Introduction
Parkinson’s disease (PD), a progressive movement
disorder, is one of the most common neurodegenerative disorders
worldwide (1). The predominant
pathological features of PD are a loss of the dopaminergic (DA)
neurons in the substantia nigra and striatum (2–5).
Thus, stem cells may offer an alternative source of novel cells for
patients with PD. It is hypothesized that the introduction of stem
cells into the brain may delay the onset or progression of PD
(6). However, the quantity of
fetal tissue available is insufficient to treat the large number of
patients with PD, and the use of neurons from fetal sources raises
ethical questions. Currently, iPS cells produced from the cells of
patients with Parkinson’s disease are being utilized to produce
diseased neurons in the laboratory, in order to determine the
mechanisms of PD and to test potential therapeutic agents (7). However, whether agents with
therapeutic potential in the PD models would be beneficial in
patients with PD has not yet been elucidated. Furthermore, with
regard to the transplantation of healthy cells into the brains of
patients with PD, further studies are required to ensure the cells
are safe. In addition, further investigation is required to improve
the effectiveness of the transplants, minimize the side-effects,
determine the mechanism of the disease and demonstrate how the
cells may aid in the development of novel therapeutic agents.
Previously, cancer studies have focused on
traditional medicinal plants to discover novel therapeutic agents
with minimal side-effects. The use of medicinal herbs has a long
history in Asia and is commonly utilized in the treatment of
various neurological diseases, including stroke and epilepsy
(8–10). According to ancient Chinese medical
literature, Tianma (Gastrodia elata Blume, Orchidaceae) is a
herbal medicine for the the treatment of PD. The dry tuber of
Tianma is officially listed in the Chinese Pharmacopoeia and is
utilized in the treatment of headaches, dizziness, tetanus,
epilepsy, infantile convulsions and numbness of the limbs (11). Recently, gastrodin, the predominant
and bioactive component of Tianma, has been demonstrated to inhibit
neuroinflammation in a PD model in rats (12).
A mitochondrial complex I inhibitor, rotenone, led
to the selective death of DA neurons and Parkinsonism in rodents
(13,14). This PD model is superior for use in
the present study on the effects of gastrodin on PD. Accumulating
data have indicated the importance of astrocytes in Parkinsonism
(15–17). It has also been demonstrated that
several connexins are expressed in neurons and astrocytes, and
these may be involved in the release of ATP and glutamate (18–21).
In addition, astrocytes have been shown to be involved in
neurological disorders, including PD (15,16),
and astrocyte gap junctions may be formed of multiple connexins
(22). The metabolic and ionic
coupling provided by these diverse types of gap junctions may
provide intercellular signaling required for brain development and
cortical lamination (19).
Furthermore, astrocytes in PD are demonstrated to upregulate the
expression of gap junction connexin 43 (Cx43) genes (23).
Gastrodin may inhibit Cx43 expression in the
temporal lobe and hippocampus, inhibit the formation of abnormal
gap junctions and achieve anti-epileptic formation with the
suppression of aberrant new cell formation (24). The aim of the present study was to
determine whether gastrodin prevents PD via its effect on the
expression of Cx43. Thus, following gastrodin treatment, the
changes in astrocyte gap junctional intercellular communication
(GJIC) and Cx43, and the phosphorylation status of Cx43 were
determined in a rat model of PD (induced by chronic exposure to
rotenone) and in cultured astrocytes stimulated with rotenone. This
model has been previously utilized to investigate the etiology of
Parkinsonism (13,25,26)
and will aid in the study of the function of gastrodin in treating
PD.
Materials and methods
Drugs and chemicals
Gastrodin injections were purchased from Nanchong
Central Hospital (Nanchong, China). Rotenone and dimethylsulfoxide
(DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rotenone was dissolved in DMSO and stored at −20°C.
Lewis rats
Lewis rats (weight, 200–250 g) were purchased from
the Shanghai Laboratory Animal Centre (Chinese Academy of Sciences,
Shanghai, China) and maintained in specific pathogen-free
conditions. The rats were acclimated and maintained at 23°C under a
12-h light/dark cycle (lights on, 08:00–20:00). Rats were housed in
standard laboratory cages with free access to food and water. The
rats were randomly divided into experimental (n=6) and control
(n=12) groups. The experimental group subcutaneously received
rotenone and gastrodin (2.5 and 5.0 mg/kg, respectively, in
Panacet) and the control group received Panacet only. All
procedures were conducted in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (27) and were approved by
the animal care committee of China Medical University (Shenyang,
China).
Primary astrocyte cultures
Primary astrocytes were prepared from the brains of
neonatal Wistar rats (age, 1–2 days) (28), which were purchased from Shanghai
SLAC Laboratory Animal Co. (Shanghai, China). Briefly, the brains
were digested with 0.05% trypsin-EDTA at 37°C for 10 min,
dissociated by gentle pipetting and passed through a 100-μm-pore
nylon mesh. Cells were plated onto 75-cm2 plastic flasks
and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented
with 10% v/v fetal bovine serum and 1% penicillin/streptomycin, at
37°C in a humidified 5% CO2 atmosphere. The medium was
changed every three days. Cells were harvested when they reached
80% confluence and seeded into a secondary culture. The purity of
the primary astrocyte cultures was determined by immunocytochemical
staining using an antibody against an astrocyte-specific marker,
glial fibrillary acidic protein (GFAP; dilution, 1:1000; product
number G 3893, Sigma) or a microglia-specific marker (anti-CD11b;
dilution, 1:200; Serotec, Oxford, UK). At 30 days in vitro,
99% of the primary cultured cells were GFAP-positive and no
detectable CD11b-positive cells (microglia) were identified
(29). Cultured astrocytes were
treated with rotenone (8 nM) and gastrodin (10 or 20 nM; molecular
formula, C13H18O7; molecular
weight, 286.25), or with 8 nM rotenone only for 2 days.
