Introduction
Chronic pressure overload induces myocardial
hypertrophy, also called ‘pathological’ cardiac hypertrophy, which
is compensatory. Excessive hypertrophy, however, is associated with
significantly poor prognosis including increased cardiac morbidity,
mortality and ultimate heart failure in humans (1,2).
3-Hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, also
known as statins, exert a lipid-lowering effect in the blood which
has been extensively investigated (3). Additionally, recent experimental
evidence indicates that certain statins restore or improve
endothelial function (4), decrease
smooth muscle cell (SMC) content and collagen accumulation in
atherosclerotic plaques (5), play
an anti-inflammatory role associated with acute coronary events
(6) and activate the expression of
peroxisome proliferator-activated receptors (PPARs) (7,8).
Moreover, according to previous studies, statins have been shown to
attenuate cardiac hypertrophy (9)
and to delay the progression from cardiac hypertrophy to failure
under pressure overload in aortic banding models (4,10).
Additional evidence suggests that phosphorylated Akt/glycogen
synthase kinase 3β (GSK3β), extracellular signal-regulated kinases
1 or 2 (ERK1/2) and GATA binding protein 4 (GATA4) pathways play
important roles in the process of cardiac hypertrophy and
ventricular remodeling induced by pressure overload (11–13).
However, the molecular mechanisms of action of rosuvastatin (RSV),
a hydrophilic statin which is involved in the hypertrophic response
to pressure overload, remains to be fully elucidated in
vivo. Therefore, we hypothesized that statins inhibit cardiac
hypertrophy through the inhibition of numerous activation signaling
pathways differentially associated to myocardial hypertrophy, such
as the Akt, ERK1/2 and GATA4 signaling pathways.
The present study was divided into two major parts.
We firstly investigated the transition from normal to hypertrophic
myocardium and evaluated the effects of RSV on rats subjected to
abdominal aortic constriction. In the second part of this study, we
investigated the variation of activation pathways including Akt,
ERK1/2 and GATA4 in four groups. We also explored whether RSV could
reverse myocardial hypertrophy of the remodeling process following
long-term treatment.
Materials and methods
Reagents
RSV was kindly provided by AstraZeneca (London, UK).
Primary antibodies for total ERK1/2, AKT and their phosphorylated
forms were purchased from Cell Signaling Technology, Inc. (Beverly,
MA, USA). GATA4 and its phosphorylated forms were purchased from
Abcam (Cambridge, MA, USA). Rabbit anti-glyceraldehyde-3-phosphate
dehydrogenase (anti-GAPDH) antibody was provided by Hangzhou
Goodhere Biotechnology Co., Ltd. (Hangzhou, China). Secondary
horseradish peroxidase (HRP)-conjugated anti-rabbit antibody was
ordered from Multisciences Biotech Co., Ltd. (Hangzhou, China).
Enhanced chemiluminescence (ECL) reagent was obtained from
Millipore (Billerica, MA, USA). All the reagents were used
according to the manufacturer's instructions.
Animals
Male Wistar rats were supplied by the Novel
Pharmaceuticals Research Center of Shandong University (Jinan,
Shandong, China). The study was approved by the ethics committee of
Shandong Provincial Qianfoshan Hospital, Affiliated Hospital of
Shandong University, Jinan, China and the protocols were in
compliance with the Guidelines for the Care and Use of Laboratory
Animals published by the National Academy Press (NIH publication
no. 85-23, revised 1996).
Animal experimental protocols
Twenty-eight male Wistar rats, weighing 180–220 g,
were allowed free access to food and water, and were maintained on
a 12-h light/dark cycle at room temperature (21–23°C). When the 28
male Wistar rats weighed 300±10 g, they were randomly allocated
into 4 groups: the sham operation-vehicle (SH-V, n=7), abdominal
aortic constriction-vehicle (AAC-V, n=7), abdominal aortic
constriction-RSV 10 mg/kg/day (AAC-LO, n=7) and the abdominal
aortic constriction-RSV 20 mg/kg/day (AAC-HI, n=7) group. RSV was
dissolved in stroke-physiological saline solution daily; the
freshly prepared RSV solution was not preserved for >30 min
prior to gavage. Five days prior to aortic constriction, the rats
of the AAC-LO and AAC-HI groups were administered various doses of
RSV, while the rats of the SH-V and AAC-V groups were administered
an equal volume of vehicle by a lavage needle, until 4 weeks after
surgery. Drug or vehicle were administered once daily during the
entire experimental period. The rats underwent aortic constriction
or sham operation 5 days after initiation of gavage and were
sacrificed 4 weeks following surgery. Although higher doses of RSV
(30 and 50 mg/kg/day) were also administered, male Wistar rats did
not suffer any side-effects, such as intestinal obstruction of the
drug, during the the experiment.
Abdominal aortic constriction model
Abdominal aortic constriction-induced pressure
overload has been previously described (14). The rats were anesthetized with 10%
chloral hydrate [0.3 ml/100 g, intraperitoneal (i.p.) injection].
The adequacy of anesthesia was measured by assessing cardiac and
respiratory rates as well as pattern, lash reflex and muscle
relaxation. Following asepsis, abdominal aorta was exposed through
a ventral median line incision. Subsequently, ligatures were placed
around both the abdominal aortas (0.5 cm above renal artery
bifurcation) and an obtuse 22-gauge needle (outside diameter, 0.7
mm) with a 4-0 silk suture. The needle was promptly removed
following constriction. The rats of the SH-V group underwent
abdominal aortic constriction without ligation.
Echocardiographic evaluation
All the rats were anesthetized with 10% chloral
hydrate (0.3 ml/100 g, i.p.) and echocardiography was performed to
evaluate left ventricular function 4 weeks following the operation
(15). Left parasternal short-axis
views of the left ventricle were obtained using Sonos 5500
Ultrasound Machine with a S12 Pediatric Sector Probe at 10 MHz
(Hewlett-Packard Development Company, L.P., Palo Alto, CA, USA), at
the level of the papillary muscles. Dimensions of end diastolic
interventricular septum (IVSd), diastolic left ventricular
posterior wall (LVPWd) and left ventricular ejection fraction
(LVEF) were noninvasively measured. Three consecutive cycles of
measurements were performed and the results were averaged (16). Echocardiography and the measurement
procedure were performed in a double-blind manner.
