Introduction
The treatment of composite bone and soft tissue
defects continues to challenge orthopedic surgeons (1). Traditionally, tissue flaps and open
bone grafts (OBGs) were commonly used to restore structure and
function. Composite tissue flap transplantation may minimize the
injury, but it is complex and unpopular (2). Conventional installment therapy does
not have an extended healing time and has poor efficacy. Novel
methods for the treatment of bone and soft tissue defects are
required.
OBG is a procedure that replaces the missing bone in
order to repair the bone defects (3). OBG is feasible due to the fact that
bone tissue has the ability to completely regenerate. OBG has been
reported to significantly shorten hospital stays (4). The ensuing dressing changes did not
efficiently control infections, particularly for free bone
fragments without a blood supply; therefore, healing time for the
wound was prolonged. This increases the risk of infection and
chronic osteomyelitis. Negative pressure wound therapy (NPWT) has
been widely used on various complex wounds (5). NPWT reduces local edema and bacterial
load, promotes the growth of granulation tissue and increases blood
supply topically, and thus promotes wound healing (6). We hypothesized that NPWT combined
with OBG (NPWT-OBG) may be a novel and effective method for the
management of bone and soft tissue defects.
Animal models with polluted wounds and bone and soft
tissue defects were treated by either NPWT-OBG or OBG. The
efficiency was assessed by bacteria counting, X-ray and evaluation
of the levels of CD34 and vascular endothelial growth factor
(VEGF).
Materials and methods
Animal model
In the present study, 24 New Zealand rabbits
weighing 2.8–3.5 kg were purchased and housed in an approved animal
care facility (eligibility certification: No. 4210000313). The
rabbits were anesthetized with a 1% pentobarbital sodium (30 mg/kg)
intraperitoneal injection. The forearm hair of all the rabbits was
removed using 8% NaS solution prior to each surgical procedure. An
incision, 2.5 cm in length including skin and subcutaneous tissue,
was made in the radialis side of each forearm. The radius was
partially amputated by removing 1 cm. A sufficient quantity of
autologous ilium was obtained and grafted into the defects. The
wounds were daubed with Staphylococcus aureus
(1×103) to produce polluted wounds. After 1 h, the
polluted wounds were debrided and a bacteria counting test was
performed in order to determine whether the model was successful.
We randomly selected two forearms to treat with OBG or NPWT-OBG.
Rabbits in the NPWT-OBG group were then treated with VAC foam (VSD
Medical Science Technology Co., Ltd., Wuhan, China). The negative
pressure value was −75 mmHg. Wounds in the OBG group were covered
with conventional gauze. The day on which the surgery was performed
was defined as day 0. Vacuum sealing drainage (VSD) dressing change
was performed on days 3, 7 and 14 and granulation tissue, with a
volume of 2 × 5 × 10 mm3, was obtained under aseptic
conditions and divided into three. The samples were immediately
analyzed by bacteria counting, stored at −80ºC for western blot
analysis and immersed in 4% paraformaldehyde for
immunohistochemical analysis, respectively. From day 21, the gauze
dressings were changed every three days.
Bacteria counting
Samples were weighed immediately, minced,
homogenized and diluted. Diluents (5 μl) were placed on a normal
agar plate and incubated at 37ºC with 5% CO2 for 48 h.
The number of bacteria in each wound was calculated by counting the
colony forming units (CFUs) on each plate.
X-ray imaging
The lateral films for the upper extremities were
performed for each rabbit on days 0, 7, 14, 21 and 28. The
condition of the fracture and its healing rate were recorded on the
28th day.
Immunohistochemical analysis
All samples fixed in 4% paraformaldehyde were
embedded in paraffin and routinely sectioned (5 μm). Staining was
performed using the SABC method (7). The primary monoclonal anti-rabbit
CD34 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA;
1:1000) was applied to the sections and incubated for 1 h at room
temperature, rinsed three times with PBS and the sections were then
incubated with fluorescein isothiocyanate (FITC)- or
rhodamine-conjugated secondary antibody (Santa Cruz Biotechnology,
Inc.) for 30 min. The complex was visualized using the
avidin-biotinylated enzyme complex. The stained blood microvessels
were counted in the section.