Fluorescence recovery after
photobleaching (FRAP) assay for GJIC
The quantitative FRAP assay for GJIC was performed
as previously described (23),
using a laser-scanning confocal microscope (LSCM, Olympus Fluoview
FV300; Olympus, Ltd., Beijing, China). Following the bleaching of
randomly selected cells with a micro-laser beam, the rate of
transfer of 5,6-carboxyfluoresceindiacetate (Sigma-Aldrich) from
adjacent labeled cells back into the bleached cells was calculated.
The recovery of fluorescence was examined after 0.5 min and the
recovery rate (RR) was calculated as the percentage of
photobleached fluorescence/min. The RR was adjusted for the loss of
fluorescence measured in unbleached cells and the results are
expressed as the fold increase in the RR compared with that of the
untreated control cells (23).
Extraction of Cx43 RNA and the
quantification of Cx43 mRNA
Cells were grown in 6-cm cell culture dishes for ≥48
h. The cells were trypsinized and suspended in DMEM containing 10%
fetal calf serum. Total RNA was isolated from the cells using the
QIAshredder and RNeasy mini kits (Qiagen, Inc., Almeda, CA, USA).
The initial strand of cDNA was synthesized from 500 ng RNA extracts
in a volume of 20 μl using AMV reverse transcriptase XL (Takara
Biotechnology Co., Ltd., Dalian, China) priming with random nonamer
primers (9-mers) at 42°C for 10 min. The cDNA strand was stored at
20°C until use. The expression of Cx43 mRNA was determined by qPCR.
PCR was performed in an ABI Prism 7900 sequence detector (Applied
Biosystems, Foster City, CA, USA) in a final volume of 20 μl. The
PCR mixture contained 10 mM Tris-HCl buffer (pH 8.3), 50 mM KCl,
1.5 mM MgCl2, 0.2 mM dNTP mixture, 0.5 units Ampli Taq
gold enzyme (Applied Biosystems) and 0.2 M primers. The primer and
probe sequences for gene amplification were as follows: Cx43
forward, 5′-ATCAGCATCCTCTTCAAGTCTGTCT-3′ and reverse,
5′-CAGGGATCTCTCTTGCAGGTGTA-3′ (22); and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′-CCCTTCATTGACCTCAACTAC-3′ and
reverse, 5′-CCACCTTCTTGATGTCATCAT-3′. GAPDH was used as an internal
control. The Ampli Taq gold enzyme was activated by heating for 10
min at 95°C, and all genes were amplified by 50 cycles of heating
for 15 sec at 95°C, followed by 1 min at 60°C.
For the construction of standard curves for the
positive controls, the total RNA of the primary astrocytes was
reverse transcribed into cDNA and serially diluted in water in five
or six log steps to achieve four-fold serial dilutions of cDNA from
~100 ng to 100 pg. These cDNA serial dilutions were stored at
−20°C. The coefficient of linear regression for each standard curve
was calculated, and the cycle threshold value of a sample was
substituted into the formula for each standard curve to calculate
the relative concentration of Cx43 or GAPDH. To normalize the
differences in the quantity of total RNA added to each reaction
mixture, GAPDH was used as an endogenous control. The data
represent the average expression of target genes relative to GAPDH,
from three independent cultures.
Western blot analysis
Cells and rat brains were lysed in ice-cold buffer
(50 mmol/l Tris-HCl, pH 7.4; 150 mmol/l NaCl; 1% [v/v] NP40; 5
mmol/l EDTA; 5% [v/v] glycerol; 10 μg/ml leupeptin; 10 μg/ml
aprotonin; 1 mmol/l phenylmethylsulfonyl fluoride and 1 mmol/l
Na3VO4) using a polytron. The lysates were
then sonicated, the samples were diluted 1:4 in water and their
protein concentrations were determined using the Bradford method
(30), with affinity-purified
bovine serum albumin as a standard. Samples (10 g) were dissolved
in Laemmli sample buffer (60 mM Tris-Cl pH 6.8, 2% SDS, 10%
glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue), separated
on 12% acrylamide gel and transferred to polyvinylidene fluoride
(PVDF) membranes. Blots were incubated with anti-Cx43 antibody
(Shengshizhongfang BioSci and Tech. Co., Ltd., Beijing, China)
overnight at 4°C, followed by three 15 min washes with
phosphate-buffered saline and 0.1% Triton X-100 (PBST). As an
internal control, to determine whether equal amounts of protein had
been loaded onto the gel, the PVDF membranes were stripped and
reprobed with anti-tubulin (T5168; Sigma-Aldrich). Blots were
incubated with goat anti-rabbit antibody conjugated horseradish
peroxidase (AP307P, Merck Millipore, Billerica, MA USA). The
immunoreactive bands were visualized by enhanced chemiluminescence
(ECL; GE Healthcare, Shanghai, China) and quantified by
densitometry with ImageJ 1.45 software (National Institutes of
Health, Bethesda, USA) according to the manufacturer’s
instructions.