Body weight and cardiac
characteristics
All of the rats were precisely weighed prior to
echocardiography and the weight recorded was considered as the
terminal body weight. The rats were perfused with
stroke-physiological saline solution through the left ventricle
immediately after echocardiography was performed and were then
sacrificed by decapitation. The hearts of decollated rats were
carefully excised and washed in double distilled water. The left
ventricle including the septum was carefully clipped from the
atrium and the right ventricle, and then weighed. The ratio of the
left ventricular weight to body weight (LVW/BW) was described as a
relative ratio.
Hematoxylin and eosin (H&E)
staining
For the investigation of the myocyte cross-sectional
area (CSA) of the different groups of rats, the heart tissues were
stained with H&E. The cross-sections of the left ventricle of
the rats in all 4 groups were submerged in 4% paraformaldehyde
solution for fixation and then embedded in wax. They were
transversely cut into 4-μm slices and the cross-sections were
selected adequately. The slices were dehydrated through a series of
graded alcohols (100, 95 and 75%), 15 min for each process. The
slices were then stained with Mayer's hematoxylin for 10–15 min and
washed under running tap water for 5–10 min. Each slice was
submerged in warm water until it appeared bright purple and then
counterstained in eosin solution for 2–3 min. After gently washing
with water, each slice was submerged in 85% alcohol, 100% alcohol I
and II for 15 min each. Finally, all of the slices were submerged
in Xylene I-Xylene II, for 15 min each. Photomicrographs were
obtained using an Olympus FSX100 microscope (magnification, ×30;
Tokyo, Japan) under the same conditions. The surplus tissues were
stored at −80°C for subsequent molecular biology research.
Histopathological characteristics
Myocyte CSAs in epicardial, midwall and endocardial
regions were randomly selected once for each location in the rats
of all 4 groups. In each selected location, a visual field of
≥30–50 cardiomyocytes was observed. Thus, ≥100 cardiomyocytes were
observed in all the visual fields in each sample of heart tissue.
The myocyte CSA (magnification, ×30) was investigated using the
Image-Pro Plus software (Media Cybernetics, Carlsbad, CA, USA).
RT-PCR of cellular RNA
Total cellular RNA was extracted and purified from
freezing left ventricular tissues of rats using TRIzol reagent
(TransGen Biotech, Beijing, China). Primers for atrial natriuretic
factor (ANF), β-myosin heavy chain (β-MHC) and peroxisome
proliferator-activated receptor α (PPARα) were designed and
synthesized by Sangon Biotechnology (Shanghai, China; Table I).
 | Table IPrimer sequences used for the target
genes and internal standard GAPDH gene in rats. |
Table I
Primer sequences used for the target
genes and internal standard GAPDH gene in rats.
| Gene | Primer | Size (bp) |
|---|
| PPARα | F:
5′-GTGGCTGCTATAATTTGCTGTG-3′
R: 5′-GGAGTTTTGGGAAGAGAAAGGT-3′ | 145 |
| ANF | F:
5′-AGGAGAAGATGCCGGTAGAAG-3′
R: 5′-AGAGCCCTCAGTTTGCTTTTC-3′ | 211 |
| GAPDH | F:
5′-ACAGCAACAGGGTGGTGGAC-3′
R: 5′-TTTGAGGGTGCAGCGAACTT-3′ | 256 |
| β-MHC | F:
5′-TCCAGAAGAGAAGAACTCCATTT-3′
R: 5′-ATACTCGTTGCCCACTTTGACT-3′ | 205 |
Oligo(dT)-primed RNA (2 μg) was reverse transcribed
to cDNA using a ReverTra Ace® qPCR RT kit (Toyobo Co.,
Ltd., Life Sciences Department, Osaka, Japan) in a volume system of
20 μl. As a template for PCR, cDNA was amplified with a Taq PCR
Master mix (CWBIO, Beijing, China) using a Gene-Pro™ PCR Thermal
Cycler. The thermocycling conditions were as follows: PCR products
were predegenerated at 94°C for 2 min, followed by 30 cycles of 30
sec at 94°C, 30 sec at different annealing temperatures (60°C for
ANF, β-MHC and GAPDH; 62°C for PPARα), then 30 sec at 72°C, and
finally a 2-min cycle at 72°C. Amplified PCR products were
electrophoresed on 2% agarose gels and stained with ethidium
bromide; the agarose gels were then visualized with a UV
illuminator (Tiangen Biotech, Inc., Beijing, China). To quantify
the relative density of each DNA band, all of the gel images were
analyzed using ImageJ software (NIH Image, Madison, WI, USA). GAPDH
was used as an internal control. The band intensities were measured
and normalized to the intensity of the respective GAPDH signal.
Three independent experiments were performed in each group.
Western blot analysis
Total Akt, total ERK1/2 and their phosphorylated
proteins (activation forms) were obtained using RIPA Lysis Buffer
[50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride,
EDTA, leupeptin] containing 1 mM phenylmethanesulfonyl fluoride
(PMSF). GATA4 and its phosphorylated protein (activation form) were
extracted from the nuclei of cardiomyocytes using the Nuclear and
Cytoplasmic Protein Extraction kit (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentrations were
determined by the BCA protein assay (Beyotime Institute of
Biotechnology). Equivalent amounts of protein samples (20 μg) from
homogenates were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) at a 12% acrylamide resolving gel.
