Introduction
It is well established that vascular smooth muscle
cells (VSMCs) are not terminally differentiated (1). Their transition and maintenance from
the differentiated state to the dedifferentiated phenotype occurs
on accelerated proliferation, migration and synthesis of
extracellular matrix (ECM) components. The phenotypic modulation of
VSMCs plays a crucial role in the pathogenesis of a wide range of
cardiovascular diseases, including atherosclerosis, in-stent
restenosis and hypertension (2).
However, despite advances in this research area, the molecular
mechanisms underlying VSMC phenotypic modulation have not been
completely understood.
MicroRNAs (miRNAs or miRs) are endogenous, small,
single-stranded, 20 to 26 nucleotides in length, evolutionarily
conserved RNAs. These noncoding RNAs, which have been shown to be
ubiquitous in all eukaryotes, including vertebrates, negatively
regulate more than one-third of protein-coding genes in the human
genome by degrading or inhibiting their specific target genes at
the post-transcriptional level (3–5).
Their essential roles in physiological and pathophysiological
processes, initially revealed in cancer, sepsis, viral infection
and development, were further explored in cardiovascular diseases,
including cardiogenesis, cardiac hypertrophy, arrhythmia, heart
failure and cardiovascular remodeling (6–10).
Recent studies have revealed that miR-146a was aberrantly expressed
and upregulated in rat arteries after balloon injury (11). Evidence has also demonstrated that
miR-146a was involved in innate immunity and inflammatory responses
(12). However, little is known
about the effect of miR-146a on VSMC phenotypic modulation, as well
as its molecular basis.
In the present study, we explored the possible role
of miR-146a in the proliferative and migratory properties of VSMCs
by transfecting small interfering RNA (siRNA) to knockdown
miR-146a. Nuclear factor κBp65 (NF-κBp65), proliferating cell
nuclear antigen (PCNA) and Bax, which are critical pro-inflammatory
factors and transcriptional factors in the induction of vascular
dysfunction and remodeling, were investigated in order to better
understand the regulatory mechanisms of miR-146a that are
responsible for regulating VSMC phenotypes.
Materials and methods
Cell culture
Primary VSMCs were obtained and isolated from the
thoracic aortic media of male Sprague-Dawley rats (100–150 g) and
were then propagated in high-glucose Dulbecco’s modified Eagle’s
medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS;
purchased from Gibco-BRL, Carlsbad, CA, USA), 100 U/ml of
penicillin and 100 μg/ml of streptomycin in a humidified incubator
containing 5% CO2 at 37°C. Cells between passages 3 and
8 were used for the subsequent experiments. The study was approved
by the Ethics Committee of Shenzhen People’s Hospital, Shenzhen,
China.
Quantification of miR-146a
To identify the potential role of miR-146a in VSMC
biology, the expression signature of miR-146a in quiescent and
proliferative cells was first surveyed by real-time PCR. After
culture in various concentrations of FBS (5, 10, 20%) for 48 h,
total RNA of VSMCs was extracted by using the RNeasy Mini kit
(Qiagen, Hilden, Germany) and reverse transcribed to cDNA using the
One Step PrimeScript miRNA cDNA Synthesis kit (Takara Bio, Inc.,
Shiga, Japan). Quantitative real-time PCR of miR-146a was
accomplished by using the ABI PRISM 7900 Sequence Detection System
(Applied Biosystems, Foster City, CA, USA) with the SYBR Premix Ex
Taq kit (Takara Bio, Inc.). miR-146a was amplified using the
PrimeScript Universal Primer (Takara Bio, Inc.) together with
miRNA-specific primer (5′-TGAGAACTGAATTCCATGGGTT-3′). U6 RNA was
used as an internal control. miRNA-146a-specific primer and U6
primers were synthesized by Sangon (Shanghai, China). The relative
expression levels of miRNA-146a were calculated based on the
following equation: relative miR-146a level = 2−(ΔCt
sample−ΔCt control). All PCR was performed according to the
manufacturer’s instructions.
Knockdown of miR-146a
The antisense miR-146a oligonucleotide (miR-146a
inhibitor) and the negative control miR-146a oligonucleotide
(miR-146a control) were synthesized by GenePharma (Shanghai,
China). The sequences of these oligonucleotides were as follows:
5′-AACCCAUGGAAUUCA GUUCUCA-3′ (miR-146a inhibitor) and 5′-CAGUACUUU
UGUGUAGUACAA-3′ (miR-146a control). The miR-146a oligonucleotides
contained 2/-O-methyl modifications at every base. At 24 h prior to
transfection, VSMCs were cultured in DMEM without antibiotics and
serum. The cells were classified into three groups: normal VSMCs,
miR-146a control (50 nM) and miR-146a inhibitor (10, 50, 100 nM).
All cells were transfected using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. At 5 h after transfection, the
supernatants were decanted and replaced by new DMEM containing 10%
FBS. After incubation for 48 h, relative expression of miR-146a in
different VSMCs was investigated by real-time PCR according to the
methods described previously.