Statistical analysis
The correlation between Cx43 levels and gastrodin
and rotenone treatment in the different groups was compared by a
one-way analysis of variance followed by post hoc analysis with a
protected Fisher’s least significant difference test. P<0.05
indicates a statistically significant difference.
Results
Gastrodin inhibits the rotenone-induced
levels of Cx43 expression in astrocytes
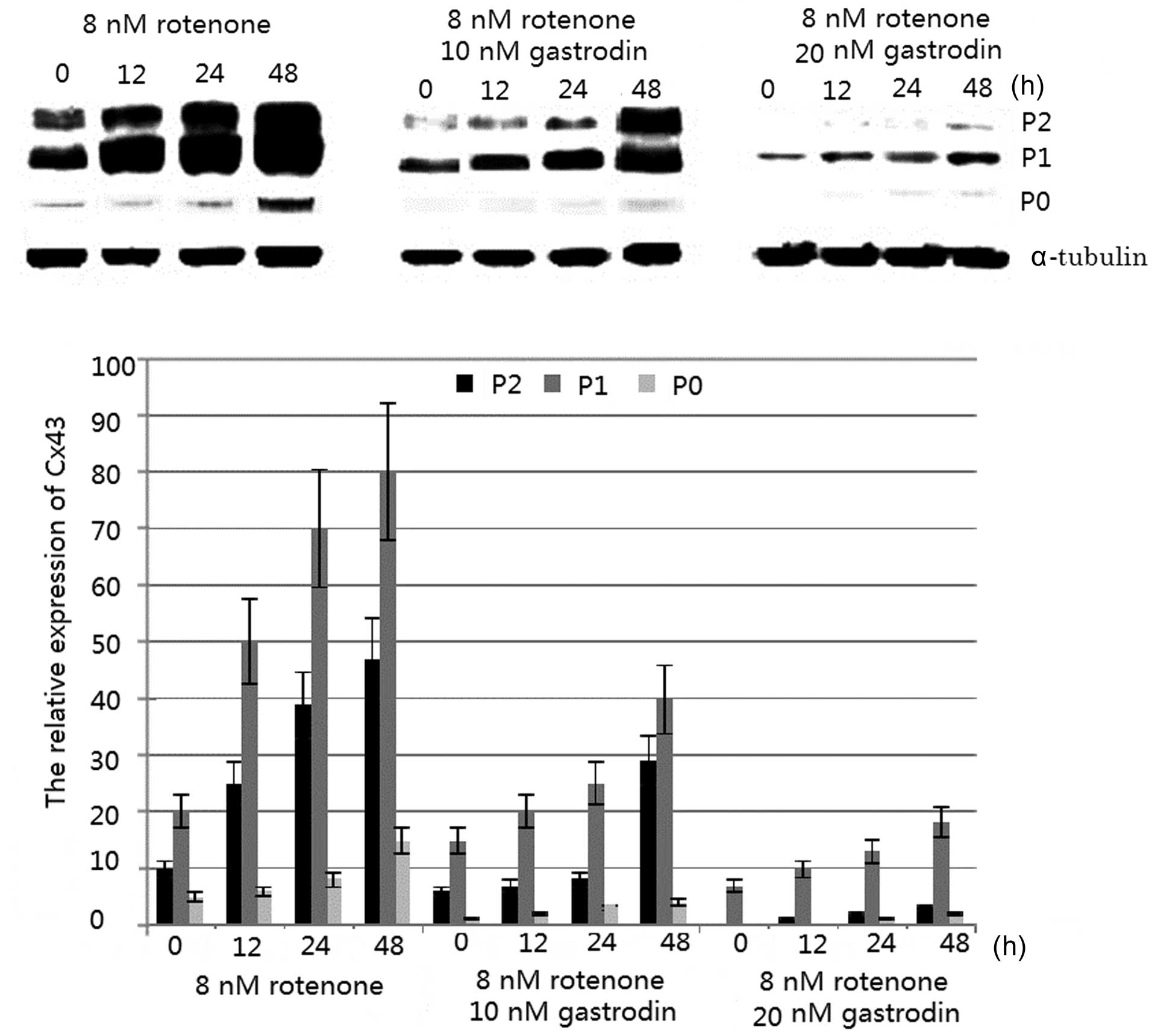
Western blot analysis demonstrated that three forms
of the Cx43 immunoreactive protein (Mr 40,000–43,000) were observed
in all samples; a fast-migrating band (non-phosphorylated form, P0;
Fig. 1) and two slower migrating
bands (phosphorylated forms, P1 and P2; Fig. 1). Phosphorylated Cx43 was observed
to localize at the plasma membrane and gap junctions (23). Densitometric analysis demonstrated
that rotenone induced a significant dose- and time-dependent
increase in phosphorylated Cx43 levels compared with that of the
control cells (23). The levels of
the non-phosphorylated form, P0, appeared to marginally change
(Fig. 1). The effect of rotenone
on Cx43 protein levels was also determined, and the phosphorylated
Cx43 level was demonstrated to be modulated by rotenone treatment.
The expression of phosphorylated Cx43 reached high levels when the
astrocytes were treated with 8 nm rotenone for 48 h (Fig. 1). However, the enhanced expression
level of phosphorylated Cx43 was inhibited by gastrodin. The
increased inhibitory rate was correlated with an increasing
concentration of gastrodin, and was greatest when 20 nM gastrodin
was added (Fig. 1).