Separated target proteins were transferred onto polyvinylidene
difluoride membranes (Millipore). The membranes were blocked with
5% bovine serum albumin (BSA) in TBST buffer (20 mM Tris-HCl, pH
7.6, 150 mM NaCl, 0.05% Tween-20) for 2 h at room temperature. The
membranes were then probed with primary antibodies against ERK1/2,
phospho-ERK1/2 (Thr202/Tyr204), Akt, phospho-Akt (Ser473)
(dilution, 1:1,000), GATA4, phospho-GATA4 (Ser105) (dilution, 1
μg/ml) and GAPDH (dilution, 1:1,000) followed by incubation with
the proper secondary horseradish peroxidase (HRP)-conjugated
antibodies (dilution, 1:5,000). Subsequently, the membranes were
washed with TBST thrice at room temperature, each for 5 min. The
reactive proteins were visualized using enhanced chemiluminescence
(ECL) reagent. The intensity of the bands was analysed using ImageJ
software (NIH Image) and the relative expression levels of proteins
were determined using proportionality of target proteins and
GAPDH.
Statistical analysis
The results are presented as the mean ± SEM.
Comparisons between groups were conducted using one-way analysis of
variance (ANOVA) followed by Bonferroni's post hoc test. P<0.05
and P<0.01 were considered to indicate statistically significant
and highly significant differences, respectively, for all the
analyses. All of the statistical analyses were conducted with SPSS
17.0 statistical software package (SPSS, Inc., Chicago, IL,
USA).
Results
Echocardiographic analysis
Echocardiographic analysis indicated an increased
thickness of IVSd (2.31±0.12 vs. 1.73±0.09 mm, P<0.01) and LVPWd
(2.15±0.07 vs. 1.68±0.09 mm, P<0.01) in vehicle-treated banded
rats compared with rats in the sham-operated group 4 weeks
following surgery. Treatment with RSV at a low and high dose
resulted in decreased IVSd (1.95±0.08 and 1.84±0.04 vs. 2.31±0.12
mm, P<0.01) and LVPWd (1.84±0.06 and 1.77±0.04 vs. 2.14±0.07 mm,
P<0.01) compared with rats in the untreated aortic banding group
4 weeks following surgery. Among the rats with aortic banding, LVEF
evaluated by transthoracic echocardiography was found to be
significantly decreased compared with the sham-operated rats
(69.41±2.85 vs. 91.49±2.18%, P<0.01). Additionally, LVEF of
heart tissues from rats in the RSV-treated aortic banding group was
higher compared with vehicle-treated banded rats (85.07±1.87 and
89.83±1.11 vs. 69.41±2.85%, P<0.01) 4 weeks following surgery
(Table II).
 | Table IIEchocardiographic analysis of IVSd,
LVPWd and LVEF, and measurements of LVW/BW in the rats of all
groups. |
Table II
Echocardiographic analysis of IVSd,
LVPWd and LVEF, and measurements of LVW/BW in the rats of all
groups.
| Group | IVSd (mm) | LVPWd (mm) | LVEF (%) | LVW/BW (mg/g) |
|---|
| SH-V | 1.73±0.09 | 1.68±0.09 | 91.5±2.2 | 1.93±0.17 |
| AAC-V | 2.31±0.12a | 2.15±0.07a | 69.4±2.8a | 3.14±0.39a |
| AAC-LO | 1.95±0.08a,c | 1.84±0.06a,c | 85.1±1.9a,c | 2.36±0.05a,b |
| AAC-HI | 1.84±0.04c | 1,77±0.04c | 89.9±1.1c | 2.15±0.06c |
Animal characteristics (LVW/BW)
LVW/BW was determined to evaluate the extent of
myocardial hypertrophy. Constriction of abdominal aorta led to
higher LVW/BW compared with the ratio of the sham-operated rats
(3.14±0.39 vs. 1.93±0.17 mg/g, P<0.01) 4 weeks following
surgery. A significantly decreased LVW/BW was observed in
RSV-treated aortic banding rats when compared with the ratio of the