Western blot analysis
All samples were homogenized in buffer with an added
protease inhibitor cocktail (Roche Inc., Basel, Switzerland), 10 mM
NaCl, 1% NP40, 0.02% sodium azide and 50 mM Tris. Homogenates were
centrifuged at 8,465 × g for 10 min at 4ºC. The supernatant was
stored at −20ºC prior to use. The loading sample volume was 50 μg
and the protein was separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a polyvinylidene difluoride (PVDF) membrane. The
membranes were blocked with 5% fat milk at 37ºC for 2 h and
incubated in a shaker (WD-9405A, Beijing World Biomedical
Instruments Co., Ltd., Beijing, China). for a further 2 h. The
membranes were incubated with a primary goat anti-rabbit antibody
against either VEGF (Santa Cruz Biotechnology, Inc.; 1:20,000) or
β-actin (Santa Cruz Biotechnology, Inc.; 1:10,000) overnight at
4ºC. Blots were washed for 5–10 min with Tris-buffered saline
containing 0.05% Tween-20 (TBST). Following washing, the blots were
incubated for 1 h with a horseradish-peroxidase (HRP)-coupled
secondary antibody and subsequently washed again with TBST. The
blots were incubated with a luminescent HRP substrate for 1 min
until positive signals were detected. The optical densities of at
least three replicates of each sample were quantified. Samples were
stored at −80ºC prior to use.
Statistical analysis
All data are presented as the mean ± SD, and were
analyzed by the Student's t-test. Enumeration data are presented as
percentages and were analyzed by the Chi-square test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Wound condition
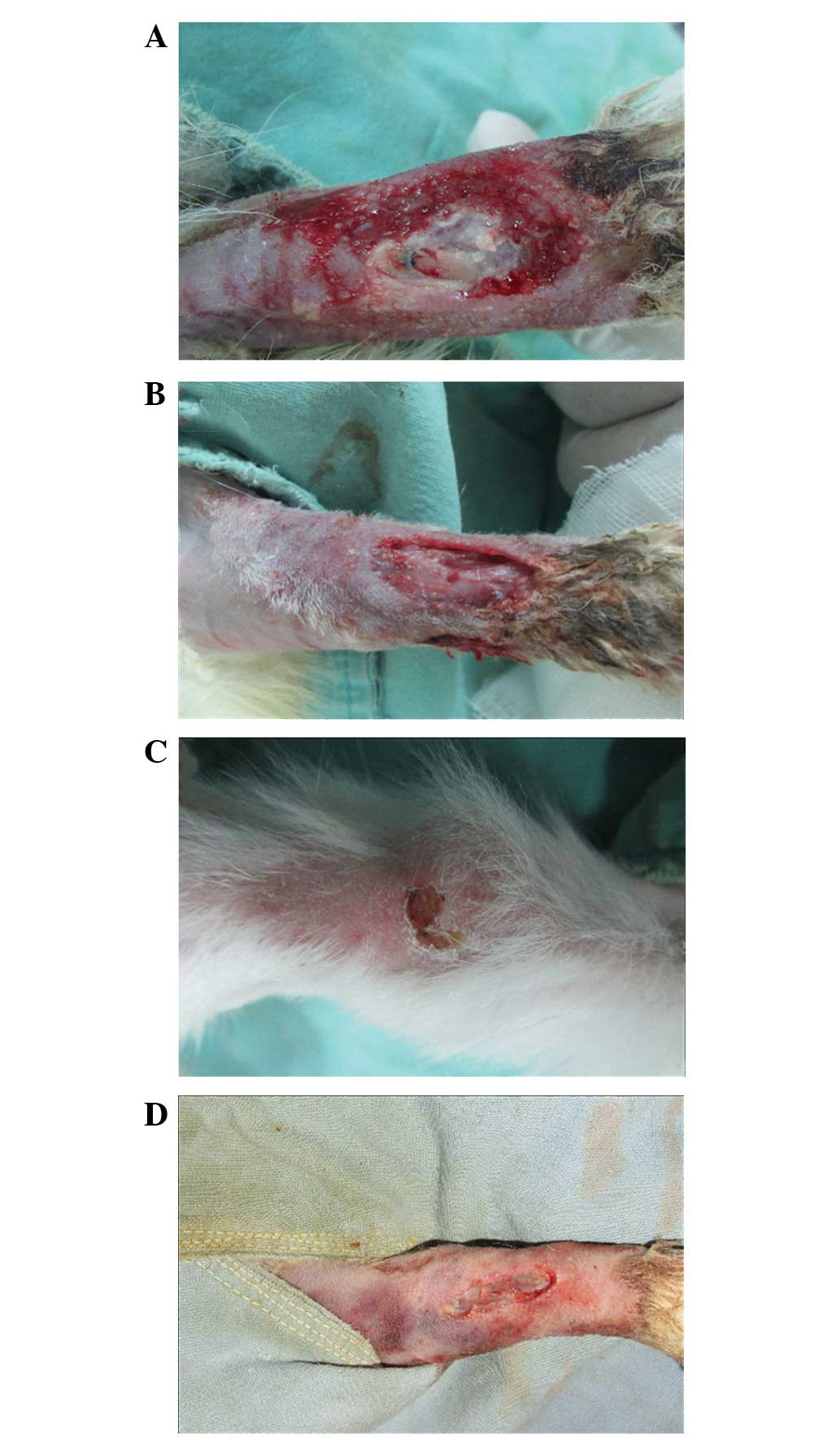
On days 3, 7, 14 and 21 following the surgical
procedure, the wounds in the NPWT-OBG group were cleaner and drier
with less exudate compared with the control group (Fig. 1). In general, the quality of
granulation tissue was improved following NPWT; the wound healing
rate significantly increased and the wound healing time decreased
(18.5±9.4 for the NPWT-OBG group vs. 24.2±6.5 days for the control
group).
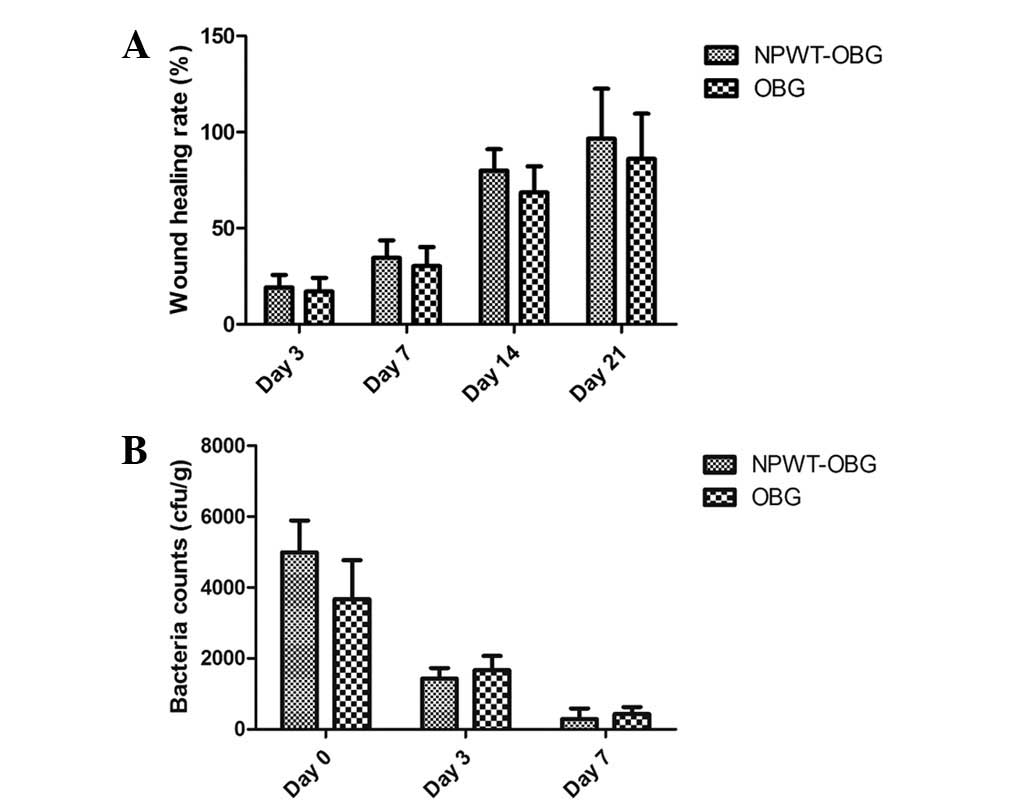
Bacterial colony counts
The tissues obtained from the wounds were cultured
and analyzed 1 h after the surgical procedure. Staphylococcus
