischemia by occlusion of the SMA for 30 min and then the
clamp was released and the mice were maintained for 1, 3, 6 and 12
h, respectively. In the baseline group, the same surgery was
performed, with the exception of the clamping of the SMA, and the
animals were maintained for 1 h. The mice were injected
subcutaneously with 0.1 ml physiological saline after the clamp was
released. Following completion of the experiments, the mice were
sacrificed and the intestinal tissues were obtained for further
study. The animals were maintained at 37°C by using a warm pad
during the procedure.
Histopathological examination of
intestine
A segment of 1.0 cm intestine (from 5 cm of the
terminal ileum) was harvested and fixed in 10% formaldehyde. The
small intestine tissues were paraffin-embedded and then stained
with hematoxylin and eosin for light microscopy. Intestinal mucosal
damage was evaluated by two pathologists, who were blinded
initially to the experiment, using the criteria of Chiu’s method
(14) as follows: Grade 0, normal
mucosa villi; Grade 1, development of subepithelial Gruenhagen’s
space at the tip of villus; Grade 2, extension of the subepithelial
space with moderate epithelial lifting; Grade 3, large epithelial
lifting, possibly with a few denuded villi; Grade 4, denuded villi
with lamina propria and exposed capillaries; Grade 5,
disintegration of the lamina propria, ulceration and
hemorrhage.
Immunohistochemical detection of tryptase
in intestine and IMMC counts
Sections (5 μm) of small intestine were prepared
from paraffin-embedded tissue according to previous instructions
(9,10), with minor revisions. Briefly,
endogenous peroxidase was quenched with 3%
H2O2 in deionized water for 10 min after
deparaffinization. Non-specific binding sites were blocked by
incubating the sections in 10% normal rabbit serum for 1 h. The
sections were then incubated with polyclonal rat anti-mast cell
tryptase (dilution 1:2,000) at 37°C for 20 min, followed by
incubation with biotinylated mouse-anti-rat IgG for 10–15 min at
room temperature. The horseradish peroxidase-conjugated
streptavidin solution was added and incubated for 10–15 min at room
temperature after three 5 min PBS rinses. The antibody binding
sites were visualized by incubation with a
diaminobenzidine-H2O2 solution. Sections
incubated with PBS instead of the primary antibody were used as
negative controls. Brown-yellow granules in the cytoplasm were
identified as positive staining for tryptase. The counts of
tryptase-positive mast cells were calculated in five randomly
selected areas by Image-Pro Plus 5.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) software at a ×400 magnification (15).
Western blot analysis of intestinal
MCP7
Total proteins were extracted from frozen intestine
tissues using protein extraction kits for MCP7 measurement (KenGen
Biotech Company, Nanjing, China). Protein concentration was
measured by BCA Protein Assay reagent kit (KenGen Biotech Company).
Protein (60 μg) was loaded onto a 4–20% SDS-PAGE premade gel
(Invitrogen, Carlsbad, CA, USA) for polyacrylamide gel
electrophoresis and then transferred to a polyvinylidene fluoride
(PVDF) membrane pretreated with 100% methanol. Membranes loaded
with proteins of interest were incubated with 5% skimmed milk, and
then rat monoclonal anti-MCP7 antibody (1:500 dilution, Santa Cruz,
USA) was added to the supernatant and the mixture was incubated on
a rotating wheel at 4°C overnight. On the second day, membranes
were washed with TBST three times and incubated with a second
antibody conjugated to horseradish peroxidase (1:2,000 dilution,
Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room
temperature. Immunoblots were washed and then were incubated with
an enhanced chemiluminescence detection system (KeyGen Biotech).
After exposure to hyperfilm-ECL, the membranes were stripped and
reprobed with β-actin antibody (1:2,000 dilution, Santa Cruz
Biotechnology). Densitometry was analyzed using NIH ImageJ software
(http://rsb.info.nih.gov/ij/index.html) and normalized
by β-actin immunoreactivity to correct sample differences (16).
Detection of histamine and TNF-α levels
in intestine
The other segment of intestine tissue was
homogenized with frozen normal saline, then centrifuged at 1,500 ×
g for 15 min. Supernatants were transferred into fresh tubes for
detection of histamine and TNF-α. Intestinal protein was determined
using a BCA Protein Assay Kit (KenGen Biotech Company). The
concentrations of histamine and TNF-α were measured using an
enzyme-linked immunosorbent assay (ELISA) kit (R&D systems
Inc., Minneapolis, MN, USA). The absorbance was read at 450 nm by a
Biokinetics microplate reader Model EL340 (Biotek Instruments,
Anaheim, CA, USA). The histamine and TNF-α levels were expressed as
ng/ml and pg/ml, respectively. The concentrations of histamine and
TNF-α in the intestine were calculated as ng/mg protein and pg/mg
protein, respectively.
Statistical analysis
Data were expressed as the means ± SD, and were
analyzed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA).