vehicle-treated banded rats (2.36±0.55 vs. 3.14±0.39 mg/g,
P<0.05 and 2.15±0.06 vs. 3.14±0.39 mg/g, P<0.01) 4 weeks
following surgery.
Assessment of cardiomyocyte CSA
As expected, following histological analysis, a 406%
increase in myocyte CSA of the AAC-V rats was observed compared
with the CSA of SH-V rats 4 weeks following surgery. The CSA of
RSV-treated banded rats treated with low and high RSV doses was
decreased by ~39 and 66%, respectively, compared with the CSA of
the AAC-V rats 4 weeks following surgery (Fig. 1).
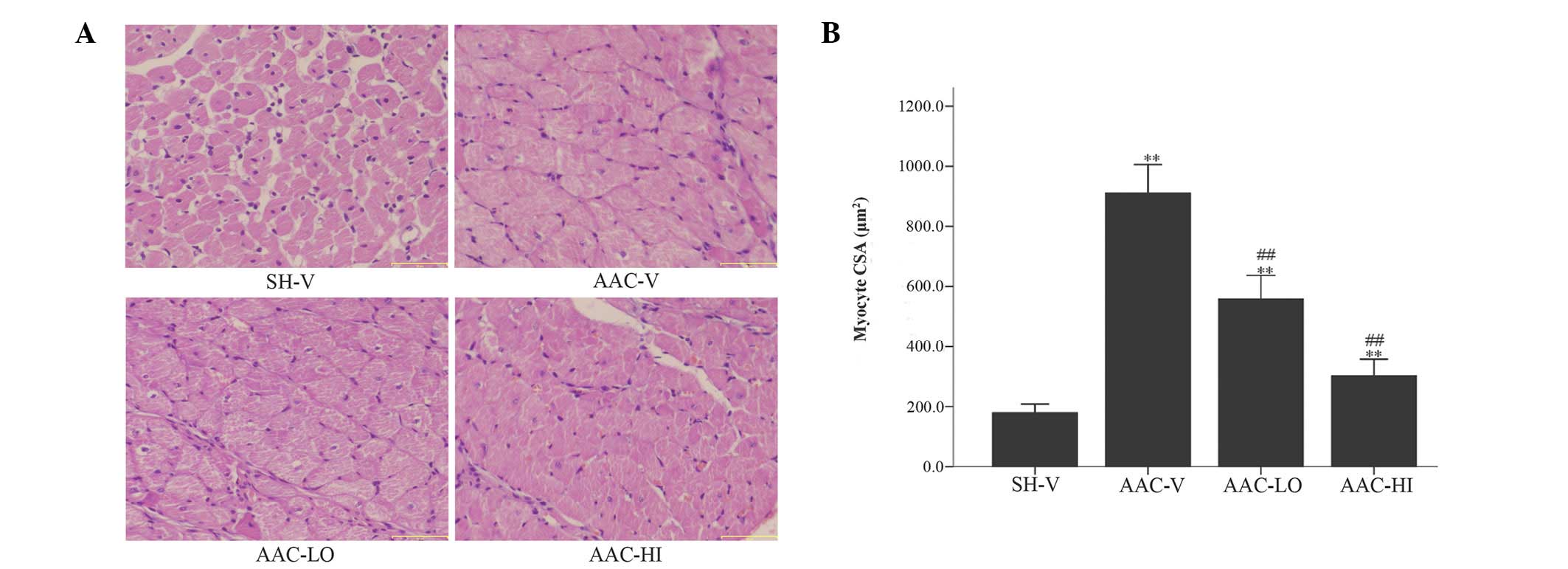 | Figure 1Mean histological CSA following
H&E staining at high magnification (x30) was assessed in the
left ventricles of rats in the SH-V, AAC-V, AAC-LO and AAC-HI
groups. (A) H&E staining (magnification, ×30); scale bars, 50
μm and (B) quantitative analysis of mean CSA (μm2) of
cardiac myocytes in the 4 groups. Results are expressed as the mean
± SEM. **P<0.01 vs. SH-V group;
##P<0.01 vs. AAC-V group. CSA, cross-sectional area;
H&E, hematoxylin and eosin; RSV, rosuvastatin; SH-V, sham
operation-vehicle control group; AAC-V, abdominal aortic
constriction-vehicle control group; AAC-LO, abdominal aortic
constriction-RSV 10 mg/kg group; AAC-HI, abdominal aortic
constriction-RSV 20 mg/kg group. |
ANF, β-MHC and PPARα mRNA expression
In addition to the morphological changes, ANF and
β-MHC mRNA expression levels as hypertrophic markers were
significantly increased in rats with aortic banding compared with
sham-operated rats (ANF/GAPDH, 1.09±0.08 vs. 0.26±0.05; P<0.01
and β-MHC/GAPDH, 1.19±0.07 vs. 0.32±0.02; P<0.01). However,
PPARα expression was decreased in aortic banded rats compared with
the sham-operated rats (PPARα/GAPDH, 0.60±0.07 vs. 1.21±0.03;
P<0.01). Furthermore, ANF and β-MHC expression levels were
decreased according to the RSV doses used in banded rats compared
with vehicle-treated rats (ANF/GAPDH, 0.73±0.04 and 0.53±0.03 vs.
1.09±0.08; β-MHC/GAPDH, 0.63±0.04 and 0.46±0.03 vs. 1.19±0.07;
P<0.01), in contrast with PPARα mRNA expression, which was
significantly increased (PPARα/GAPDH, 0.99±0.04 and 1.02±0.04 vs.
0.60±0.07; P<0.01). No significant differences in PPARα
expression were observed between the two RSV-treated groups
(P>0.05; Fig. 2).
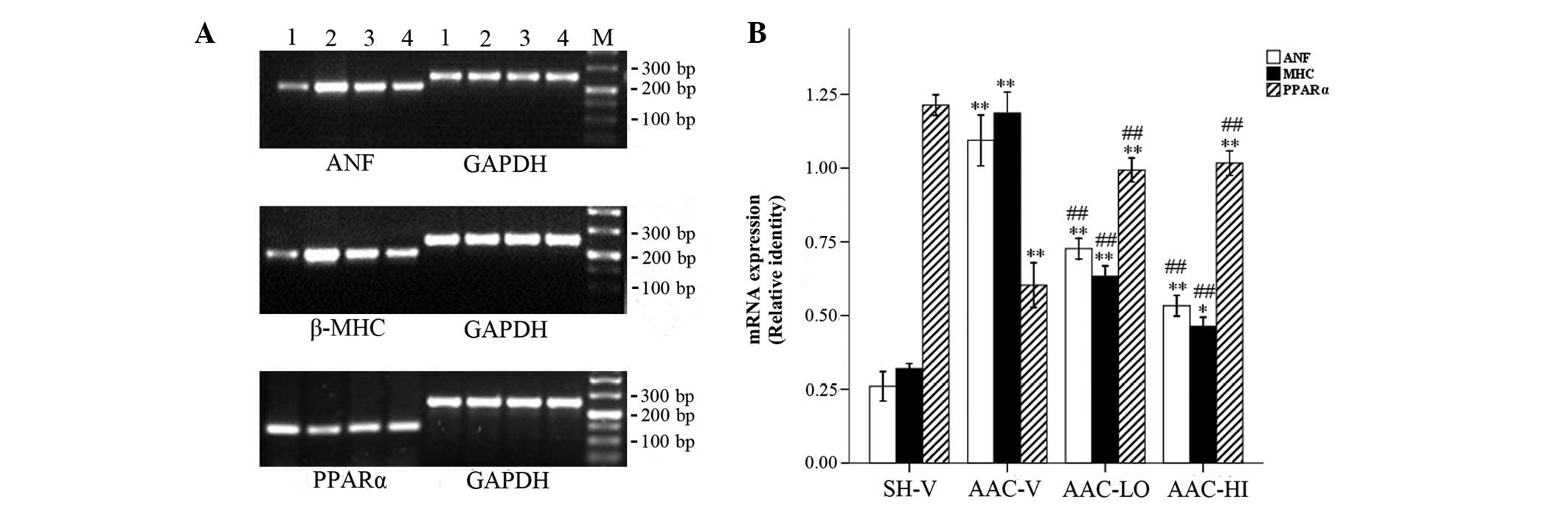 | Figure 2RT-PCR detection of ANF, β-MHC and
PPARα mRNA relative levels in SH-V, AAC-V, AAC-LO and AAC-HI
groups. (A) mRNA expression of ANF, β-MHC and PPARα. M, marker;
lane 1, SH-V; lane 2, AAC-V; lane 3, AAC-LO; lane 4, AAC-HI. (B)
Relative qualitative identities of mRNAs were normalized to the
intensity of GAPDH. The adjusted mRNA level for every group was the
average of three measurements. Results are expressed as the mean ±
SEM. **P<0.01 vs. SH-V group; ##P<0.01
vs. AAC-V group. RSV, rosuvastatin; ANF, atrial natriuretic factor;
β-MHC, β-myosin heavy chain; PPARα, proliferator-activated receptor
α; SH-V, sham operation-vehicle control group; AAC-V, abdominal
aortic constriction-vehicle control group; AAC-LO, abdominal aortic
constriction-RSV 10 mg/kg group; AAC-HI, abdominal aortic
constriction-RSV 20 mg/kg group. |
Analysis of Akt, ERK1/2 and GATA4 protein
activation
As shown in Figs. 3
and 4, quantitative total Akt and
ERK1/2 protein expression (relative identities compared with GAPDH)
were not significantly altered among the 4 groups (P>0.05).