aureus, with a density <1×105 CFU/ml, was
detected in all wounds. This suggested that the polluted wound
model had been successfully established. Staphylococcus
aureus was also detected on days 3 and 7 in both groups;
however, the difference was not identified to be statistically
significant (Fig. 2). On days 14
and 21, no bacteria were detected. Therefore, the bacteria count
was not performed on day 28.
VEGF expression
VEGF has been confirmed to promote angiogenesis
during the wound healing process (8). Overexpression of VEGF was observed in
wounds treated by NPWT. In this study, in order to examine the
exact mechanism underlying the effect of VEGF on wound healing,
western blot analysis of VEGF was performed. At the examined times,
the expression of VEGF was higher in the NPWT-OBG group than in the
OBG group. The results indicated that VEGF overexpression
contributed to the wound healing process.
Microvascular density
On days 3, 7 and 14, the microvessels of the wounds
were counted via immunohistochemical staining of CD34 (Fig. 4). The results demonstrated that the
densities of microvessels in the NPWT-OBG group were higher than
those in the OBG group throughout the entire process. Moreover, the
density increased as the experiment proceeded, and the difference
was statistically significant (P<0.05).
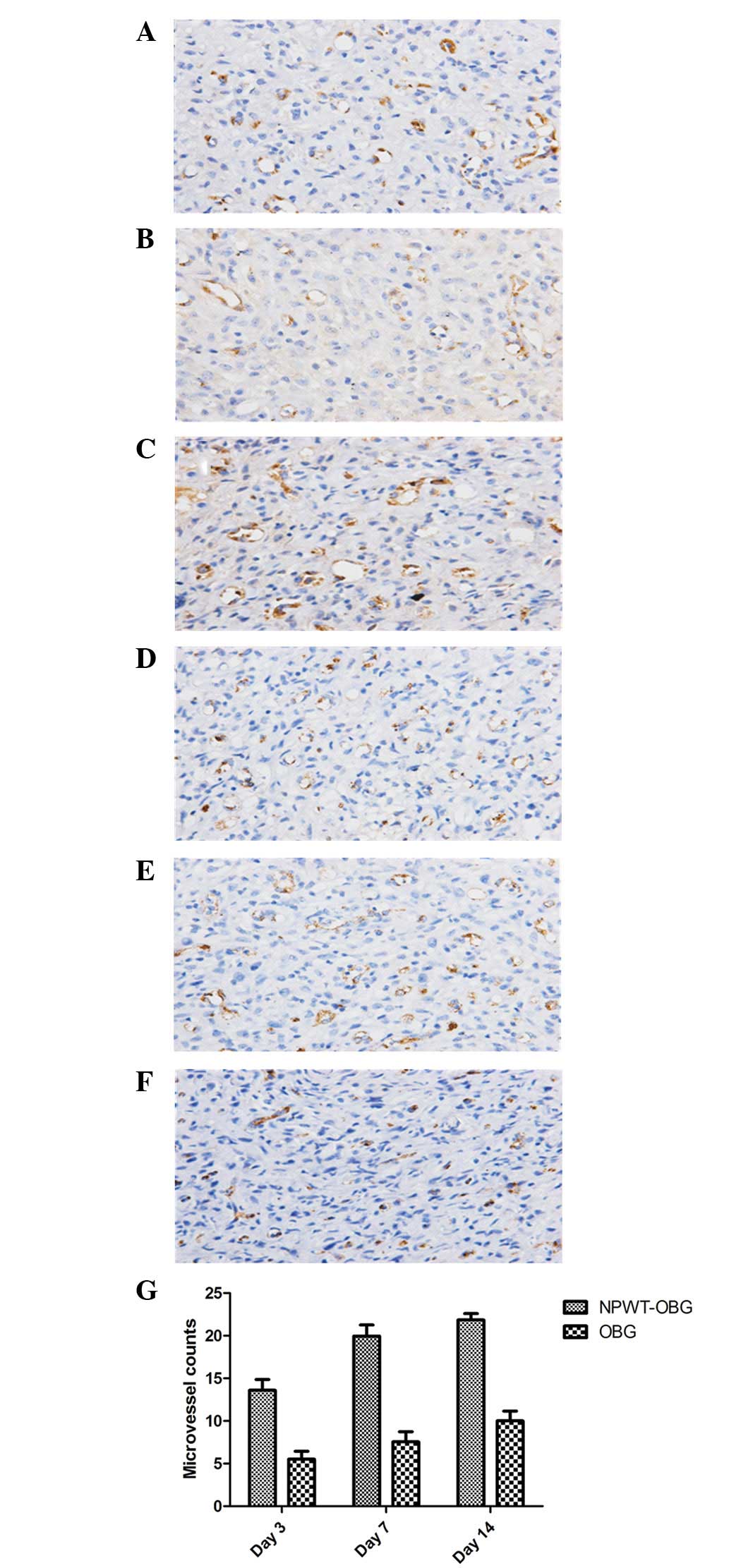 | Figure 4Microvascular density in the
granulation tissues was revealed by staining for CD34. (A, C and E)
Microvascular density on days 3, 7 and 14, respectively, in the
NPWT-OBG group. (B, D and F) Microvascular density on days 3, 7 and
14, respectively, in the OBG group (magnification, ×400). (G) The
number of blood vessels at different times for both groups.
NPWT-OBG, negative pressure wound therapy-open bone graft. |
X-ray examination
For all rabbits, the fracture healing processes were
monitored by X-ray examination on days 0, 7, 14, 21 and 28,
respectively (Fig. 5). The films
revealed that the callus growth in the experimental group had
improved to a greater extent than that in the control group. On the
28th day, the healing rate of the NPWT-OBG group was 75% (9/12),
which was higher than the OBG group [25% (3/12)].