Repeated measurements were used for intra-group comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes of intestinal mucosa under light
microscopy and Chiu’s scores
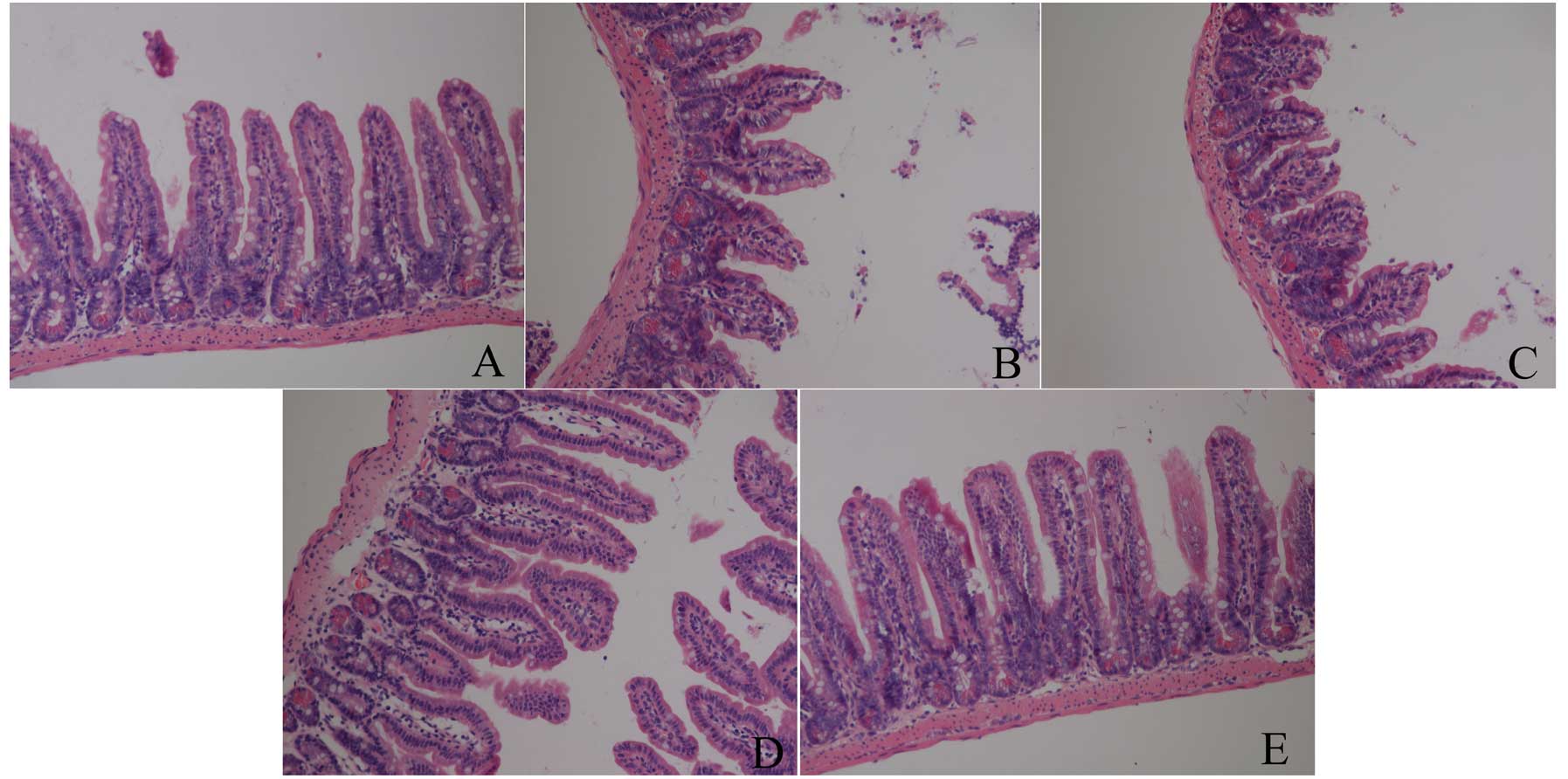
As shown in Fig. 1,
there was no damage in the baseline group, which showed normal
villus and glands. However, IIR induced intestinal structural
destruction, particularly at 3 h after reperfusion, in which all
the animals showed massive epithelial lifting down the sides of the
villi, accompanied with some denuded villi and lamina propria in
the IIR 1 h, IIR 3 h and IIR 6 h groups. Furthermore, the most
severe injury was assessed in the IIR 3 h group, in which
disintegration of the lamina propria and hemorrhage was observed.
Nevertheless, there was less damage in the IIR 12 h group, which
showed only an extension of the subepithelial space with lifting of
the epithelial layer in the intestine.
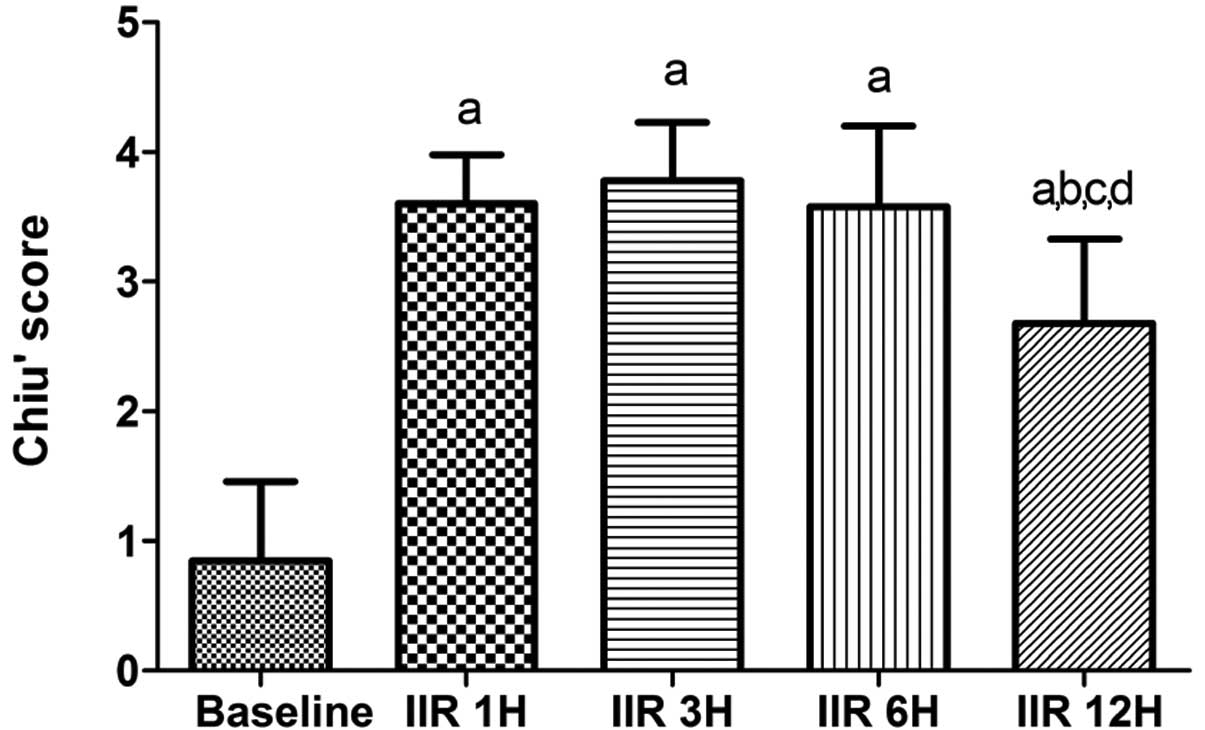
IIR led to marked increases in the Chiu’s scores 12
h after the clamp was released as compared with the baseline group
(all P<0.05 vs. baseline group). As shown in Fig. 2, the Chiu’s scores peaked at 3 h
after reperfusion and then decreased gradually, and the Chiu’s
scores were markedly lowered at 12 h after reperfusion as compared
with the IIR 1 h, IIR 3 h and IIR 6 h groups (P<0.05).