Phospho-Akt/Akt (p-Akt/Akt), (phospho-ERK1/2)/ERK1/2
[(p-ERK1/2)/ERK1/2] and phospho-GATA4/GATA4 (p-GATA4/GATA4)
relative protein levels were significantly increased in aortic
banded rats compared with the sham-vehicle rats [p-Akt/Akt,
2.40±0.06 vs. 0.74±0.05; (p-ERK1/2)/ERK1/2, 0.71±0.06 vs.
0.33±0.02; p-GATA4/GATA4, 0.30±0.03 vs. 0.06±0.02; P<0.01].
However, the relative expression of the three proteins was
significantly reduced in the aortic banded rats treated with low
and high doses of RSV compared with the sham-operated rats
[p-Akt/Akt, 1.05±0.05 and 0.83±0.04 vs. 2.40±0.06;
(p-ERK1/2)/ERK1/2, 0.45±0.06 and 0.41±0.01 vs. 0.71±0.06;
p-GATA4/GATA4, 0.22±0.02 and 0.13±0.02 vs. 0.30±0.03,
P<0.01].
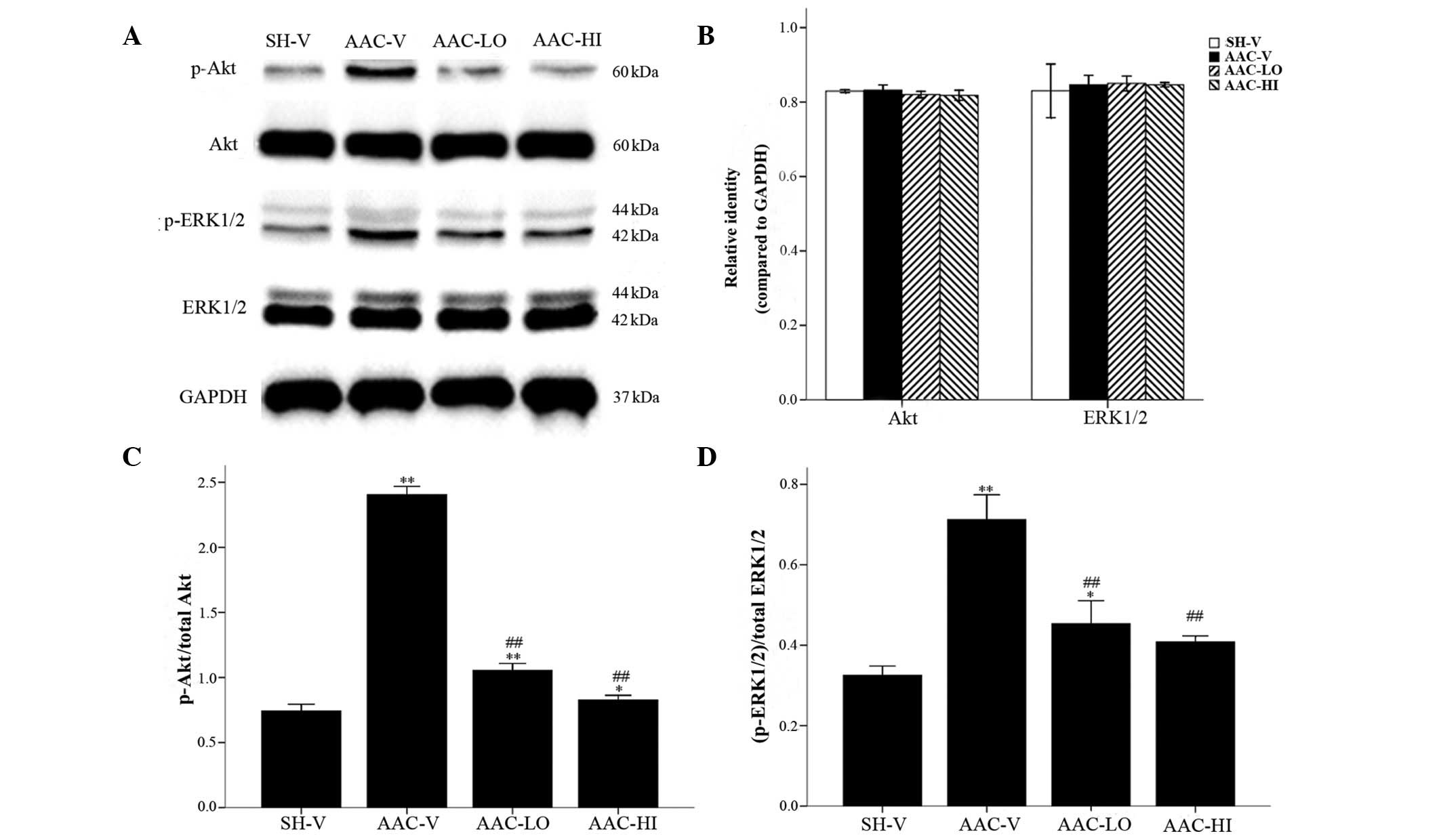 | Figure 3Western blot analysis of total Akt and
ERK1/2, and their phosphorylated isoform protein levels in the
cardiac tissue of rats in the SH-V, AAC-V, AAC-LO and AAC-HI
groups. (A) Akt, ERK1/2, p-Akt and p-ERK1/2 protein levels. (B)
Quantification of Akt and ERK1/2 compared with GAPDH.