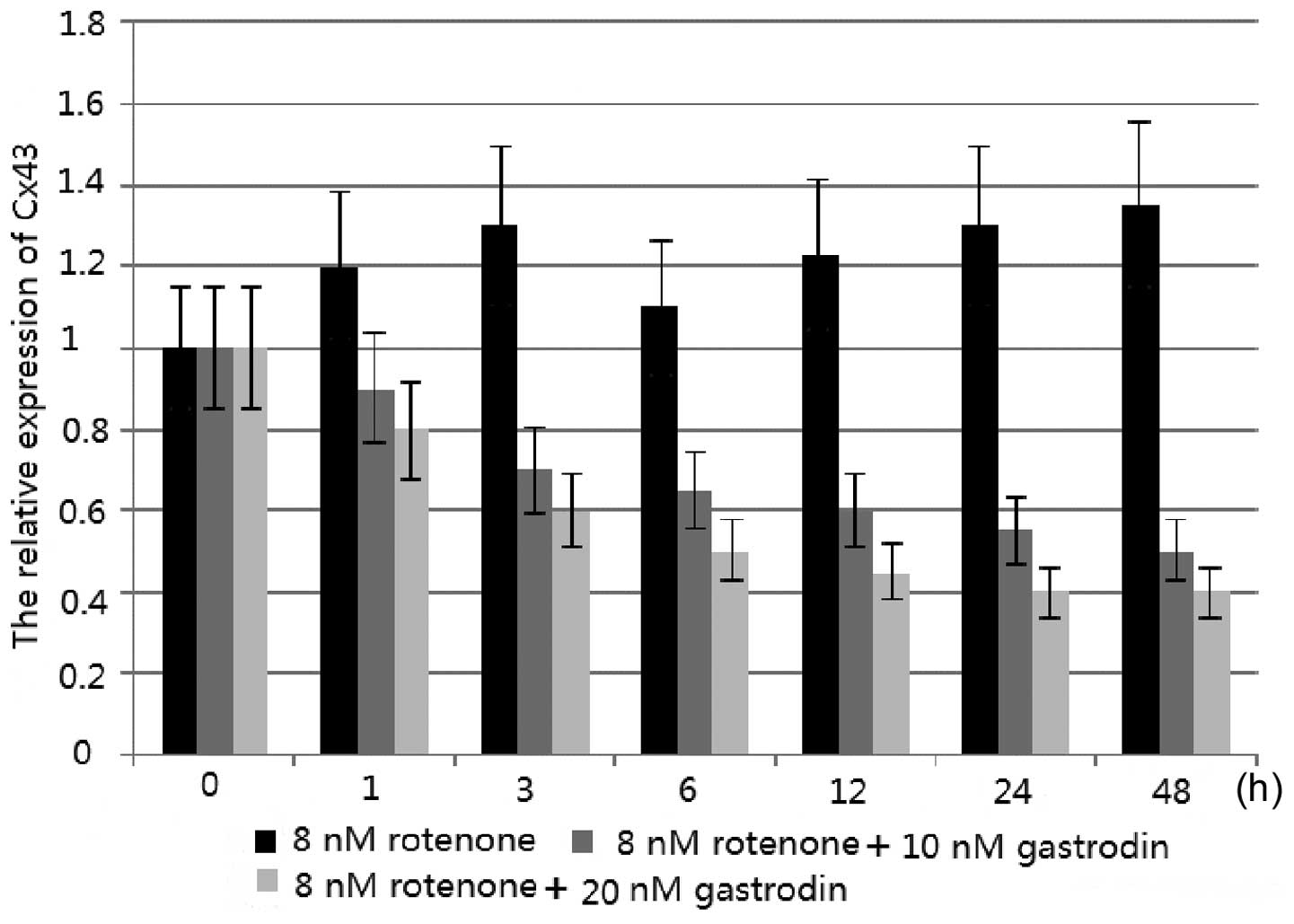
Quantification analysis also demonstrated that
gastrodin inhibited the rotenone-induced levels of Cx43 expression
in astrocytes. The effect of rotenone on Cx43 mRNA levels was also
investigated by qPCR. Rotenone treatment was observed to modulate
the Cx43 mRNA levels. Following the treatment of astrocytes with 8
nm rotenone for 48 h, the Cx43 mRNA levels increased (Fig. 2). The enhanced mRNA levels of Cx43
were shown to be inhibited by gastrodin. In addition, the increased
inhibitory rate was correlated with increasing concentrations of
gastrodin; levels of inhibition were greatest following the
addition of 20 nM gastrodin (Fig.
2).