Immunohistochemical detection of tryptase
and IMMC counts in the intestine
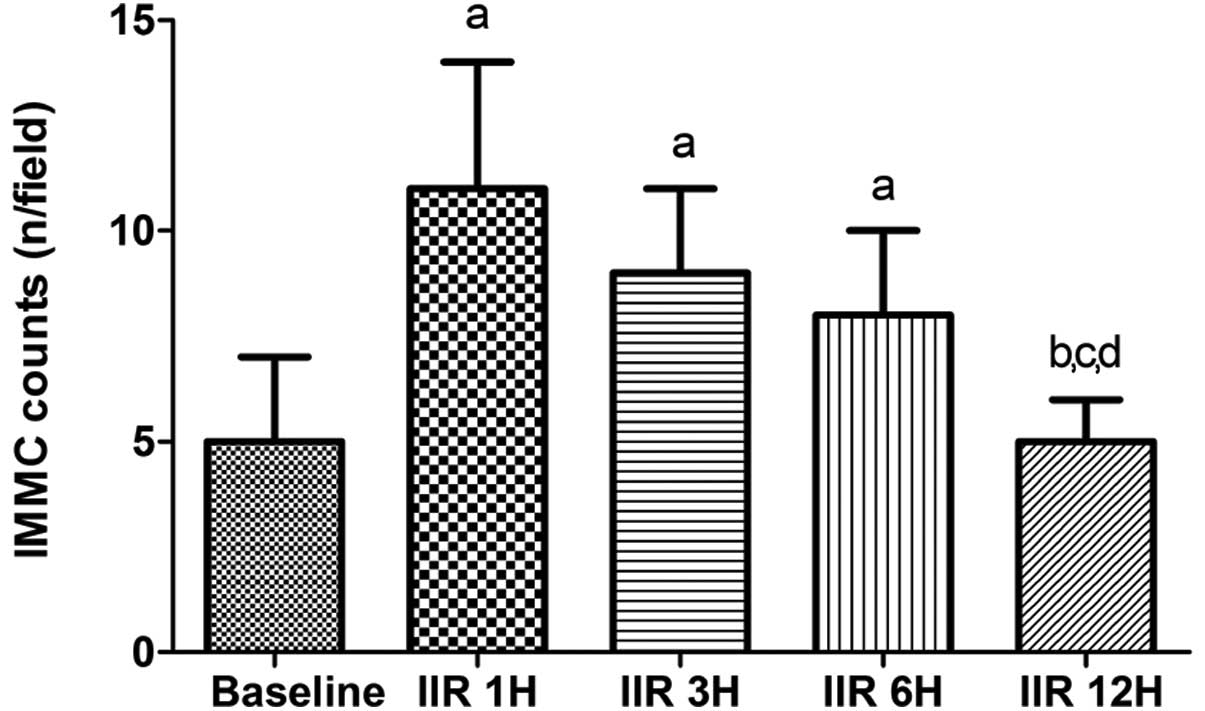
IIR induced the IMMC counts to increase
significantly up to 6 h after reperfusion, particularly at 1 h,
compared with the baseline group, and the counts were slightly
decreased by 6 h after reperfusion. The counts in the IIR 1 h, IIR
3 h and IIR 6 h groups were comparable, while the counts were
decreased to the baseline level at 12 h after reperfusion in the
IIR 12 h group (Fig. 3).