VSMC proliferation
VSMC proliferation was determined by cell counting
and bromodeoxyuridine (BrdU) incorporation assay (Cell
Proliferation ELISA; Roche, Basel, Switzerland). Briefly, VSMCs
were seeded in a 96-well plate at a density of 5×103
cells per well for 24 h. Cells were then transfected with miR-146a
inhibitor (50 nM), miR-146a control (50 nM) or phosphate-buffered
solution (PBS). After cell transfection, VSMCs were incubated in
DMEM containing 10% FBS for 12, 24 and 48 h. For cell counting,
VSMCs were trypsinized and resuspended in PBS. The number of VSMCs
was subsequently counted using a hemocytometer under a microscope.
BrdU incorporation analysis was performed according to the
manufacturer’s instructions. The absorbance was measured at 450 nm
with a reference wavelength at 690 nm. Cell viability experiments
were performed by trypan dye after BrdU treatment, and cell
viability was always >98%. All experiments were performed in
sextuplicate.
VSMC migration
The effect of miR-146a on VSMC migration was
assessed using the Transwell system (Corning, NY, USA) with
polycarbonate filters (6.5-mm diameter, 8.0-μm pore size). At 48 h
after treatment with PBS, miRNA-146a control or miRNA-146a
inhibitor, VSMCs were harvested by trypsinization and resuspended.
Cell suspensions at a density of 1×104 cells were added
into the upper chambers of the wells in 200 μl DMEM medium
containing 5% FBS and incubated for 12 h, while the lower chambers
were filled with DMEM medium containing 20% FBS in order to induce
cell migration. The VSMCs on the upper surface of the membrane were
scraped softly. Cells that migrated to the lower surface were fixed
with methanol and stained with 0.1% crystal violet dye. Cells were
counted in 5 random views under a light microscope. Experiments
were performed on 6 wells for each condition and repeated 3
times.
Flow cytometry
VSMC apoptosis was investigated by flow cytometry
analysis with an Annexin V-PE/7-AAD Apoptosis Detection kit (KeyGEN
Biotech, Nanjing, China). A total of 48 h after transfection in
6-well plates, VSMCs were harvested and washed twice in cold PBS,
subsequently centrifuged and resuspended in 500 μl binding buffer.
Annexin V-PE (1 μl) and 7-amino-actinomycin D (5 μl) were
successively added in order to stain the cells. The samples were
measured by flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA) according to the manufacturer’s instructions. The apoptotic
percentage was then calculated. Each group possessed 6 samples.
Western blot analysis
VSMCs treated with PBS, miR-146a control or miR-146a
inhibitor were cultured in DMEM medium containing 10% FBS for 48 h.
Total protein (20 μg) extracted from the cells was resolved on 10%
SDS-polyacrylamide gels and electrotransferred onto nitrocellulose
membranes by electrophoresis. The membranes were blocked in 5%
non-fat milk in TBS containing 0.3% Tween-20, then incubated
overnight with polyclonal rabbit anti-rat NF-κBp65 (1:1000
solution, Abcam, Cambridge, MA, USA), PCNA (1:500 solution, Santa
Cruz Biotechnology, Santa Cruz, CA, USA) and Bax (1:500 solution,
Santa Cruz Biotechnology). The blots were subsequently incubated in
1:6,000 dilutions of goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies. Enhanced
chemiluminescence (ECL) developing solution (100 μl) was added in
each sample to detect the protein signal with a Quant RT ECL cold
CCD imaging system (General Electric, Fairfield, CT, USA).
Statistical analysis
All statistical analysis was performed with SPSS
16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the
means ± standard error. For relative gene expression, the mean
value of the vehicle group is defined as 100%. Differences were
compared by t-test and ANOVA. P<0.05 was considered to indicate
a statistically significant result. All experiments were performed
at least three times.
Results
Relative expression of miR-146a in
VSMCs
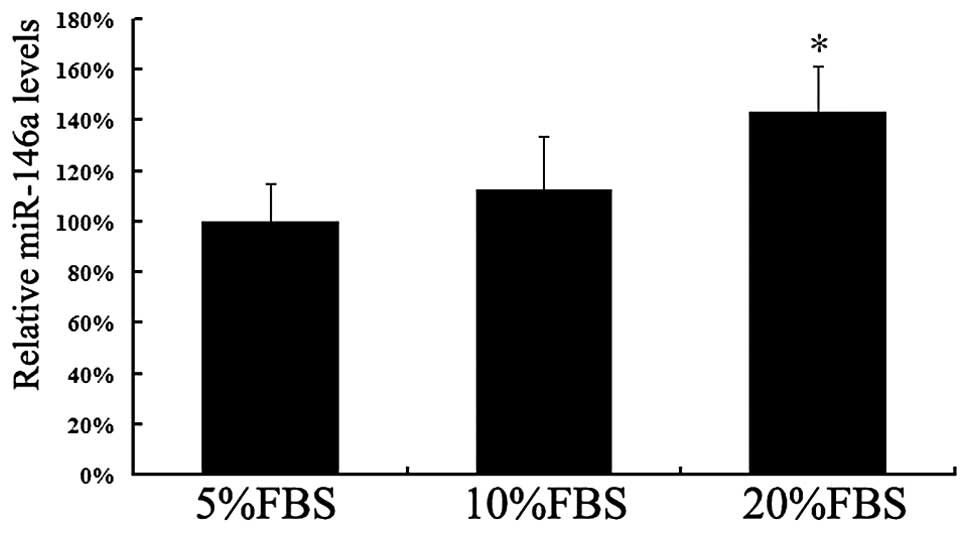
FBS is well acknowledged as a proproliferative
factor. We first examined the expression of miR-146a in VSMCs
cultured in different concentrations of FBS. The expression levels
of miR-146a were increased by FBS in a concentration-dependent
manner. The miR-146a level in VSMCs cultured with 20% FBS was
notably higher than that in VSMCs cultured with 5% FBS (P<0.05;
Fig. 1). The significantly
increased expression level of miR-146a in proliferative VSMCs
indicates that miR-146a may promote the proliferation of VSMCs and
reduce their apoptosis.