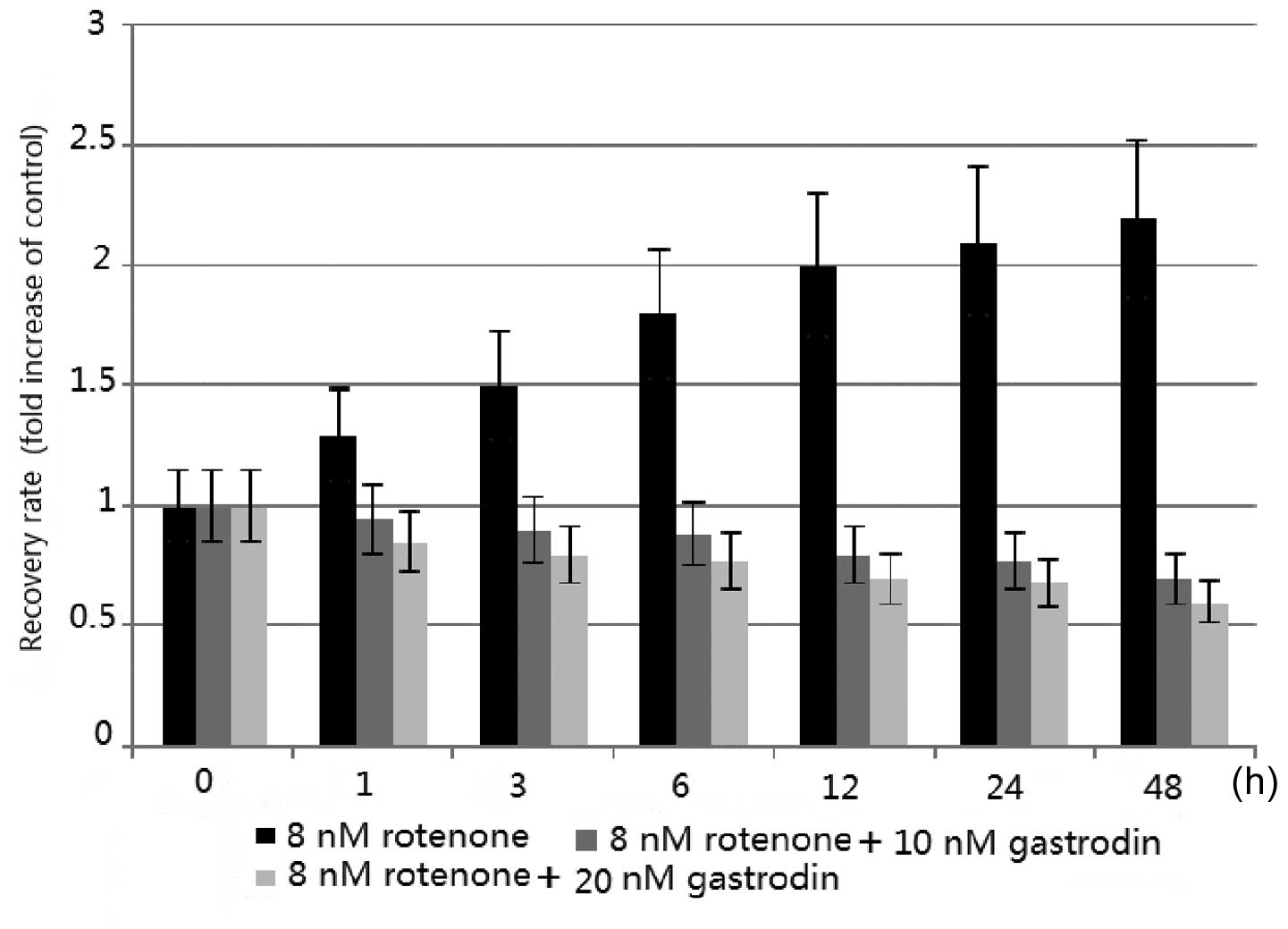
GJIC is upregulated by rotenone and
downregulated by gastrodin
The effect of rotenone and gastrodin on GJIC in
cultured astrocytes was observed. The GJIC was quantitatively
assessed in living cells by a FRAP assay, as previously described
(23), in terms of the RR.
Following photobleaching, sequential scans detected the recovery of
fluorescence in the bleached cells as the dye was transferred from
the surrounding non-bleached cells to the photobleached cells
through GJIC. The RR at 48 h of treatment showed a dose-dependent
increase up to 8 nM rotenone (23). In addition, time course analysis
showed a time-dependent increase in GJIC following rotenone
treatment (23). The quantity of
GJIC was consistent with the expression levels of phosphorylated
Cx43 induced by rotenone (Figs. 1
and 3). The results suggested that
rotenone treatment of cultured astrocytes generated increased
levels of phosphorylated proteins and a broadened membrane
distribution of Cx43, which in turn led to the enhancement of
GJIC.
By contrast, the concentration of gastrodin was
inversely correlated with the levels of GJIC. The data from
Fig. 1 suggested that gastrodin
treatment of cultured astrocytes generated reduced levels of
phosphorylated Cx43, and this in turn led to the reduction in GJIC
(Fig. 3). Thus, gastrodin may
prevent PD by inhibiting the phosphorylation of Cx43 and reducing
the expression of Cx43.