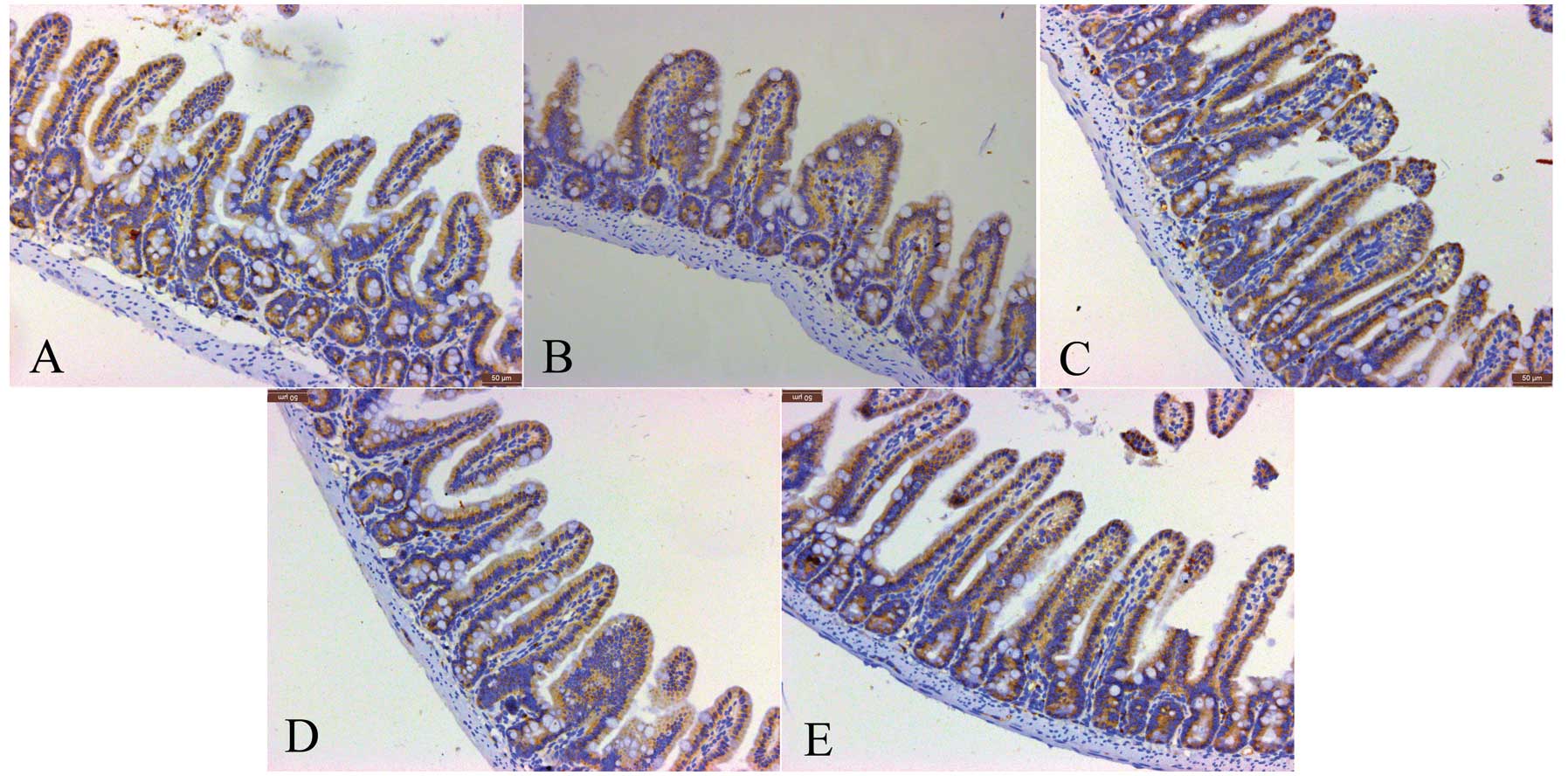
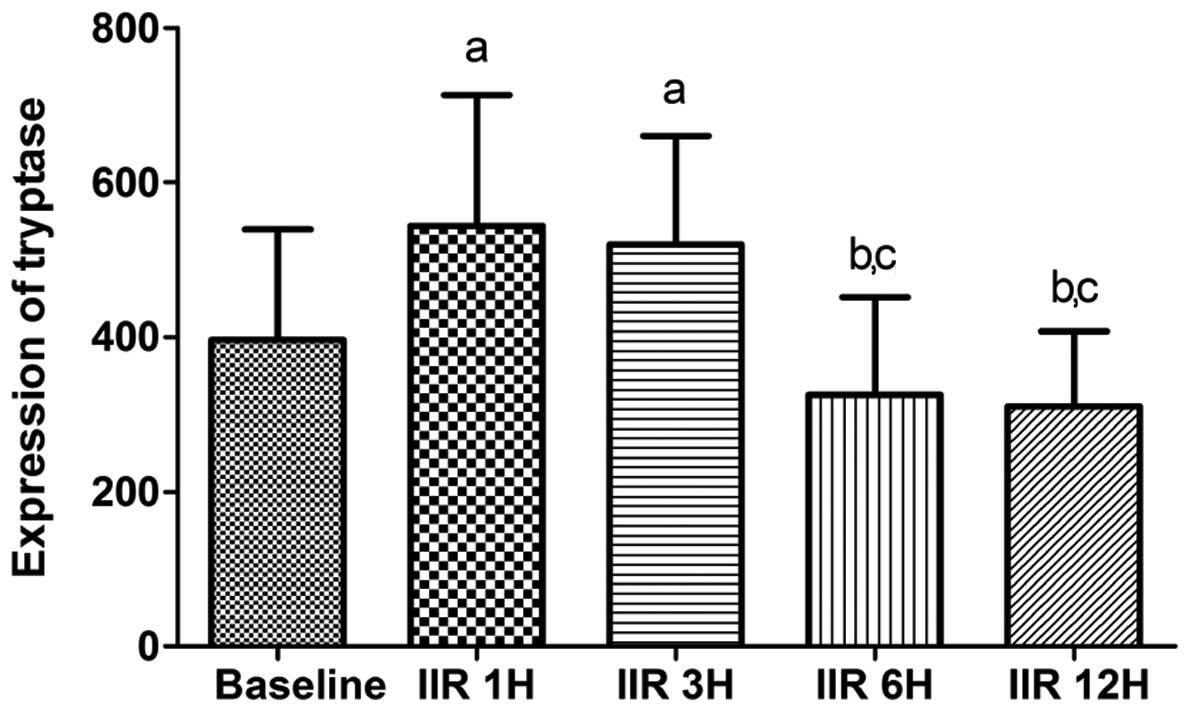
Consistent with the IMMC counts, the tryptase
protein expression in the IIR 1 h group was higher than that in the
baseline group, and was slightly but not significantly decreased in
the IIR 3 h and IIR 6 h groups. Furthermore, the tryptase protein
expression in the IIR 12 h group was markedly reduced compared with
the IIR 1 h, IIR 3 h and IIR 6 h groups, and was decreased to
baseline levels (Figs. 4 and
5).