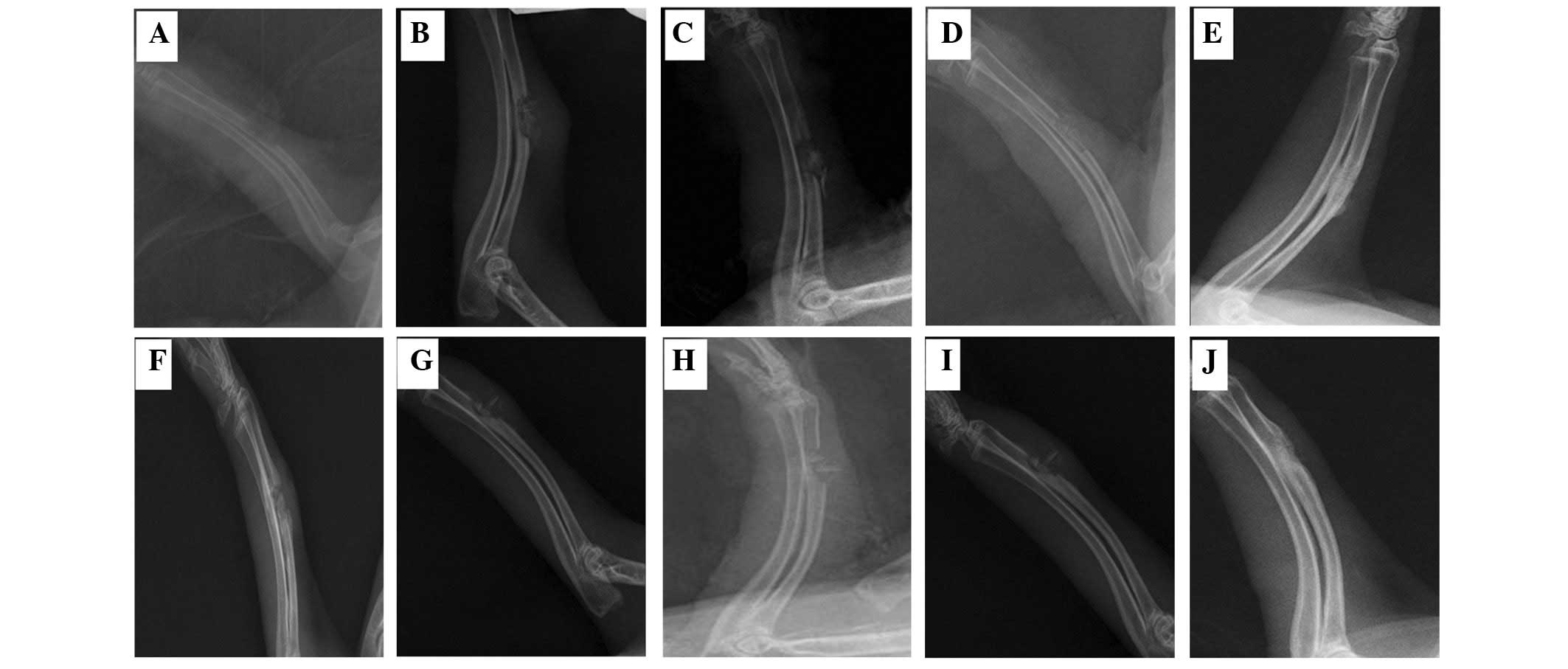 | Figure 5X-ray imaging of the forearm of
rabbits at various times. (A-E) X-ray images of the radius on days
0, 7, 14, 21 and 28, respectively, in the NPWT-OBG group. (F-J)
X-ray images of the radius on days 0, 7, 14, 21 and 28,
respectively, in the OBG group. NPWT-OBG, negative pressure wound
therapy-open bone graft. |
Discussion
The pivotal issue when repairing composite bone and
soft tissue defects is to restore the outline and function of the
bone and soft tissue as soon as possible (1). Composite tissue flap transplantation
achieves this goal in one stage. Unfortunately, the surgery is
unpopular. Furthermore, donor-site problems cause pain in the
patients.
OBG has long been used for the treatment of bone
defects. Papineau et al(9)
were the first to report on the application of open cancellous bone
grafting in patients with bone defects. However, for bone defects
with soft tissue defects, single open bone grafts only repair the
bone defect temporarily. As the grafted bone is not covered by soft
tissue, the procedure is deemed to be a failure. It indicates that
the healing process of soft tissue is important. With this method,
the wound was debrided and covered with a conventional dressing.
The skin graft was not performed until granulation tissue had
filled the wound bed. When the wound had completely healed, the
cancellous bone was transplanted. Although the method was simple,
the process was too long (10).
Thereafter, tissue flap transplantation was developed, which
shortened the overall treatment time (2). Unfortunately, the surgery was
difficult to master and high-risk.