Gastrodin and rotenone demonstrate
antagonistic functions in a PD model
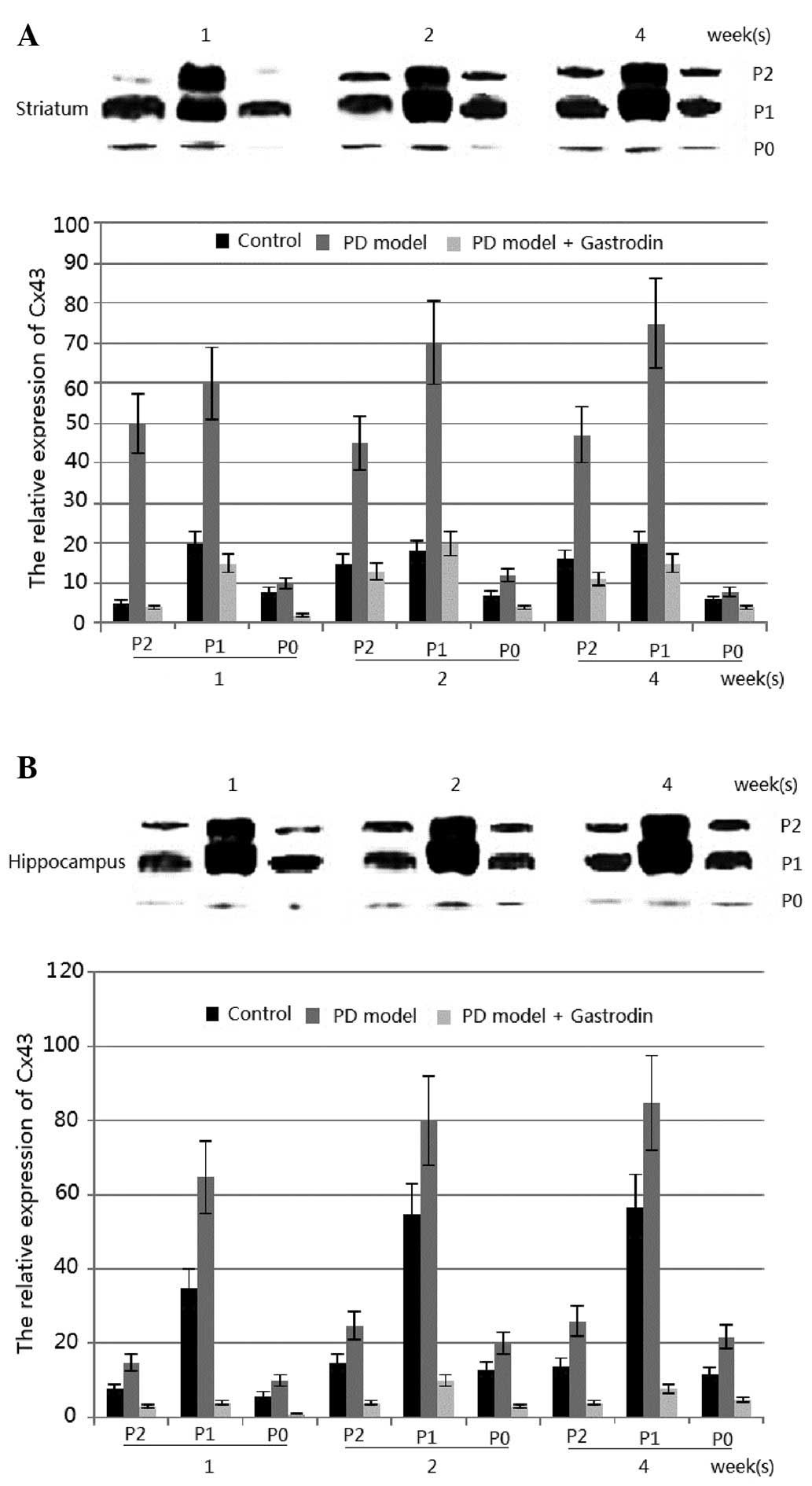
To investigate whether Cx43 levels may be altered in
Parkinsonism, the Cx43 protein level in the rotenone-induced model
of PD in rats was observed. In this model, Cx43 was identified in
all regions (although at different levels) and the Cx43 protein
level was significantly lower in the striatum and hippocampus than
in the other brain regions (data not shown) (23). The levels of phosphorylated Cx43
were markedly enhanced in the striatum of the treated group.
Significant differences in the total Cx43 levels were observed in
the striatum of rotenone-treated rats at 1, 2 and 4 weeks, as well
as in the hippocampus of rotenone-treated rats at those weeks
(P<0.01; Fig. 4). However, no
significant changes were observed in other regions (data not shown)
(23). The results from Fig. 4 suggested that treatment of the rat
model with gastrodin reduced the protein levels of phosphorylated
Cx43, which was lower than that of the control group
(P<0.01).
By contrast, the concentration of gastrodin was
inversely correlated with the expression of phosphorylated Cx43 in
the PD model induced by rotenone. The results from Fig. 4 suggested that gastrodin treatment
in the rat model generated reduced protein levels of phosphorylated
Cx43 (P<0.01), which may be less than those of the control group
(Fig. 4). Thus, gastrodin may be
used for the prevention of PD by inhibiting the phosphorylation of
Cx43 and reducing the expression of Cx43 in the PD model.
Discussion
Cx43 electrophoresis studies have identified three
forms of Cx43, non-phosphorylated Cx43 (P0) and two slower
migrating forms (commonly termed P1 and P2). The P1 and P2 isoforms
were found to be associated with gap junction structures (23). In the present study, rotenone
treatment induced an increase in Cx43 P1 and P2 levels in
astrocytes and PD models (Figs. 1
and 4), and the number of
localized foci of the total and phosphorylated Cx43 on the plasma
membrane was increased. Furthermore, astrocyte GJIC was increased
with rotenone treatment (Fig. 3).
These results are consistent with those of a previous study
(23). Figs. 1–4
suggest that all increases in Cx43 levels induced by rotenone were
inhibited by gastrodin. Thus, gastrodin may be used for the
prevention of PD, as it inhibited the phosphorylation of Cx43 and
reduced the expression of Cx43 in the models. Therefore, it may be
a potential therapeutic alternative for PD.