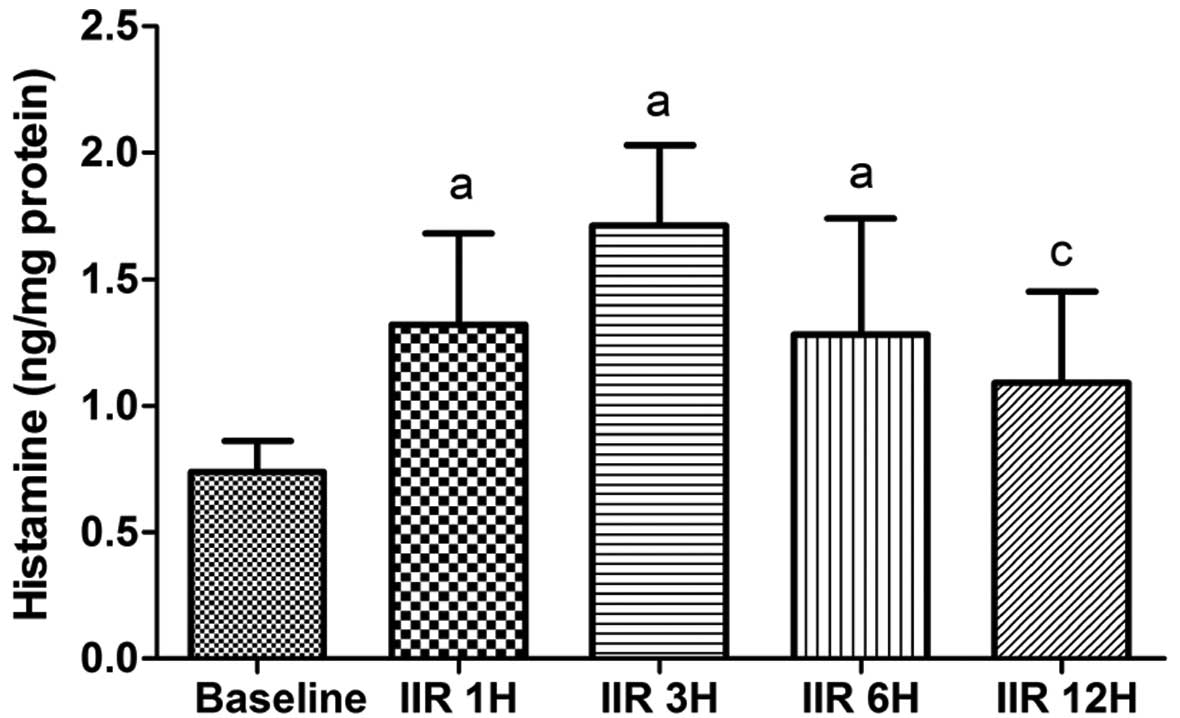
Histamine contents in the small
intestine
Compared with the baseline group, the histamine
contents in the small intestine were significantly higher in the
IIR 1 h, IIR 3 h and IIR 6 h groups (P<0.05). Moreover, the
histamine contents reached a maximum level at 3 h after
reperfusion, as shown in Fig. 5,
but this level was not significantly increased as compared with the
IIR 1 h and IIR 6 h groups. The histamine content in the IIR 12 h
group was significantly lower than that in the IIR 3 h group
(P<0.05; Fig. 6).
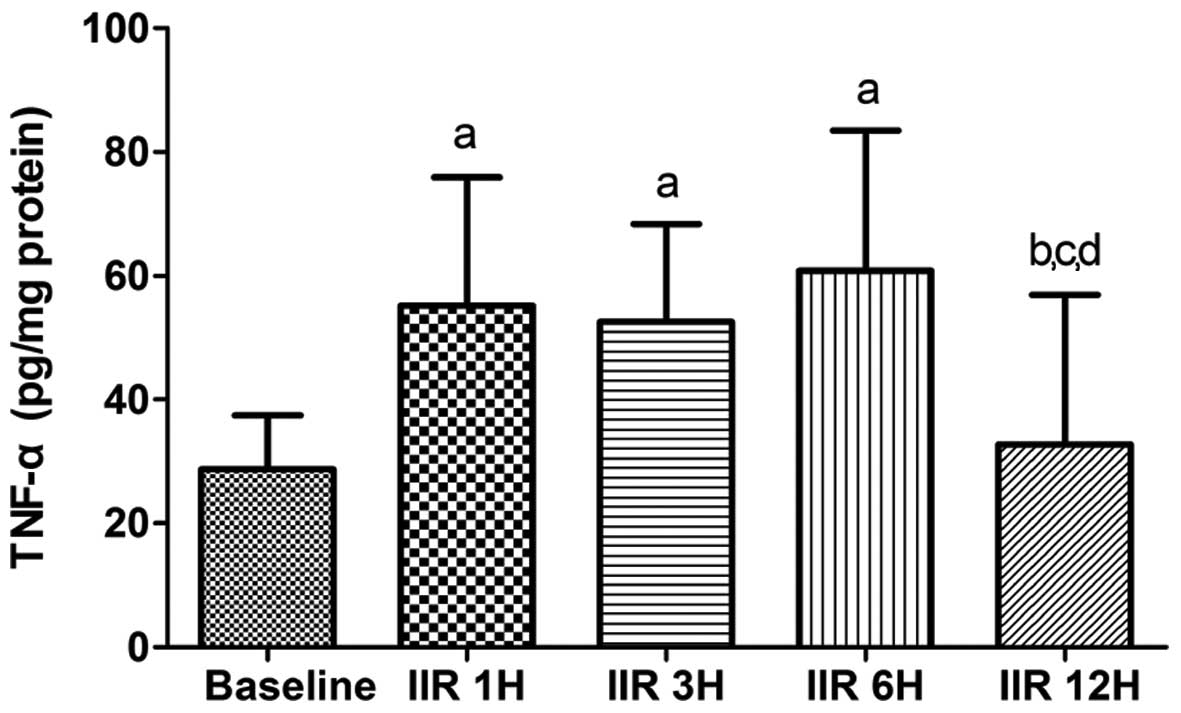
TNF-α levels in small intestine
As shown in Fig. 6,
IIR-induced TNF-α levels markedly increased in the IIR 1 h, IIR 3 h
and IIR 6 h groups as compared with the baseline group (P<0.05).
