Introduction
Burn injuries, which are characterized by
heat-induced tissue coagulation at the time of injury, constitute a
global public health problem (1).
The systemic effects of burn injuries include the release of
inflammatory cytokines produced by inflammatory cells and the
vascular endothelium. These cytokines regulate lymphocyte function
through the activation of tumor necrosis factor (TNF)-α,
interleukin (IL)-1b, IL-2, IL-4, IL-6, IL-8, IFN-a and IFN-b, or
the inhibition of IL-10 and TGF-β immune responses (2). Inflammation is important in
pathogenesis, however, the imbalance between inflammatory and
anti-inflammatory cytokines induced by burn injury aggravates the
inflammatory response. This may result in the development of sepsis
or systemic inflammatory response syndrome due to immune
dysfunction, which increases the risk of mortality (3,4).
Thus far, advances remain limited in the manipulation of the
inflammatory response to treat burn injuries.
Previously, the third signaling gasotransmitter,
hydrogen sulfide (H2S), was demonstrated to exhibit
physiological and physiopathological roles in vivo and in
vitro(5,6). An increasing number of studies
suggest that H2S exerts protective effects against
various stimuli-triggered injuries in numerous organs, including
the heart, liver and kidneys (7,8).
However, the importance of H2S in inflammation is only
recently beginning to be elucidated, and the exact role of
H2S in inflammation remains controversial, as
pro-inflammatory and anti-inflammatory effects have been
demonstrated (9). Certain studies
have determined the pro-inflammatory effects of H2S.
These studies showed that inflammation was correlated with
increased levels of plasma H2S and tissue H2S
synthesizing enzyme activity. In addition, the inhibition of
H2S synthesis by DL-propargylglycine (PAG) treatments
led to reduced inflammation (10–12).
However, other studies have observed anti-inflammatory effects of
H2S. Treatments with either H2S releasing
non-steroidal anti-inflammatory drugs or H2S donors
(sodium hydrosulfide, NaHS) showed anti-inflammatory activity in
various models of inflammation (13–15).
In addition, in lipopolysaccharide-stimulated microglias and
astrocytes, H2S exerted an anti-inflammatory effect
(16).
In the present study, it was hypothesized that
H2S is important in the regulation of the inflammatory
response induced by burn injury. Thus, the therapeutic potential of
NaHS, an H2S donor in an in vivo model of
burn-related inflammation in mice, was investigated. It was
determined that H2S was anti-inflammatory and suppressed
the inflammatory response associated with burn injury.
Materials and methods
Animals
Adult male C57BL/6 mice (age, 6–8 weeks; weight,
21–24 g) were obtained from the Second Military Medical University
(Shanghai, China). Mice were individually housed in laminar flow
cabinets under specific pathogen-free conditions with access to
food and water ad libitum. The animals were acclimatized for
1 week prior to the experiment, and maintained throughout at
standard conditions of 50% relative humidity, 24±1°C and a 12-h
light-dark cycle. The mice were randomized into two groups; the
first was a sham group that did not receive burn injuries (n=7) and
the second group received burn injuries. Mice in the second group
were then randomly subdivided into three groups (n=7 per group): An
untreated group, a saline group that received the vehicle (0.9%
sodium chloride, NaCl; 100 ml/kg body weight; subcutaneously, s.c.)
and an NaHS group that was treated with NaHS (2 mg/kg body weight;
s.c.). All animal experiments were conducted according to the
National Institutes of Health’s Guide for the Care and Use of
Laboratory Animals (2011, ISBN-13: 978-0-309-15400-0), and were
approved by the Institutional Animal Care and Use Committee of the
Second Military Medical University.
H2S
The dehydrated NaHS powder (anhydrous; Beijing
Chemical Reagents Company, Beijing, China) was dissolved in
isotonic saline (0.9%) immediately prior to administration. For the
treatment groups, either normal saline or NaHS were subcutaneously
injected into the mice using a 32-gauge needle.
Mouse injury model
The burn injury model was generated as described
previously (17). Briefly, mice
were anesthetized by intraperitoneal injection of pentobarbital
sodium (75 mg/kg), then the dorsum was cleansed and shaved with
electrical hair clippers. Subsequently, 40% of the total body
surface area (TBSA) was exposed to a 95°C water bath for 10 sec,
followed by 4°C water for 45 sec to halt the burning process. A
full-thickness skin burn was confirmed by its characteristics with
the loss of epidermis and dermis. Mice were revived from
unconsciousness by intraperitoneal application of 1 ml sterile
saline 1 h after burning. The group of mice without exposure to
boiling water served as controls. Mice were then immediately
injected with the vehicle or sodium sulfide (2 mg/kg body weight,
s.c.). After 10 h, mice were sacrificed by CO2
asphyxiation. Blood samples were taken via direct cardiac puncture
for analysis by enzyme-linked immunosorbent assay (ELISA) assay.