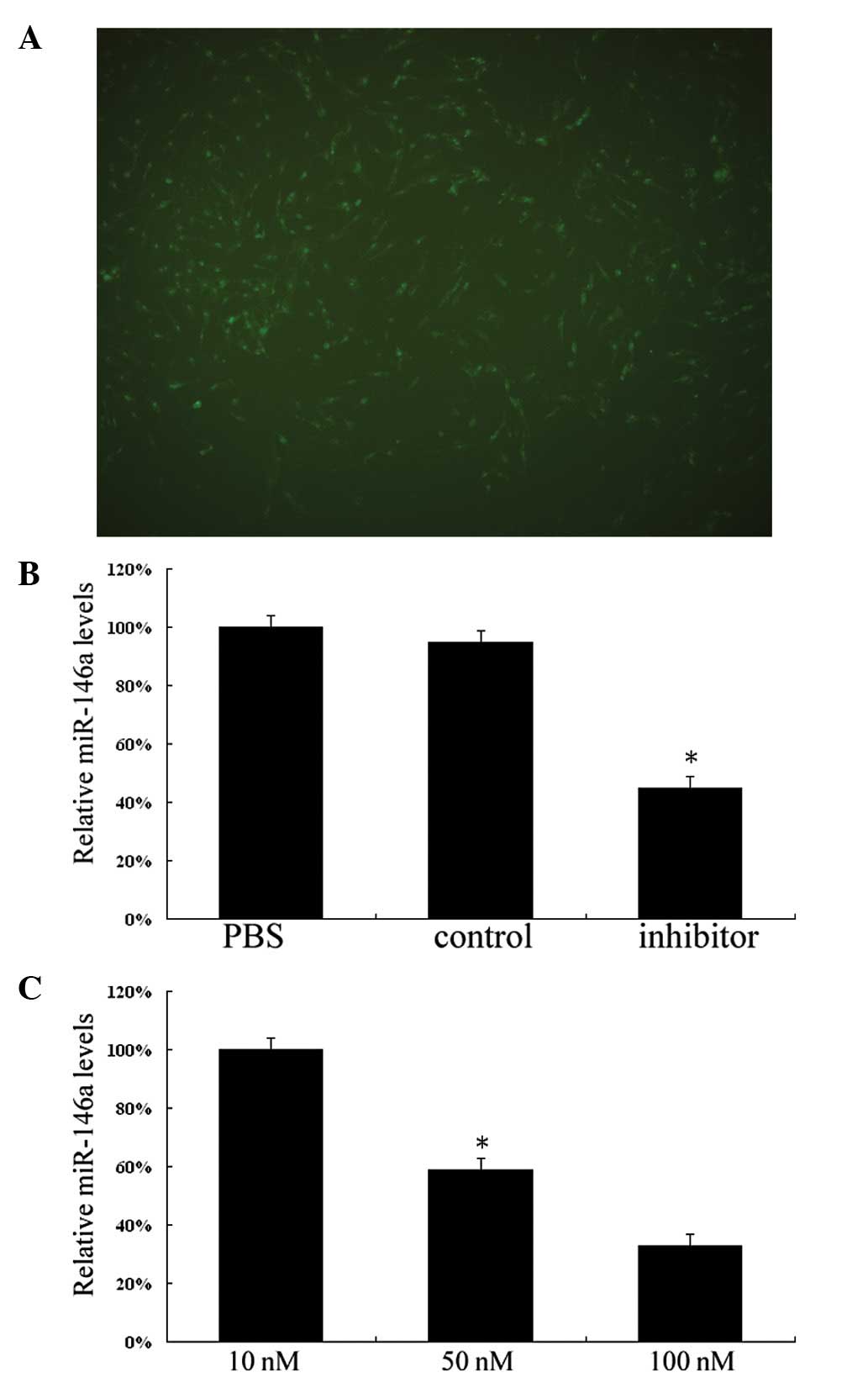
To knockdown miR-146a in vitro, we employed
siRNA-labeled 6-carboxyfluorescein to treat VSMCs by using
Lipofectamine 2000. As shown in Fig.