After reaching a plateau level, the contents of TNF-α in the
intestine were markedly decreased to the baseline level (Fig. 7).
Expression of MCP7 in intestine
MCP7 is a subtype of tryptase. As shown in Fig. 7, the expression of MCP7 was
gradually increased in the 6 h following the clamp release, and
reached maximum levels in the IIR 3 h and IIR 6 h groups as
compared to the baseline group. Notably, the expression of MCP7 was
markedly decreased to baseline levels at 12 h after reperfusion
(Fig. 8).
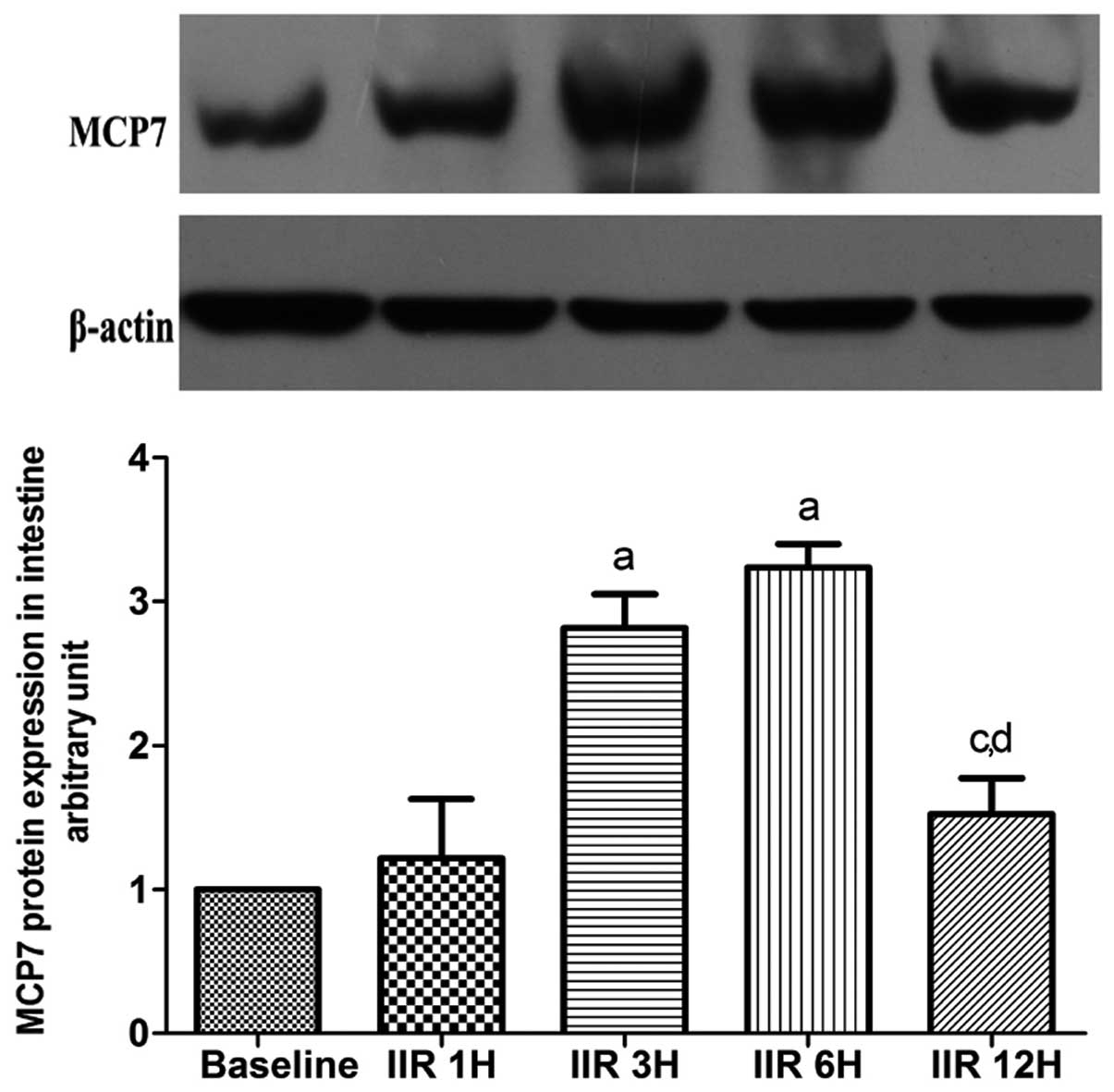 | Figure 8Western blot analysis of expression of
small intestine MCP7 (means ±SD, n=7). Baseline, baseline group;
IIR 1H, 1 h after reperfusion; IIR 3H, 3 h after reperfusion; IIR
6H, 6 h after reperfusion; IIR 12H, 12 h after reperfusion.
aP<0.05, compared with the baseline group;
bP<0.05, compared with the IIR 1H group;
cP<0.05, compared with the IIR 3H group;
dP<0.05, compared with IIR 6H group. |
Discussion
The damage to small intestinal mucosa and the
recovery after IIR depend on the duration of ischemia and the
animal species involved. In the present study, we observed that IIR
led to the greatest destruction of pathological structure at 6 h
after reperfusion. As shown in Fig.