Quantification of (C) p-Akt and (D) p-ERK1/2 protein levels
compared with their nonphosphorylated forms. Results are expressed
as the mean ± SEM. *P<0.05, **P<0.01
vs. SH-V group; ##P<0.01 vs. AAC-V group. RSV,
rosuvastatin; ERK1/2, extracellular signal-regulated kinases 1 or
2; SH-V, sham operation-vehicle control group; AAC-V, abdominal
aortic constriction-vehicle control group; AAC-LO, abdominal aortic
constriction-RSV 10 mg/kg group; AAC-HI, abdominal aortic
constriction-RSV 20 mg/kg group. |
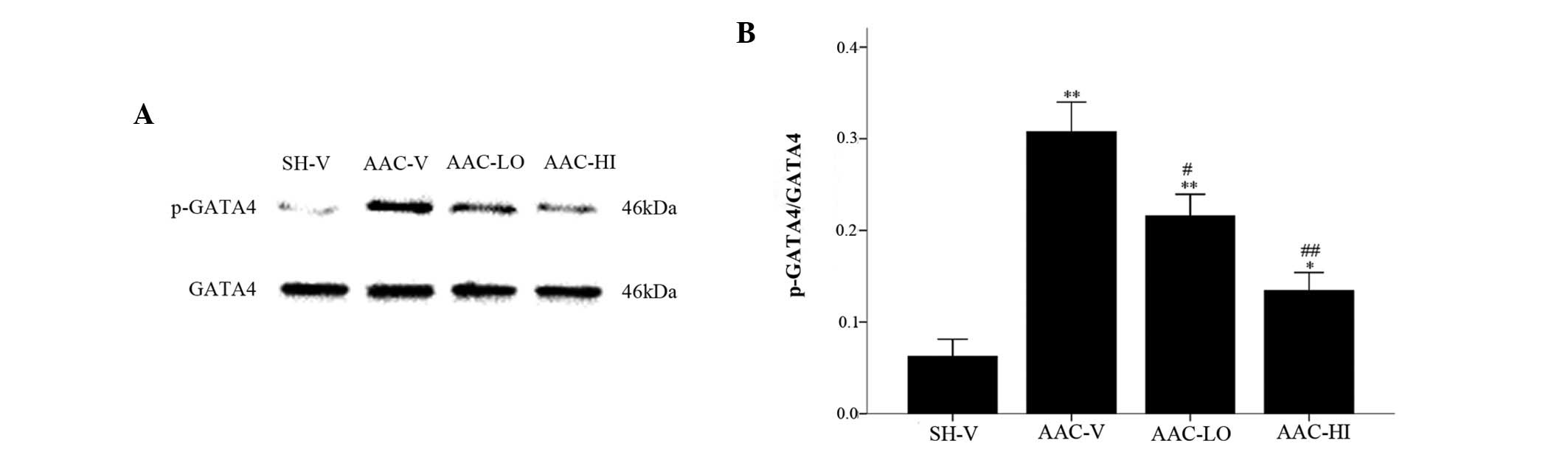 | Figure 4Nonphosphorylated and phosphorylated
isoform protein levels of GATA4 in the SH-V, AAC-V, AAC-LO and
AAC-HI groups. (A) GATA4 and p-GATA4 protein levels. (B)
Quantification of p-GATA4 protein levels compared with GATA4.
Results are expressed as the mean ± SEM. *P<0.05,
**P<0.01 vs. SH-V group; #P<0.05,
##P<0.01 vs. AAC-V group. RSV, rosuvastatin; GATA4,
GATA binding protein 4; SH-V, sham operation-vehicle control group;
AAC-V, abdominal aortic constriction-vehicle control group; AAC-LO,
abdominal aortic constriction-RSV 10 mg/kg group; AAC-HI, abdominal
aortic constriction-RSV 20 mg/kg group. |
Discussion
It is well known that ventricular hypertrophy
followed by heart failure constitute familiar outcomes of abdominal
aortic constriction induced by pressure overload in rats (17,18).
Previous studies on this model have shown that β-adrenergic (AR)
blockers and angiotensin converting enzyme inhibitors suppress
myocardial hypertrophy (19,20).
To the best of our knowledge, this model was used for the first
time in the present study to investigate the anti-myocardial
hypertrophy effect of a novel HMG-CoA reductase inhibitor, RSV, and
the potential underlying molecular mechanisms of action.
The beneficial effects of statins on left
ventricular hypertrophy (LVH) regression in various hypertrophy
models has been previously shown. Particularly, statins have been
reported to enhance endothelial nitric oxide synthase (eNOS)
expression and to inhibit NADPH oxidase activity, which has been
associated with myocardial hypertrophy endothelial function and the
development of myocardial hypertrophy (9,21).
Statins have also been reported to inhibit myocardial hypertrophy
via the ERK1/2 activation signaling pathway in spontaneously
hypertensive rats (22). However,
there have been controversial results regarding the effect of RSV
on cardiac hypertrophy, which have been associated with the
different types of animal hypertrophy models and the different
experimental protocols used. RSV has been shown to inhibit cardiac
hypertrophy via suppression of Gh and cardiac oxidative stress
(23,24). However, Chang et al(25) showed that RSV treatment does not
reverse hypertension-induced LVH; no beneficial effects on heart
failure and survival were also observed (25). The underlying mechanisms of action
of RSV on abdominal aortic constriction-induced myocardial
hypertrophy have yet to be fully elucidated. Therefore, the Akt,
ERK1/2 and GATA4 signaling pathways were investigated in the
present study.
Akt is a serine/threonine protein kinase and acts as
an important pathway which is involved in the regulation of heart
development. Short-term Akt activation, occurring in postnatal
cardiac development and trained athletes, promotes physiological
hypertrophy, while long-term activation of Akt occurring in
unbounded hypertension, myocardial infarction and aortic stenosis,
results in pathological hypertrophy and heart failure (26). In the present study, we found that
the increased phosphorylated Akt protein level is associated with
cardiac hypertrophy (pathological hypertrophy) in response to
pressure overload (11).