Liver samples from each group were collected and frozen immediately
in liquid nitrogen for subsequent measurement of tissue
myeloperoxidase (MPO) activity.
Detection of plasma H2S
content
Blood samples for each group were taken in
heparinized tubes via direct cardiac puncture and centrifuged at
726 × g for 5 min. Plasma H2S concentration was measured
with an ELIT Ion Analyzer (ELIT 9801; Electro Analytical
Instruments Ltd., London, UK) as described previously (18). In brief, 0.5 ml sulfide antioxidant
buffer (SAOB; NaOH 2.35 mol/l and EDTA 0.27 mol/l) was added to 0.5
ml H2S standard solutions (10, 20, 30, 40, 50, 60 and 80
μmol/l, respectively). A sulfide-sensitive electrode (ELIT 8225)
and a reference electrode (ELIT 003n; Electro Analytic Instruments
Ltd.) were rinsed in deionized water, blotted dry and immersed in a
mixture of SAOB and 10 μmol/l H2S standard solution.
When a stable reading was displayed, the voltage value (mV) was
recorded. This procedure was repeated for the other combinations of
SAOB and different concentrations of H2S standard
solutions. When all the standards were measured, the standard curve
of voltage versus concentration was plotted. The electrodes were
washed as previously, and the samples were measured in the same way
as the measurement for the standard solution. The sample data were
plotted on the standard curve and the sample concentration was
obtained.
Measurement of MPO activity
Neutrophil sequestration in the liver was quantified
by measuring the tissue MPO activity as previously described
(19). Liver samples were thawed,
homogenized in 20 mM phosphate buffer (pH 7.4), centrifuged (10,000
× g for 10 min at 4°C) and the resulting pellet was resuspended in
50 mM phosphate buffer (pH 6.0) containing 0.5% (w/v)
hexadecyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO,
USA). The suspension was subjected to four cycles of freezing and
thawing, and further disrupted by sonication (40 sec). The sample
was then centrifuged (10,000 × g for 5 min at 4°C) and the
supernatant was used for the MPO assay. The reaction mixture
consisted of the supernatant (50 μl), 1.6 mM tetramethylbenzidine
(Sigma-Aldrich), 80 mM sodium phosphate buffer (pH 5.4) and 0.3 mM
hydrogen peroxide (50 μl). This mixture was incubated at 37°C for
110 sec, the reaction was terminated with 50 μl of 0.18 M
H2SO4 and the absorbance was measured at 450
nm. The absorbance was then corrected for the DNA content of the
tissue sample and results were expressed as the enzyme
activity.
Measurement of plasma inflammatory and
anti-inflammatory cytokine levels by ELISA
Blood samples from each group were collected in
heparinized tubes via direct cardiac puncture and centrifuged at
726 × g. Cytokine levels were quantified by a sandwich ELISA using
ELISA kits (Quantikine® Colorimetric Sandwich ELISAs;
R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions. Briefly, samples (100 μl) and IL-6
standards (0, 62.5, 125, 250, 500 and 1000 pg/ml) were added to the
wells. Each was tested in duplicate. Following 1 h of incubation at
37°C, samples were removed and the plates were washed with a
washing buffer (consisting of PBS, 10 mmol/l, pH 7.4 and Tween 20,
0.1%). Anti-rat IL-6 biotin (100 μl) was added to each well of the
plates and left for 30 mins at 37°C. Following five additional
washing steps, 100 μl horseradish peroxidase was added to the wells
and left for 30 mins at 37°C. Subsequent to a further wash, 100 μl
tetramethylbenzidine substrate was added to each well for color
development. The mixture was incubated in the dark for 30 mins at
room temperature. Following the termination of the reaction by the
addition of 100 μl stop solution to each well, the optical density
(OD) values at 450 nm were measured by a Bio-Rad ELISA reader
(iMark Microplate Absorbance Reader; Bio-Rad, Richmond, CA, USA).
The standard curve of the OD value versus the concentration of IL-6
was obtained. The sample data were plotted on the standard curve
and the sample IL-6 concentration was determined. The same method
was utilized for the analysis of the plasma levels of TNF-α, IL-8
and IL-10.
Statistical analysis
SPSS Version 17.0 software (SPSS for Windows, Inc.,
Chicago, IL, USA) was used for all statistical analyses. All
results are presented as the mean ± SEM. Statistical analysis of
the data was performed using standard one-way analysis of variance
followed by a least significant difference post hoc test.