1, massive collapsed epithelia and villi, as well as denuded
villi and lamina propria were observed, and the structure of
intestinal mucosa, as assessed by light microscopy, appeared to
recover to normal at 12 h after release of the clamp, which is in
agreement with the study of Chang et al(12), in which they reported that the
small intestinal mucosal injury in rats that were subjected to 60
min of hemorrhagic shock was severe at the onset after
resuscitation, and the structure of small intestinal mucosa began
to recover gradually at 6 h after resuscitation and almost normal
intestinal mucosa was observed at 24 h after reperfusion.
IMMCs, widely distributed in the intestinal lamina
propria, are adjacent to blood vessel and nerve fiber cells. IMMCs
interact with neuropeptides and cytokines through the immune and
neural pathways, which play an important role in regulating the
physical function of the gastrointestinal tract, as well as the
development of inflammatory bowel diseases (17,18).
A study has previously demonstrated that the ratio of IMMCs to
intestinal mucosal lamina propria cells is ~2–3% under normal
conditions. However, this ratio can increase by 10-fold in subjects
suffering from intestinal diseases (19). Several lines of evidence have
demonstrated that mast cells play a critical role in small IIRI by
using mast cell-deficient rats or by inhibiting mast cells
(8–10).
Mast cells are important pro-inflammatory cells.
Previous studies (20,21) have reported that the number of mast
cells were increased in lungs, intestines or other organs subjected
to ischemia-reperfusion injury. In the present study, the findings
showed that 30 min of ischemia followed by 1, 3 or 6 h of
reperfusion induced a significant increase in the number of IMMCs
by analyzing time-course changes of mast cell counts. Moreover, the
IMMC counts were higher at 1 h after reperfusion, and then the IMMC
counts gradually decreased to baseline at 12 h after reperfusion,
which is consistent with the Chiu’s scores, although severe
intestinal mucosal destruction was found at 6 h after initiation of
reperfusion.
Mast cells are generated in the bone marrow, and
then distributed to tissues and organs, and play various roles in
regulating physiological function (22). A previous study (23) has reported that bone marrow-derived
mast cells (BMMCs) migrate to the injury sites to serve their
function. MCP7 is a subtype of tryptase and is only expressed in
immature BMMCs (24). Accordingly,
the expression of MCP7 can partly identify the number of BMMCs that
have migrated to injured tissues (25). The findings in the present study
have shown that the MCP7 expression was substantially increased at
3 h after reperfusion, slowly decreased at 6 h and had almost
reached baseline level at 12 h after reperfusion. The findings
suggest that small intestinal ischemia-reperfusion may result in
BMMC migration to the injured intestine tissue, which further
aggravates small intestine injury by releasing numerous chemokines
and cytokines.
Levels of histamine and tryptase are characteristic
markers of mast cell activation and degranulation. Previous studies
(26,27) have demonstrated that histamine and
tryptase, which are involved in tissue injury, was able to increase
microvascular permeability, induce inflammatory cell infiltration,
and amplify the effects of mast cells. Several studies (9,10,28)
have confirmed that the application of mast cell membrane
stabilizers or antihistamines were capable of inhibiting mast cell
degranulation and attenuate IIRI. In the present study, we observed
that the levels of histamine and tryptase in the small intestine
were markedly and rapidly increased after IIR, and peaked within 3
h after reperfusion, and then gradually decreased to the baseline
level at 12 h. Furthermore, these changes were consistent with the
pathological damage. The results indicated that the best time to
target mast cells is at ~3 h after reperfusion.
Inflammatory reactions mediated by cytokines are one
of the main mechanisms of IIRI. Among the many mediators that
contribute to IIR, TNF-α is one of the key mediators and initiates
the cascade effect (29). A number
of cells, such as endothelial cells as well as inflammatory cells,
are capable of producing TNF-α when they are subjected to injury.
Mast cells can also produce and release TNF-α. Furthermore,
Bischoff et al(30)
speculated that the concentration of TNF-α in the small intestine
is largely from IMMC degranulation. The findings in the present
study showed that the levels of TNF-α in intestine were greatly
increased at 6 h after reperfusion, and then decreased gradually at
12 h after reperfusion. Of note, the results were in agreement with
the changes of mast cell counts and intestinal injury scores.
Therefore, the results suggest that mast cell degranulation leads
to small intestinal ischemia reperfusion injury.
In conclusion, small intestinal ischemia reperfusion
results in substantial increases in the mast cell counts within 6 h
after reperfusion, which contributes to the small intestine mucosal
destruction by degranulation.
Acknowledgements
The study was in part supported by the National
Natural Science Foundation of China (NSFC), 30901408 and
30972858.
References
|
1
|
Schoots IG, Koffeman GI, Legemate DA, Levi
M and van Gulik TM: Systematic review of survival after acute
mesenteric ischaemia according to disease aetiology. Br J Surg.
91:17–27. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Acosta-Merida MA, Marchena-Gomez J,
Hemmersbach-Miller M, Roque-Castellano C and Hernandez-Romero JM:
Identification of risk factors for perioperative mortality in acute
mesenteric ischemia. World J Surg. 30:1579–1585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pierro A and Eaton S: Intestinal ischemia
reperfusion injury and multisystem organ failure. Semin Pediatr
Surg. 13:11–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kassahun WT, Schulz T, Richter O and Hauss
J: Unchanged high mortality rates from acute occlusive intestinal
ischemia: six year review. Langenbecks Arch Surg. 393:163–171.