Mitogen-activated protein kinases (MAPKs) are 3-tiered kinase
cascades that are classified into 3 distinct subfamilies, including
extracellular signal receptor-regulated kinases (ERKs), c-jun
NH2-terminal kinases (JNKs) and p38 MAPK. The three MAPK pathways
have been shown to be activated in the myocardium of mice following
transverse aortic constriction (TAC) operation (12). The phosphorylated transcription
factor GATA4 is required for pressure overload-induced cardiac
hypertrophy in vivo(13).
It has been proved that the phosphorylation of GATA4 at Ser105
directly activated by ERK1/2, improves the transcriptional activity
and DNA-binding affinity of GATA4 in vivo and in
vitro(27,28). The activated Akt pathway managed to
enforce its hypertrophic effect via GSK3β/GATA4 phosphorylation
activity in cardiac myocytes in vivo and in vitro.
Activity of the Akt signal pathway is not involved with activity of
the ERK1/2 signal pathway. They are two different signal pathways
which lead to GATA4 activation (29,30).
These data suggest that the activation of the Akt, ERK1/2 and GATA4
signaling pathways is involved in pressure overload-induced cardiac
hypertrophy. Thus, we further aimed to investigate the activation
of Akt, ERK1/2 and GATA4 activation signaling pathways in rats
disposed by abdominal aortic constriction, and to examine whether
RSV reduces pressure overload-induced cardiac hypertrophy by
preventing the activation of these molecular pathways. PPARα is a
member of the nuclear receptor of ligand-activated transcription
factors. Relevant studies have shown that deficiency of PPARα leads
to a more significant hypertrophic growth response, suggesting that
PPARα attenuates pathological cardiac remodeling induced by
pressure overload. Thus, PPARα has been suggested to exert
beneficial effects on cardiac hypertrophy (31).
In the present study, abdominal aortic
constriction-induced pressure overload resulted in left ventricular
myocardial hypertrophy due to the increase in LVW/BW,
echocardiography characteristics, cardiomyocyte area and mRNA
expression levels of the hypertrophy markers ANF and β-MHC. In
pressure overload-induced hypertrophic hearts, phosphorylated
activation of Akt, ERK1/2 and GATA4 proteins was significantly
increased, which was in agreement with the results of previous
studies (11,32,33).
Briefly, these results showed that the phosphorylation levels of
Akt, ERK1/2 and GATA4 proteins are involved in myocardial
hypertrophy induced by abdominal aortic constriction. Treatment
with RSV at doses of 10 and 20 mg/kg/day was shown to downregulate
the phosphorylation levels of Akt, ERK1/2 and GATA4 proteins, and
to upregulate PPARα mRNA expression in rat myocardial cells.
Consequently, these may constitute the molecular mechanisms of
regression to myocardial hypertrophy. Based on this observation,
the effect of RSV on promoting cardiac function is suggested to be
parallel with the effect of myocardial hypertrophy regression. RSV
is suggested to reverse the development of cardiac hypertrophy by
affecting the phosphorylation of Akt, ERK1/2 and GATA4 molecular
activation signaling pathways in cardiomyocytes.
In conclusion, based on the significant decreases in
left ventricular mass and relative cardiomyocyte area in the
RSV-treated banded rats, RSV was shown to have cardiac
anti-hypertrophic effects and to be involved in the maintenance of
hemodynamic stability. Therefore, RSV suppresses myocardial
hypertrophy induced by pressure overload. The underlying molecular
mechanisms of action may be associated to the regulation of
activation of Akt, ERK1/2 and GATA4 pro-hypertrophic signaling
pathways. However, further studies are needed for the investigation
of the association between Akt, ERK1/2 and GATA4 molecular
activation signaling pathways. RSV treatment also increases the
mRNA expression levels of PPARα, which is beneficial to the
regression of cardiac hypertrophy. To the best of our knowledge,
this is the first time that RSV has been shown to prevent and
reverse cardiovascular remodelling induced by abdominal aortic
constriction initiated by pressure overload. The results of the
present study provide additional evidence regarding the pleiotropic
effects of statins. RSV is suggested to constitute a novel drug
suitable for the clinical reversal of cardiac hypertrophy. However,
further studies are needed for the in-depth investigation of the
role of RSV in myocardial hypertrophy.
Acknowledgements
This study was supported by the General Programs of
the Natural Science Foundation of Shandong Province of China (no.
ZR2010HM116).
References
|
1
|
Levy D, Garrison RJ, Savage DD, Kannel WB
and Castelli WP: Prognostic implications of echocardiographically
determined left ventricular mass in the Framingham Heart Study. N
Engl J Med. 322:1561–1566. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okin PM, Devereux RB, Jern S, et al:
Regression of electrocardiographic left ventricular hypertrophy
during antihypertensive treatment and the prediction of major
cardiovascular events. JAMA. 292:2343–2349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auer J, Berent R, Weber T and Eber B:
Clinical significance of pleiotropic effects of statins: lipid
reduction and beyond. Curr Med Chem. 9:1831–1850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laufs U, Fata VL and Liao JK: Inhibition
of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks
hypoxia-mediated down-regulation of endothelial nitric oxide
synthase. J Biol Chem. 272:31725–31729. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukumoto Y, Libby P, Rabkin E, et al:
Statins alter smooth muscle cell accumulation and collagen content
in established atheroma of watanabe heritable hyperlipidemic
rabbits. Circulation. 103:993–999. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ridker PM, Rifai N, Clearfield M, et al:
Measurement of C-reactive protein for the targeting of statin
therapy in the primary prevention of acute coronary events. N Engl
J Med. 344:1959–1965. 2001. View Article : Google Scholar
|
|
7
|
Planavila A, Laguna JC and Vázquez-Carrera
M: Atorvastatin improves peroxisome proliferator-activated receptor
signaling in cardiac hypertrophy by preventing nuclear factor-kappa
B activation. Biochim Biophys Acta. 1687:76–83. 2005. View Article : Google Scholar
|
|
8
|
Young ME, Laws FA, Goodwin GW and
Taegtmeyer H: Reactivation of peroxisome proliferator-activated
receptor alpha is associated with contractile dysfunction in
hypertrophied rat heart. J Biol Chem. 276:44390–44395. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takemoto M, Node K, Nakagami H, et al:
Statins as antioxidant therapy for preventing cardiac myocyte
hypertrophy. J Clin Invest. 108:1429–1437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kameda Y, Hasegawa H, Kubota A, et al:
Effects of pitavastatin on pressure overload-induced heart failure
in mice. Circ J. 76:1159–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu R, Lin F, Zhang S, Chen X, Hu S and
Zheng Z: Signal pathways involved in reverse remodeling of the
hypertrophic rat heart after pressure unloading. Int J Cardiol.