The NPWT technique, first applied clinically by
Fleischmann et al(11) in
1993, is now widely used in surgical drainage, even for complicated
wounds. The characteristics that promote wound healing by NPWT are
well recognized. Using the NPWT technique, the wound was sealed
with a membrane to promote healing. All drawbacks mentioned
previously have been solved. Therefore, we combined OBG with NPWT
in order to assess whether it was feasible for the treatment of
composite bone and soft tissue defects.
Due to the diversity of injuries, wounds are often
in contact with the environment without being covered. As a result,
the majority of wounds are contaminated with dirt, dust and other
pollutants. The majority of open wounds were considered clinically
to be polluted wounds. Thus in this study, we investigated polluted
wounds in an animal model.
We have identified few relevant reports regarding
the use of polluted wound models. Generally, a polluted wound was
defined as a wound, open for <6 h, not severely polluted and
without any signs of infection following debridement (12). Thus, we smeared Staphylococcus
aureus at a concentration of 1×103 cells/ml on
wounds for 1, 2 and 3 h. We found that in open wounds debrided
after 1 h, bacteria counting was positive but not infective. This
confirmed that the polluted wound model had been successfully
established.
It has been recognized that NPWT promotes wound
healing. The mechanism been studied thoroughly, with the main focus
on the following issues: i) Reducing the edema of tissue
surrounding the wound (13); ii)
mechanical traction and stimulation with negative pressure; iii)
improving local microcirculation in the wound (14); iv) keeping the wound clean in order
to inhibit bacterial growth (15);
and v) enhancing the expression of relevant factors associated with
wound repair (16,17). The first two mechanisms are
subjective and not easily measured. Thus we mainly focused on the
latter three. In this study, we observed that the bacteria count
was reduced in the NPWT-OBG group, but this decrease was not
statistically significant when compared with the control group. In
the NPWT-OBG group, signs of infection were observed in one rabbit
and in three of the rabbits in the OBG group. We suspect that this
was due to the small sample size and that if the sample size was
increased, greater differences may emerge. Compared with the
control group, the number of microvessels increased and VEGF
expression was upregulated in the NPWT-OBG group. Increased VEGF
typically resulted in increasingly rapid wound closure and
facilitated bone healing (18).
Thus the combined method has an advantage over conventional therapy
for managing wounds with bone and soft tissue defects, and this is
in accordance with other studies (19).
Due to its relatively large specific surface area,
cancellous bone has a greater number of connections with
surrounding tissues (20). Thus,
cancellous bone <0.5 cm can survive easily. During the surgical
procedure, we followed this principle for bone grafting. NPWT
research mainly focuses on wound healing, while reports on the
effect of NPWT on fracture healing do not exist. Under negative
pressure, blood flow is accelerated and the blood supply is
increased (21). The
anti-infection ability was enhanced. All of these factors
facilitated the healing process of bone, in accordance with our
results.
Though NPWT-OBG exhibited improved results for
composite bone and soft tissue defects in rabbits, this may not
produce the same result in humans. Composite bone and soft tissue
defects in humans are more complicated than those found in animal
models and the negative pressure varies. Thus, further research is
required.
In conclusion, NPWT may protect polluted wounds from
infection. By promoting wound healing, controlling infection and
facilitating fracture healing, NPWT-OBG is a feasible treatment for
composite bone and soft tissue defects and is more efficient than
an OBG. However, whether this remains true for human patients
requires further clinical trials.
Acknowledgements
This project was supported by the National Natural
Science Funds of China (grant no. 81171713) and the National
Natural Science Funds of Hubei Province (grant no.
2012FFB04311).
References
|
1
|
Yazar S, Lin CH and Wei FC: One-stage
reconstruction of composite bone and soft-tissue defects in
traumatic lower extremities. Plast Reconstr Surg. 114:1457–1466.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu AX, Yu GR, Deng K, Tao SX, Pan ZY and
Zhang JH: Combination of negative pressure wound therapy with
tissue flap transplantation for severe infectious bone exposure.