2A, extensive green fluorescence signals were observed in the
cytoplasm of VSMCs after treatment for 5 h. The average
transfection efficiency exceeded 90%. Subsequently, we investigated
the relative expression of miR-146a in cultured VSMCs after 48 h of
treatment by PBS, miR-146a control or miR-146a inhibitor. As shown
in Fig. 2B, the expression of
miR-146a in miR-146a inhibitor-treated VSMCs was significantly
lower than that in PBS-treated and miR-146a control-treated VSMCs
(P<0.05). The dose-dependent response of miR-146a expression to
the inhibitor was identified after treatment with various
concentrations of miR-146a inhibitors. Fig. 2C shows that expression of miR-146a
was downregulated by inhibitors in a dose-dependent manner
(P<0.05).
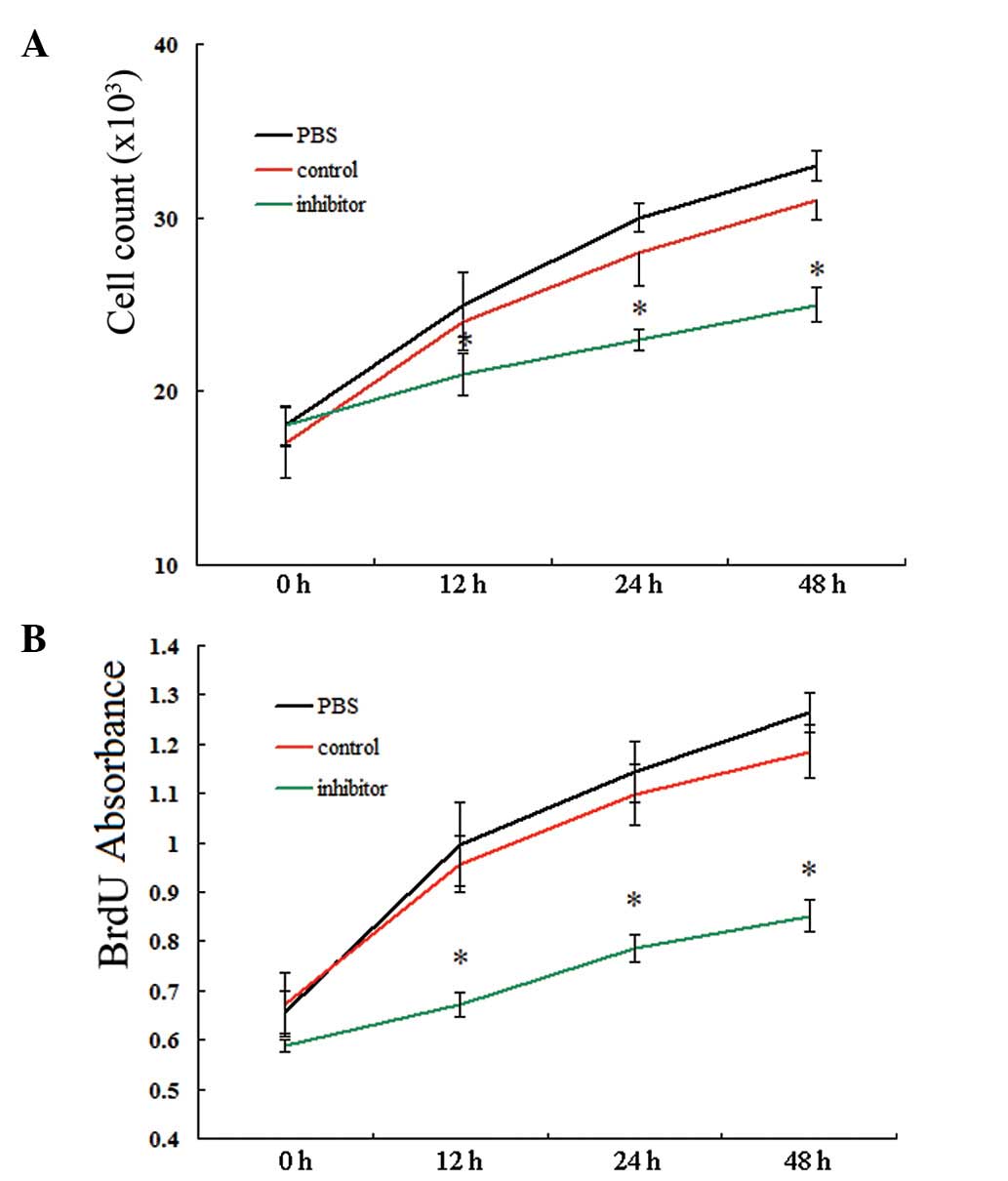
Knockdown of miR-146a inhibited
proliferation of VSMCs
In subsequent experiments, we identified the role of
miR-146a in the proliferation of VSMCs by applying two different
methods: cell counting and BrdU incorporation assay. As shown in
Fig. 3, the cell number and BrdU
ELISA absorbance showed no significant differences among VSMCs
treated with PBS, miR-146a control and miR-146a inhibitor,
respectively, prior to transfection (P>0.05). However, after
transfection, the VSMCs treated with miR-146a inhibitor exhibited
markedly lowered ability for proliferation compared with the VSMCs
treated with PBS or miR-146a control. These differences were
observed from 12 to 48 h after treatment (all P<0.05). These
results indicated that miR-146a is a proproliferative regulator for
VSMCs.