Connexins require an integrated network for protein
synthesis, assembly, gating, internalization, degradation and
feedback control, all of which are required to regulate the
biosynthesis and turnover of gap junction channels. Fundamentally,
the introduction of sequence-altering modifications results in
changes in protein conformation, activity, charge, stability and
localization. Thus, an understanding of the sites, patterns and
magnitude of protein post-translational modification, including
phosphorylation, is essential. Previously, studies of connexin
phosphorylation have suggested that one or a small number of sites
of modification strictly correspond to one molecular function;
however, connexins undergoing multiple levels of multi-site
phosphorylation are critical to improving the functions of connexin
(30). The present study on
rotenone-treated rats demonstrated the induction of phosphorylated
Cx43 in astrocytes, which may be important since astrocytes exhibit
direct, active and critical roles in mediating neuronal survival
and function in various neurodegenerative disorders, including PD
(17). The post-translational
modification was inhibited by gastrodin via the suppression of Cx43
expression.
GJIC is involved in cellular growth control and may
be restored by Cx43 protein expression; therefore, Cx43 is
correlated with GJIC (31). The
central question is whether the elevation of astrocyte GJIC is
involved in the development of PD or whether it is merely a
protective response to rotenone. The results of the present study
demonstrated that the quantity of GJIC was correlated with the
expression levels of Cx43. The expression levels of Cx43 were
enhanced in the striatum and hippocampus of the PD model (Fig. 4). Subsequently, GJIC was also
increased in the PD model, thus the elevation of astrocyte GJIC may
result in the development of PD. Immunohistological analysis
suggested that Cx43 was upregulated in astrocytes in the striatal
and hippocampal regions, while the upregulation was inhibited by
gastrodin. Therefore, this difference in the density of astrocytes
may have affected the induction of Cx43 protein by gastrodin.
Another possibility is that astrocytes in the striatum and
hippocampus demonstrated different characteristics compared with
those in other areas (33,34).
Gastrodin is the main component extracted from the
rhizome of Gastrodia elata (Orchidaceae), a Chinese herbal
medicine, which has long been used for treating dizziness,
epilepsy, stroke and dementia (35). Gastrodin exhibits a neuroprotective
action against hypoxia in cultured cortical neurons, and the
mechanism may involve decreasing the extracellular glutamate level
(35). In the treatment of PD,
gastrodin has been observed to inhibit neuroinflammation in a
rotenone-induced model of PD (12). In the present study, it was
demonstrated that gastrodin prevented the development of PD by
downregulating the expression of Cx43.
In conclusion, a rat PD model was successfully
set-up by treatment with rotenone. Using the rat PD model, the
effects of gastrodin on PD were explored. Gastrodin can ameliorate
PD by downregulating the protein levels of phosphorylated Cx43,
which is closely correlated with the amounts of GJIC. In the rat PD
model induced by rotenone, phosphorylated Cx43 was selectively
enhanced in the striatum and hippocampus. The enhanced activity
could be inhibited specifically by gastrodin treatment (P<0.01).
This study also has limitations, for instance, the inhibition of
Parkinsonism by gastrodin will need to be examined further in
patients. It will also be necessary to examine the changes in the
signal transduction of neuron cells undergoing gastrodin treatment
(36). In future, gastrodin may
offer a potential therapeutic alternative for PD.
References
|
1
|
Paisán-Ruíz C, Jain S, Evans EW, et al:
Cloning of the gene containing mutations that cause PARK8-linked
Parkinson’s disease. Neuron. 44:595–600. 2004.PubMed/NCBI
|
|
2
|
Zhang X, Lu L, Liu S, Ye W, Wu J and Zhang
X: Acetylcholinesterase deficiency decreases apoptosis in
dopaminergic neurons in the neurotoxin model of Parkinson’s
disease. Int J Biochem Cell Biol. 45:265–272. 2013.PubMed/NCBI
|
|
3
|
Salama M, Ellaithy A, Helmy B, et al:
Colchicine protects dopaminergic neurons in a rat model of
Parkinson’s disease. CNS Neurol Disord Drug Targets. 11:836–843.
2012.PubMed/NCBI
|
|
4
|
Ahn EH, Kim DW, Shin MJ, et al:
PEP-1-ribosomal protein S3 protects dopaminergic neurons in an
MPTP-induced Parkinson’s disease mouse model. Free Radic Biol Med.
55:36–45. 2013.PubMed/NCBI
|
|
5
|
Tönges L, Frank T, Tatenhorst L, et al:
Inhibition of rho kinase enhances survival of dopaminergic neurons
and attenuates axonal loss in a mouse model of Parkinson’s disease.
Brain. 135:3355–3370. 2012.PubMed/NCBI
|
|
6
|
Ali F, Stott SR and Barker RA: Stem cells
and the treatment of Parkinson’s disease. Exp Neurol. Jan
6–2013.(Epub ahead of print).
|
|
7
|
Nishimura K and Takahashi J: Therapeutic
application of stem cell technology toward the treatment of
Parkinson’s disease. Biol Pharm Bull. 36:171–175. 2013.
|
|
8
|
Kim H: Neuroprotective herbs for stroke
therapy in traditional eastern medicine. Neurol Res. 27:287–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearl PL, Drillings IM and Conry JA: Herbs
in epilepsy: evidence for efficacy, toxicity, and interactions.
Semin Pediatr Neurol. 18:203–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schachter SC: Botanicals and herbs: a
traditional approach to treating epilepsy. Neurotherapeutics.