2008.PubMed/NCBI
|
|
5
|
Cerqueira NF, Hussni CA and Yoshida WB:
Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta
Cir Bras. 20:336–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haddad JJ: Antioxidant and prooxidant
mechanisms in the regulation of redox(y)-sensitive transcription
factors. Cell Signal. 14:879–897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vollmar B and Menger MD: Intestinal
ischemia/reperfusion: microcirculatory pathology and functional
consequences. Langenbecks Arch Surg. 396:13–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andoh A, Kimura T, Fukuda M, Araki Y,
Fujiyama Y and Bamba T: Rapid intestinal ischaemia-reperfusion
injury is suppressed in genetically mast cell-deficient Ws/Ws rats.
Clin Exp Immunol. 116:90–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalia N, Brown NJ, Wood RF and Pockley AG:
Ketotifen abrogates local and systemic consequences of rat
intestinal ischemia-reperfusion injury. J Gastroenterol Hepatol.
20:1032–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hei ZQ, Gan XL, Huang PJ, Wei J, Shen N
and Gao WL: Influence of ketotifen, cromolyn sodium, and compound
48/80 on the survival rates after intestinal ischemia reperfusion
injury in rats. BMC Gastroenterol. 8:422008. View Article : Google Scholar
|
|
11
|
Boros M, Takaichi S, Masuda J, Newlands GF
and Hatanaka K: Response of mucosal mast cells to intestinal
ischemia-reperfusion injury in the rat. Shock. 3:125–131. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JX, Chen S, Ma LP, et al: Functional
and morphological changes of the gut barrier during the restitution
process after hemorrhagic shock. World J Gastroenterol.
11:5485–5491. 2005.
|
|
13
|
Noda T, Iwakiri R, Fujimoto K, Matsuo S
and Aw TY: Programmed cell death induced by Ischemia-reperfusion in
rat intestinal mucosa. Am J Physiol. 274(2 Pt 1): G270–G276.
1998.PubMed/NCBI
|
|
14
|
Chiu CJ, Mcardle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low flow states. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thakurdas SM, Melicoff E, Sanscores-Garcia
L, Moreira DC, Petrova Y, Stevens RL and Adachi R: The mast
cell-restricted tryptase mMCP-6 has a critical immunoprotective
role in bacterial infections. J Biol Chem. 282:20809–20815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kemna E, Pickkers P, Nemeth E, van der
Hoeven H and Swinkels D: Time-course analysis of hepcidin, serum
iron, and plasma cytokine levels in humans injected with LPS.
Blood. 106:1864–1866. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bischoff SC: Physiological and
pathophysiological functions of intestinal mast cells. Semin
Immunopathol. 31:185–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wierzbicki M and Brzezińska-Błaszczyk E:
The role of mast cells in the development of inflammatory bowel
diseases. Postepy Hig Med Dosw (Online). 62:642–650.
2008.PubMed/NCBI
|
|
19
|
Bischoff SC and Kramer S: Human mast
cells, bacteria, and intestinal immunity. Immunol Rev. 217:329–337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hei ZQ, Gan XL, Luo GJ, Li SR and Cai J:
Pretreatment of cromolyn sodium prior to reperfusion attenuates
early reperfusion injury after the small intestine ischemia in
rats. World J Gastroenterol. 13:5139–5146. 2007.PubMed/NCBI
|
|
21
|
Lindsberg PJ, Strbian D and
Karjalainen-Lindsberg ML: Mast cells as early responders in the
regulation of acute blood-brain barrier changes after cerebral
ischemia and hemorrhage. J Cereb Blood Flow Metab. 30:689–702.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morii E: Development of mast cells:
analysis with mutant mice. Int J Hematol. 86:22–26. 2007.
View Article : Google Scholar
|
|
23
|
Okayama Y and Kawakami T: Development,
migration, and survival of mast cells. Immunol Res. 34:97–115.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McNeil HP, Reynolds DS, Schiller V, et al:
Isolation, characterization, and transcription of the gene encoding
mouse mast cell protease 7. Proc Natl Acad Sci USA. 89:11174–11178.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Funaba M, Ikeda T, Murakami M, et al:
Transcriptional activation of mouse mast cell protease-7 by activin
and transforming growth factor-beta is inhibited by
microphthalmia-associated transcription factor. J Biol Chem.
278:52032–52041. 2003. View Article : Google Scholar
|
|
26
|
Caughey GH: Mast cell tryptases and
chymases in inflammation and host defense. Immunol Rev.
217:141–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He S, Gaca MD and Walls AF: A role for
tryptase in the activation of human mast cells: modulation of
histamine release by tryptase and inhibitors of tryptase. J
Pharmacol Exp Ther. 286:289–297. 1998.PubMed/NCBI
|
|
28
|
Gan XL, Hei ZQ, Huang HQ, Chen LX, Li SR
and Cai J: Effect of Astragalus membranaceus injection on the
activity of the intestinal muscosal mast cells after hemorrhagic
shock-reperfusion in rats. Chin Med J. 119:1892–1898.
2006.PubMed/NCBI
|
|
29
|
Pascher A and Klupp J: Biologics in the
treatment of transplant rejection and ischemia/reperfusion injury:
new applications for TNFalpha inhibitors? BioDrugs. 19:211–231.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bischoff SC, Lorentz A, Schwengberg S,
Weier G, Raab R and Manns MP: Mast cells are an important cellular
source of tumour necrosis factor alpha in human intestinal tissue.
Gut. 44:643–652. 1999.PubMed/NCBI
|