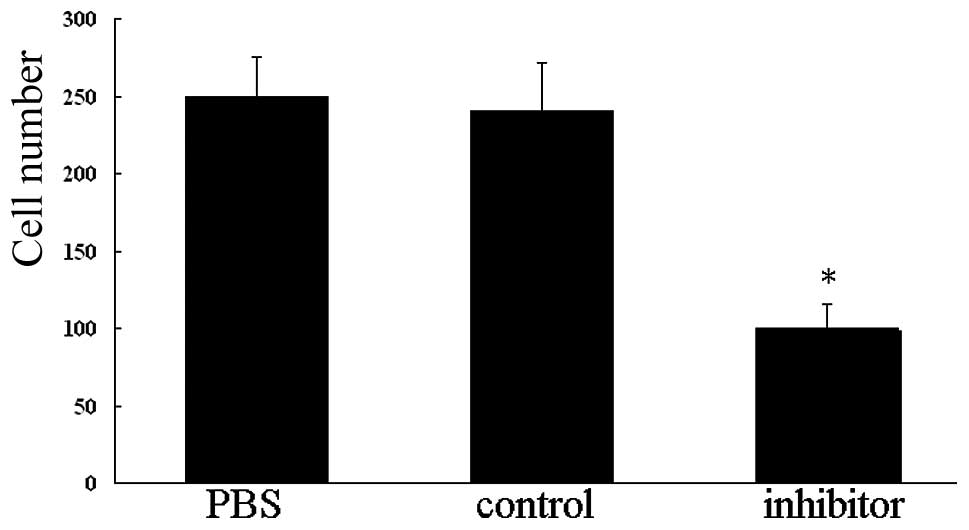
Knockdown of miR-146a inhibits migration
of VSMCs
To further confirm that knockdown of miR-146a is
capable of inhibiting VSMC migration, the Transwell system was
utilized. As expected, the number of cells that migrated to the
lower chamber in VSMCs treated with miR-146a inhibitor for 48 h was
markedly reduced compared with those in VSMCs treated with PBS or
miR-146a control (P<0.01; Fig.
4). These results indicate that miR-146a is capable of
promoting migration of VSMCs in vitro.
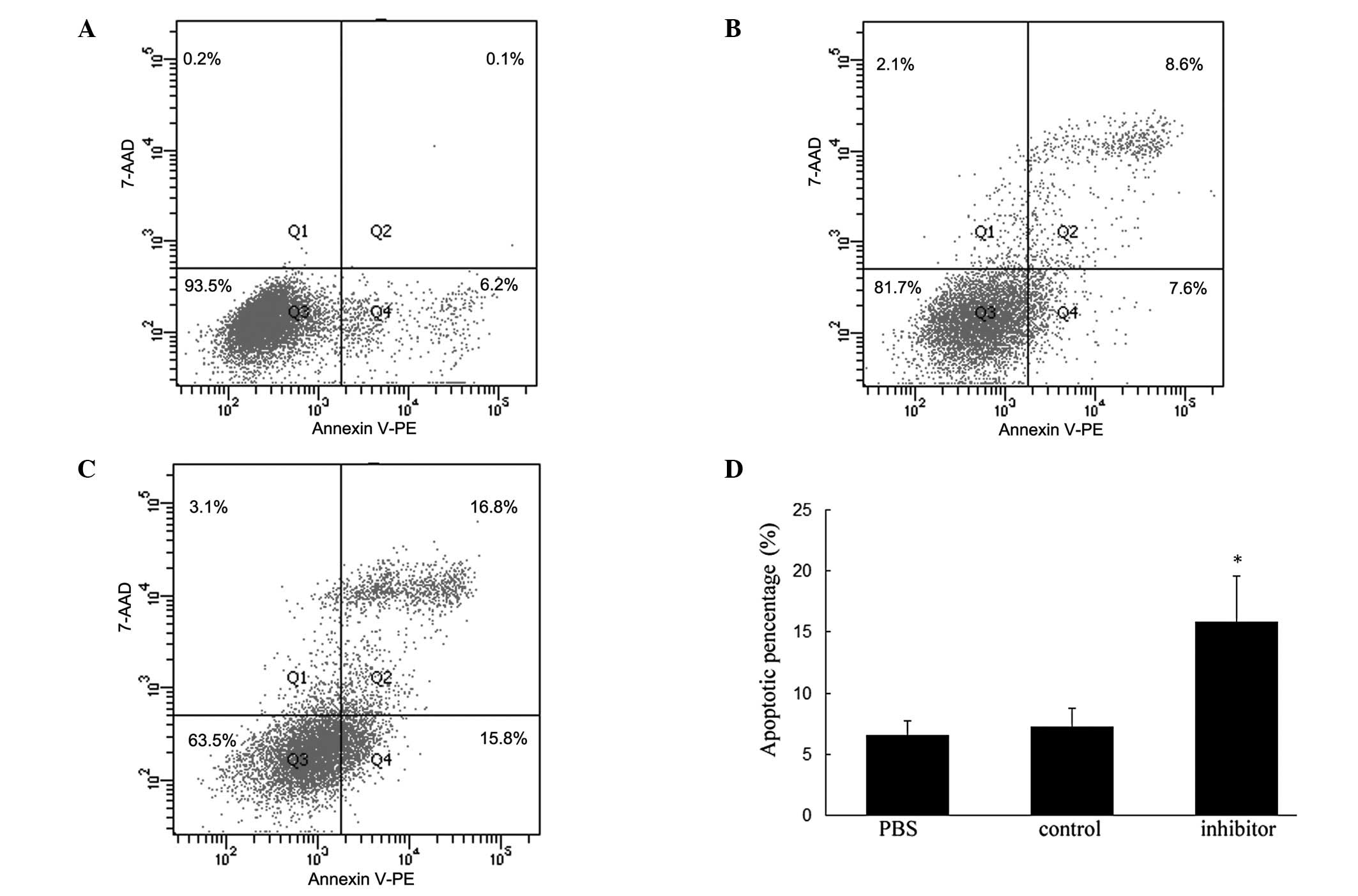
Knockdown of miR-146a promotes apoptosis
of VSMCs
To explore whether miR-146a affects VSMC survival
in vitro, we applied flow cytometry to examine the apoptotic
aspects in VSMCs after treatment for 48 h. Combining Annexin V-PE
with 7-AAD, three categories of cellular populations were detected:
viable, early apoptotic, late apoptotic or necrotic cells. As shown
in Fig. 5, the percentage of
apoptotic cells in the miR-146a-inhibitor treated group was
significantly increased compared with VSMCs treated with PBS or
miR-146a control (P<0.05). These results indicated that
knockdown of miR-146a is capable of promoting apoptosis of VSMCs