Chin J Microsurg. 29:219–220. 2006.(In Chinese).
|
|
3
|
Cabanela ME: Open cancellous bone grafting
of infected bone defects. Orthop Clin North Am. 15:427–440.
1984.PubMed/NCBI
|
|
4
|
Lei H and Yi L: One-stage open cancellous
bone grafting of infected fracture and nonunion. J Orthop Sci.
3:318–323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Streubel PN, Stinner DJ and Obremskey WT:
Use of negative-pressure wound therapy in orthopaedic trauma. J Am
Acad Orthop Surg. 20:564–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen SZ, Li J, Li XY and Xu LS: Effects of
vacuum-assisted closure on wound microcirculation: an experimental
study. Asian J Surg. 28:211–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasconcelos MG, Alves PM, Vasconcelos RG,
da Silveira EJ, Medeiros AM and de Queiroz LM: Expression of CD34
and CD105 as markers for angiogenesis in oral vascular
malformations and pyogenic granulomas. Eur Arch Otorhinolaryngol.
268:1213–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papineau IJ, Alfageme A, Delcourt JP and
Pilon BL: Chronic osteomyelitis of long bones - Resection and bone
grafting with delayed skin closure. J Bone Joint Surg (Br).
58:138–141. 1976.
|
|
10
|
Freeland AE and Mutz SB: Posterior
bone-grafting for infected ununited fracture of the tibia. J Bone
Joint Surg Am. 58:653–657. 1976.PubMed/NCBI
|
|
11
|
Fleischmann W, Strecker W, Bombelli M and
Kinzl L: Vacuum sealing as treatment of soft tissue damage in open
fractures. Unfallchirurg. 96:488–492. 1993.(In German).
|
|
12
|
Zhengbao Z and Youping Y: The clinical
significance of preventive use of antibiotics in polluted wound. J
Pharm Pract. 17:131–133. 1999.
|
|
13
|
Borgquist O, Ingemansson R and Malmsjö M:
Wound edge microvascular blood flow during negative-pressure wound
therapy: examining the effects of pressures from −10 to −175 mmHg.
Plast Reconstr Surg. 125:502–509. 2010.PubMed/NCBI
|
|
14
|
Zhou M, Yu A, Wu G, Xia C, Hu X and Qi B:
Role of different negative pressure values in the process of
infected wounds healing treated by vacuum-assisted closure: an
experimental study. Int Wound J. May 29–2012.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Weed T, Ratliff C and Drake DB:
Quantifying bacterial bioburden during negative pressure wound
therapy: does the wound VAC enhance bacterial clearance? Ann Plast
Surg. 52:276–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baldwin C, Potter M, Clayton E, Irvine L
and Dye J: Topical negative pressure stimulates endothelial
migration and proliferation: a suggested mechanism for improved
integration of Integra. Ann Plast Surg. 62:92–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Labler L, Rancan M, Mica L, Härter L,
Mihic-Probst D and Keel M: Vacuum-assisted closure therapy
increases local interleukin-8 and vascular endothelial growth
factor levels in traumatic wounds. J Trauma. 66:749–757. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng H, Usas A, Olshanski A, et al: VEGF
improves, whereas sFlt1 inhibits, BMP2-induced bone formation and
bone healing through modulation of angiogenesis. J Bone Miner Res.
20:2017–2027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tarkin IS: The versatility of negative
pressure wound therapy with reticulated open cell foam for soft
tissue management after severe musculoskeletal trauma. J Orthop
Trauma. 22:S146–S151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burchardt H: The biology of bone graft
repair. Clin Orthop Relat Res. 174:28–42. 1983.PubMed/NCBI
|
|
21
|
Page JC, Newswander B, Schwenke DC, Hansen
M and Ferguson J: Retrospective analysis of negative pressure wound
therapy in open foot wounds with significant soft tissue defects.
Adv Skin Wound Care. 17:354–364. 2004. View Article : Google Scholar : PubMed/NCBI
|