6:415–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manavalan A, Ramachandran U, Sundaramurthi
H, et al: Gastrodia elata Blume (tianma) mobilizes neuro-protective
capacities. Int J Biochem Mol Biol. 3:219–241. 2012.PubMed/NCBI
|
|
12
|
Li C, Chen X, Zhang N, Song Y and Mu Y:
Gastrodin inhibits neuroinflammation in rotenone-induced
Parkinson’s disease model rats. Neural Regen Res. 7:325–331.
2012.(In Chinese).
|
|
13
|
Karuppagounder SS, Madathil KS, Pandey M,
Haobam R, Rajamma U and Mohanakumar KP: Quercetin up-regulates
mitochondrial complex-I activity to protect against programmed cell
death in rotenone model of Parkinson’s disease in rats.
Neuroscience. 236:136–148. 2013.PubMed/NCBI
|
|
14
|
Xiong N, Long X, Xiong J, et al:
Mitochondrial complex I inhibitor rotenone-induced toxicity and its
potential mechanisms in Parkinson’s disease models. Crit Rev
Toxicol. 42:613–632. 2012.
|
|
15
|
Drinkut A, Tereshchenko Y, Schulz JB, Bähr
M and Kügler S: Efficient gene therapy for Parkinson’s disease
using astrocytes as hosts for localized neurotrophic factor
delivery. Mol Ther. 20:534–543. 2012.
|
|
16
|
Hauser DN and Cookson MR: Astrocytes in
Parkinson’s disease and DJ-1. J Neurochem. 117:357–358. 2011.
|
|
17
|
Rappold PM and Tieu K: Astrocytes and
therapeutics for Parkinson’s disease. Neurotherapeutics. 7:413–423.
2010.
|
|
18
|
Rouach N and Giaume C: Connexins and gap
junctional communication in astrocytes are targets for neuroglial
interaction. Prog Brain Res. 132:203–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dermietzel R, Gao Y, Scemes E, et al:
Connexin43 null mice reveal that astrocytes express multiple
connexins. Brain Res Brain Res Rev. 32:45–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagy JI and Rash JE: Connexins and gap
junctions of astrocytes and oligodendrocytes in the CNS. Brain Res
Brain Res Rev. 32:29–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thompson RJ and Macvicar BA: Connexin and
pannexin hemichannels of neurons and astrocytes. Channels (Austin).
2:81–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X and Simard JM: Multiple connexins
form gap junction channels in rat basilar artery smooth muscle
cells. Circ Res. 84:1277–1284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawasaki A, Hayashi T, Nakachi K, et al:
Modulation of connexin 43 in rotenone-induced model of Parkinson’s
disease. Neuroscience. 160:61–68. 2009.PubMed/NCBI
|
|
24
|
Ya-qin C, Yi-fan S, Hong C, Jian-ping W
and Jiao D: Effects of gastrodin on Cx43 expression in temporal
lobe cortex and hippocampus of pentylenetetrazole-induced epileptic
immature rats. Journal of Lanzhou University (Medical Sciences).
34:92008.
|
|
25
|
Mulcahy P, O’Doherty A, Paucard A, O’Brien
T, Kirik D and Dowd E: The behavioural and neuropathological impact
of intranigral AAV-α-synuclein is exacerbated by systemic infusion
of the Parkinson’s disease-associated pesticide, rotenone, in rats.
Behav Brain Res. 243:6–15. 2013.PubMed/NCBI
|
|
26
|
Thakur P and Nehru B: Anti-inflammatory
properties rather than anti-oxidant capability is the major
mechanism of neuroprotection by sodium salicylate in a chronic
rotenone model of Parkinson’s disease. Neuroscience. 231:420–431.
2013.PubMed/NCBI
|
|
27
|
Care IoLARCo, Animals UoL and Resources
NIoHDoR. Guide for the care and use of laboratory animals. US
Department of Health and Human Services, Public Health Service,
National Insititutes of Health; 1985
|
|
28
|
Wisniewska-Kruk J, Hoeben KA, Vogels IM,
et al: A novel co-culture model of the blood-retinal barrier based
on primary retinal endothelial cells, pericytes and astrocytes. Exp
Eye Res. 96:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takizawa T, Gudla PR, Guo L, Lockett S and
Misteli T: Allele-specific nuclear positioning of the
monoallelically expressed astrocyte marker GFAP. Genes Dev.
22:489–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
31
|
Chen VC, Gouw JW, Naus CC and Foster LJ:
Connexin multi-site phosphorylation: mass spectrometry-based
proteomics fills the gap. Biochim Biophys Acta. 1828:23–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jongen WM, Fitzgerald DJ, Asamoto M, et
al: Regulation of connexin 43-mediated gap junctional intercellular
communication by Ca2+ in mouse epidermal cells is
controlled by E-cadherin. J Cell Biol. 114:545–555. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baucum AJ II, Brown AM and Colbran RJ:
Differential association of postsynaptic signaling protein
complexes in striatum and hippocampus. J Neurochem. 124:490–501.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fidalgo C, Conejo NM, González-Pardo H and
Arias JL: Functional interaction between the dorsal hippocampus and
the striatum in visual discrimination learning. J Neurosci Res.
90:715–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu X, Lu Y and Bie X: Protective effects
of gastrodin on hypoxia-induced toxicity in primary cultures of rat
cortical neurons. Planta Med. 73:650–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Levine AJ, Harris CR and Puzio-Kuter AM:
The interfaces between signal transduction pathways: IGF-1/mTor,
p53 and the Parkinson Disease pathway. Oncotarget. 3:1301–1307.
2012.PubMed/NCBI
|