in vitro and also supported the conclusion that knockdown of
miR-146a inhibits VSMC proliferation.
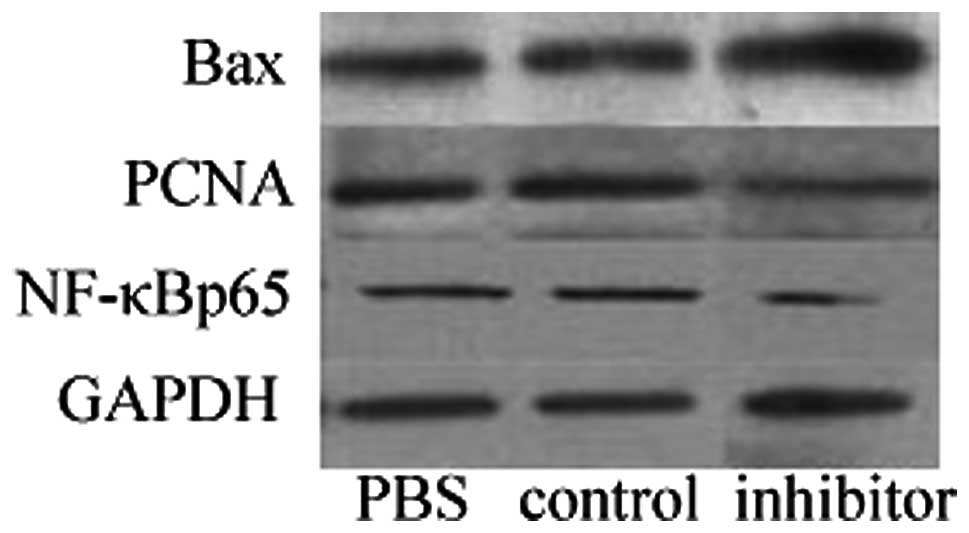
Western blot analysis
To identify the potential molecular mechanisms
underlying the regulation of proliferation and apoptosis by
miR-146a in cultured VSMCs, the protein levels of NF-κBp65, PCNA
and Bax were determined by western blot analysis. As shown in
Fig. 6, the crucial
transcriptional factor NF-κBp65 and the proliferative protein PCNA
were confirmed to be downregulated in miR-146a inhibitor-treated
VSMCs (both P<0.05), while the important proapoptotic protein
Bax was shown to be upregulated in VSMCs following miR-146a
transfection (P<0.05).
Discussion
The emergence of miRNAs has greatly increased our
knowledge of the molecular mechanisms underlying gene expression
and regulation. Studies have identified important functions of
miRNAs for the normal development and physiological homeostasis of
organisms. Increasing evidence has also highlighted their potential
roles as biomarkers in diagnosis and as targets for therapy
(13–16). However, there remain a number of
unclear details in the understanding of these complex gene
regulatory networks.