143:414–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esposito G, Prasad SV, Rapacciuolo A, Mao
L, Koch WJ and Rockman HA: Cardiac overexpression of a G(q)
inhibitor blocks induction of extracellular signal-regulated kinase
and c-Jun NH(2)-terminal kinase kinase activity in in vivo pressure
overload. Circulation. 103:1453–1458. 2001. View Article : Google Scholar
|
|
13
|
van Berlo JH, Elrod JW, Aronow BJ, Pu WT
and Molkentin JD: Serine 105 phosphorylation of transcription
factor GATA4 is necessary for stress-induced cardiac hypertrophy in
vivo. Proc Natl Acad Sci USA. 108:12331–12336. 2011.PubMed/NCBI
|
|
14
|
Siri FM: Chronic norepinephrine infusion
and adrenergic function of hypertrophied hearts. Am J Physiol.
248(4 Pt 2): H485–H492. 1985.PubMed/NCBI
|
|
15
|
Teichholz LE, Kreulen T, Herman MV and
Gorlin R: Problems in echocardiographic volume determinations:
echocardiographic-angiographic correlations in the presence of
absence of asynergy. Am J Cardiol. 37:7–11. 1976.
|
|
16
|
Leskinen H, Rauma-Pinola T, Szokodi I, et
al: Adaptive or maladaptive response to adenoviral adrenomedullin
gene transfer is context-dependent in the heart. J Gene Med.
10:867–877. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woodiwiss AJ, Tsotetsi OJ, Sprott S, et
al: Reduction in myocardial collagen cross-linking parallels left
ventricular dilatation in rat models of systolic chamber
dysfunction. Circulation. 103:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong Y, Hui SC, Chan TY and Hou JY: Effect
of berberine on regression of pressure-overload induced cardiac
hypertrophy in rats. Am J Chin Med. 30:589–599. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni L, Zhou C, Duan Q, et al: β-AR blockers
suppresses ER stress in cardiac hypertrophy and heart failure. PLoS
One. 6:e272942011.
|
|
20
|
Li J, Li P, Feng X, et al: Effects of
losartan on pressure overload-induced cardiac gene expression
profiling in rats. Clin Exp Pharmacol Physiol. 30:827–832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou MS, Jaimes EA and Raij L:
Atorvastatin prevents end-organ injury in salt-sensitive
hypertension: role of eNOS and oxidant stress. Hypertension.
44:186–190. 2004. View Article : Google Scholar
|
|
22
|
Takayama N, Kai H, Kudo H, et al:
Simvastatin prevents large blood pressure variability induced
aggravation of cardiac hypertrophy in hypertensive rats by
inhibiting RhoA/Ras-ERK pathways. Hypertens Res. 34:341–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Habibi J, Whaley-Connell A, Qazi MA, et
al: Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase
inhibitor, decreases cardiac oxidative stress and remodeling in
Ren2 transgenic rats. Endocrinology. 148:2181–2188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi EY, Chang W, Lim S, et al:
Rosuvastatin inhibits norepinephrine-induced cardiac hypertrophy
via suppression of Gh. Eur J Pharmacol. 627:56–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang SA, Kim YJ, Lee HW, et al: Effect of
rosuvastatin on cardiac remodeling, function, and progression to
heart failure in hypertensive heart with established left
ventricular hypertrophy. Hypertension. 54:591–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaanine AH and Hajjar RJ: AKT signalling
in the failing heart. Eur J Heart Fail. 13:825–829. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang Q, Wiese RJ, Bueno OF, Dai YS,
Markham BE and Molkentin JD: The transcription factor GATA4 is
activated by extracellular signal-regulated kinase 1- and
2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol
Cell Biol. 21:7460–7469. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tenhunen O, Sármán B, Kerkelä R, et al:
Mitogen-activated protein kinases p38 and ERK 1/2 mediate the wall
stress-induced activation of GATA-4 binding in adult heart. J Biol
Chem. 279:24852–24860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Condorelli G, Drusco A, Stassi G, et al:
Akt induces enhanced myocardial contractility and cell size in vivo
in transgenic mice. Proc Natl Acad Sci USA. 99:12333–12338. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morisco C, Seta K, Hardt SE, Lee Y, Vatner
SF and Sadoshima J: Glycogen synthase kinase 3beta regulates GATA4
in cardiac myocytes. J Biol Chem. 276:28586–28597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smeets PJ, Teunissen BE, Willemsen PH, et
al: Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in
response to chronic pressure overload. Cardiovasc Res. 78:79–89.
2008.
|
|
32
|
Miyamoto T, Takeishi Y, Takahashi H, et
al: Activation of distinct signal transduction pathways in
hypertrophied hearts by pressure and volume overload. Basic Res
Cardiol. 99:328–337. 2004.PubMed/NCBI
|
|
33
|
Yang D, Ma S, Tan Y, et al: Adrenergic
receptor blockade-induced regression of pressure-overload cardiac
hypertrophy is associated with inhibition of the
calcineurin/NFAT3/GATA4 pathway. Mol Med Rep. 3:497–501. 2010.
View Article : Google Scholar : PubMed/NCBI
|


