Previously identified as a novel determinant of the
molecular circuitry that governs innate immunity and inflammatory
responses, miR-146a was initially investigated in the immune
system, cancer and viral infections (12,17–23).
It has been observed that miR-146a was upregulated in THP-1 cells,
macrophages, Langerhans cells and monocytes after being induced by
lipopolysaccharide (LPS), TNF-α, IL-1β or TGF-β1 (12,18,24).
Similar outcomes were revealed in peripheral blood mononuclear
cells (PBMCs) and synovial tissue from patients with rheumatoid
arthritis (25,26). In the majority of cells, these
pro-inflammatory cytokines stimulated expression of miR-146a and
are dependent on the NF-κB signaling pathway, with the exception of
human lung alveolar epithelial cells (27). Notably, two critical downstream
molecules of cytokine and Toll-like receptors (TLRs), TNF
receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated
kinase 1 (IRAK1), were ascertained as direct target genes of
miR-146 (12,21). The activation of NF-κB is
downregulated by miR-146 through a negative feedback regulatory
loop involving TRAF6 and IRAK1. Recent evidence indicated that
miRNAs may play a role in phenotypic modulation of VSMCs (28,29).
More importantly, enhanced expression of miR-146a was confirmed in
the vascular wall after angioplasty and in PBMCs of patients with
acute coronary syndrome (ACS) (11,30).
The development of proliferative vascular diseases is an
exquisitely orchestrated process that involves a cascade of
inflammatory and immune responses, and subsequent activation,
proliferation and migration of VSMCs, as well as accelerated
synthesis of ECM components and eventual formation of neointimal
lesions. These aforementioned studies suggested that miR-146a may
play an important role in controlling VSMC fate.
In our present study, we first explored the role of
miR-146a in the proliferation process of VSMCs. A significantly
higher expression level of miR-146a was observed in proliferative
VSMCs, which indicated that miR-146a is a proproliferative factor
for VSMCs. To further verify the role of miR-146a in VSMC
proliferation and migration, we employed the well established
method of RNA interference to specifically knockdown miR-146a in
cultured VSMCs. The results revealed that miR-146a-specific siRNA
was sufficient to inhibit the proliferative and migratory capacity
of VSMCs, which indicated that miR-146a is an essential
pro-proliferative and pro-migratory regulator for VSMCs. In
addition, cell viability experiments determined by BrdU
incorporation assay showed there was no nonspecific toxicity after
miR-146a inhibitor delivery, which demonstrated that the effect of
miR-146a siRNA in regulating VSMC fate is miR-146a specific.
The equilibrium between proliferation and apoptosis
in VSMCs is responsible for neointimal fate. The increased VSMC
proliferation or decreased VSMC apoptosis contribute to neointimal
hyperplasia and vascular remodeling, which constitute the important
factors in the pathogenesis of proliferative vascular diseases. To
characterize the effect of miR-146a on VSMC functional modulation,
the apoptotic assays were performed. We observed that exogenous
inhibition of miR-146a markedly enhanced VSMC apoptosis. Moreover,
western blot analysis revealed that the Bax protein, which is a key
proapoptotic signal molecule (31), was simultaneously activated and
upregulated in VSMCs transfected with miR-146a inhibitor, although
the detailed mechanisms involved remain unclear. The anti-apoptotic
effect of miR-146a is consistent with its pro-proliferative effect
on VSMCs.
Our results from another western blot analysis also
revealed that NF-κBp65 and PCNA were involved in miR-146a-mediated
VSMC maturation and differentiation. Notably, in the majority of
immune and cancer cells, miR-146a regulated the NF-κB signal
pathway in a negative feedback manner (12,21),
while in our present study, the activity of NF-κBp65 in VSMCs was
markedly reduced by miR-146a inhibitor, which indicates that
miR-146a stimulated activation of NF-κB in VSMCs. Similar
upregulation of NF-κB induced by miR-146a was observed in PMBCs
from patients with ACS (30). It
appears that miR-146a plays different roles in diverse cells due to
its cell-type specificity. It is possible that miR-146a behaves as
a tuning mechanism to prevent overstimulated inflammatory responses
in the innate immune system, while in proliferative vascular
diseases, including atherosclerosis, post-angioplasty restenosis
and stroke, miR-146a acts as a promoter to play a role in the
pathological process at an early stage.
In conclusion, we revealed that the noncoding small
miRNA, miR-146a, is a novel regulator of VSMC proliferation,
migration and apoptosis. The modulation of VSMC fate mediated by
miR-146a was likely to be related to the activation of the NF-κB
signal transduction pathway. However, our work was only performed
in loss-of-function experiments of miR-146a, therefore, further
gain-of-function studies such as overexpression of miR-146a using
its precursor or a viral vector should be implemented in order to
clearly understand its mechanisms in regulating gene expression and
to evaluate its potential as a biomarker or therapeutic target in
proliferative cardiovascular diseases.
Acknowledgements
This study was supported by the Science Foundation
of Shenzhen (Project number: 201102158).
References
|
1
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rzucidlo EM, Martin KA and Powell RJ:
Regulation of vascular smooth muscle cell differentiation. J Vasc
Surg. 45:A25–A32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J,
Levine M, Leahy P, Davidson E and Ruvkun G: Conservation of the
sequence and temporal expression of let-7 heterochronic regulatory
RNA. Nature. 408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Ransom JF, Li A, Vedantham V, von
Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ and
Srivastava D: Serum response factor regulates a muscle-specific
microRNA that targets Hand2 during cardiogenesis. Nature.
436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Ransom JF, Li A, Vedantham V, von
Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ and
Srivastava D: Dysregulation of cardiogenesis, cardiac conduction,
and cell cycle in mice lacking miRNA-1-2. Cell. 129:303–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sayed D, Hong C, Chen IY, Lypowy J and
Abdellatif M: MicroRNAs play an essential role in the development
of cardiac hypertrophy. Circ Res. 100:416–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, Chen G and Wang Z: The
muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic
potential by targeting GJA1 and KCNJ2. Nat Med. 13:486–491. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, Chen G and Wang Z: MicroRNAs in the
human heart: a clue to fetal gene reprogramming in heart failure.
Circulation. 116:258–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of microRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-κB dependent induction of microRNA miR-146a, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
|
|
13
|
Bandiera S, Hatem E, Lyonnet S and
Henrion-Caude A: microRNAs in diseases: from candidate to modifier
genes. Clin Genet. 77:306–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar
|
|
15
|
Kaikkonen MU, Lam MT and Glass CK:
Non-coding RNAs as regulators of gene expression and epigenetics.
Cardiovasc Res. 90:430–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fichtlscherer S, Zeiher AM and Dimmeler S:
Circulating microRNAs: biomarkers or mediators of cardiovascular
diseases? Arterioscler Thromb Vasc Biol. 31:2383–2390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nahid MA, Pauley KM, Satoh M and Chan EK:
miR-146a is critical for endotoxin-induced tolerance: implication
in innate immunity. J Biol Chem. 284:34590–34599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jurkin J, Schichl YM, Koeffel R, Bauer T,
Richter S, Konradi S, Gesslbauer B and Strobl H: miR-146a is
differentially expressed by myeloid dendritic cell subsets and
desensitizes cells to TLR2-dependent activation. J Immunol.
184:4955–4965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu LF, Boldin MP, Chaudhry A, Lin LL,
Taganov KD, Hanada T, Yoshimura A, Baltimore D and Rudensky AY:
Function of miR-146a in controlling treg cell-mediated regulation
of Th1 responses. Cell. 142:914–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos
RT, Croce CM and de la Chapelle A: The role of microRNA genes in
papillary thyroid carcinoma. Proc Natl Acad Sci USA.
102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-κB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008.PubMed/NCBI
|
|
22
|
Li Y, Vandenboom TG 2nd, Wang Z, Kong D,
Ali S, Philip PA and Sarkar FH: miR-146a suppresses invasion of
pancreatic cancer cells. Cancer Res. 70:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cameron JE, Yin Q, Fewell C, Lacey M,
McBride J, Wang X, Lin Z, Schaefer BC and Flemington EK:
Epstein-Barr virus latent membrane protein 1 induces cellular
microRNA miR-146a, a modulator of lymphocyte signaling pathways. J
Virol. 82:1946–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu G, Friggeri A, Yang Y, Park YJ,
Tsuruta Y and Abraham E: miR-147, a microRNA that is induced upon
toll-like receptor stimulation, regulates murine macrophage
inflammatory responses. Proc Natl Acad Sci USA. 106:15819–15824.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pauley KM, Satoh M, Chan AL, Bubb MR,
Reeves WH and Chan EK: Upregulated miR-146a expression in
peripheral blood mononuclear cells from rheumatoid arthritis
patients. Arthritis Res Ther. 10:R1012008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakasa T, Miyaki S, Okubo A, Hashimoto M,
Nishida K, Ochi M and Asahara H: Expression of microRNA-146 in
rheumatoid arthritis synovial tissue. Arthritis Rheum.
58:1284–1292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perry MM, Moschos SA, Williams AE,
Shepherd NJ, Larner-Svensson HM and Lindsay MA: Rapid changes in
microrna-146a expression negatively regulate the IL-1β-induced
inflammatory response in human lung alveolar epithelial cells1. J
Immunol. 180:5689–5698. 2008.PubMed/NCBI
|
|
28
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
29
|
Davis BN, Hilyard AC, Nguyen PH, Lagna G
and Hata A: Induction of microRNA-221 by platelet-derived growth
factor signaling is critical for modulation of vascular smooth
muscle phenotype. J Biol Chem. 284:3728–3738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y,
Zhou W, Xiong B and Zeng Q: miR-146a in PBMCs modulates Th1
function in patients with acute coronary syndrome. Immunol Cell
Biol. 88:555–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lalier L, Cartron PF, Juin P, Nedelkina S,
Manon S, Bechinger B and Vallette FM: Bax activation and
mitochondrial insertion during apoptosis. Apoptosis. 12:887–896.
2007. View Article : Google Scholar : PubMed/NCBI
|