Bonferroni’s correction was used to adjust for multiple
comparisons. A two-tailed Student’s paired t-test was also used to
compare the difference in values between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
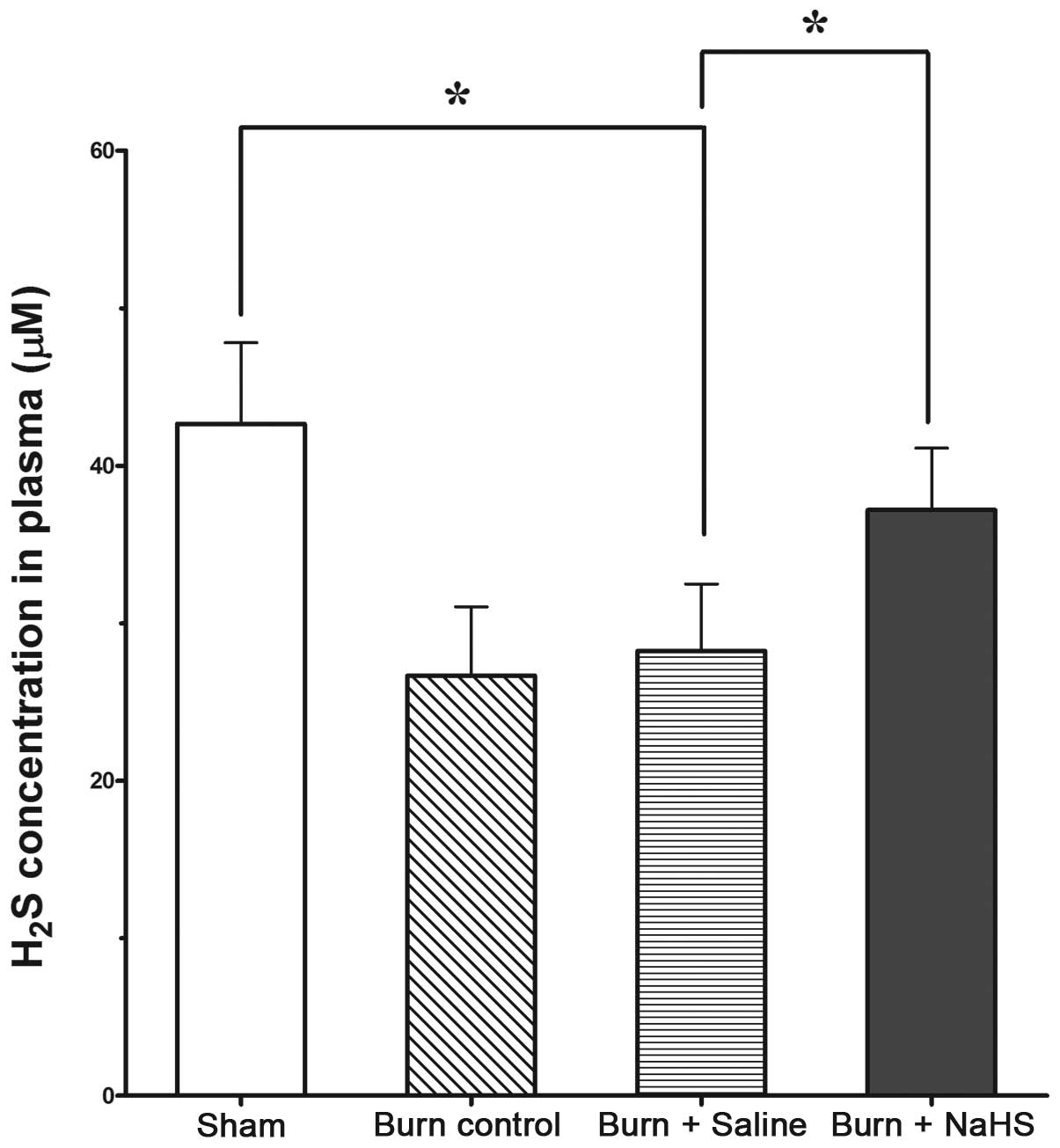
Burn injury decreases the H2S
levels in the plasma
As shown in Fig. 1,
H2S levels in the plasma of mice were significantly
lower in the groups subjected to burn injury compared with those of
the sham group (P<0.05). In addition, NaHS significantly
increased the plasma H2S levels compared with those of
the burn control group that received no treatment (P<0.05).
Burn injury promotes the inflammatory
response in rats, which is suppressed by the H2S donor,
NaHS
An ELISA assay demonstrated that the levels of IL-6,
IL-8 and TNF-α in the plasma of mice subjected to burn injuries
were significantly higher than those of the sham group that
received no injury (P<0.01, Fig.
2). Notably, the plasma levels of IL-10 in the injured group
were significantly decreased compared with those in the sham group
(P<0.01, Fig. 2).
Administration of NaHS in the mice that had received burn injuries
significantly decreased the IL-6 levels in the plasma compared with
those that received saline or no treatment (P<0.05, Fig. 2). Similarly, administration of NaHS
significantly decreased the IL-8 and TNF-α levels in the plasma of
injured mice compared with those that received saline or no
treatment (P<0.05, Fig. 2).
However, the H2S donor treatment significantly enhanced
the IL-10 levels in the plasma of injured mice compared with those
that received saline or no treatment (P<0.05, Fig. 2). No significant differences were
identified between the saline and the untreated groups.
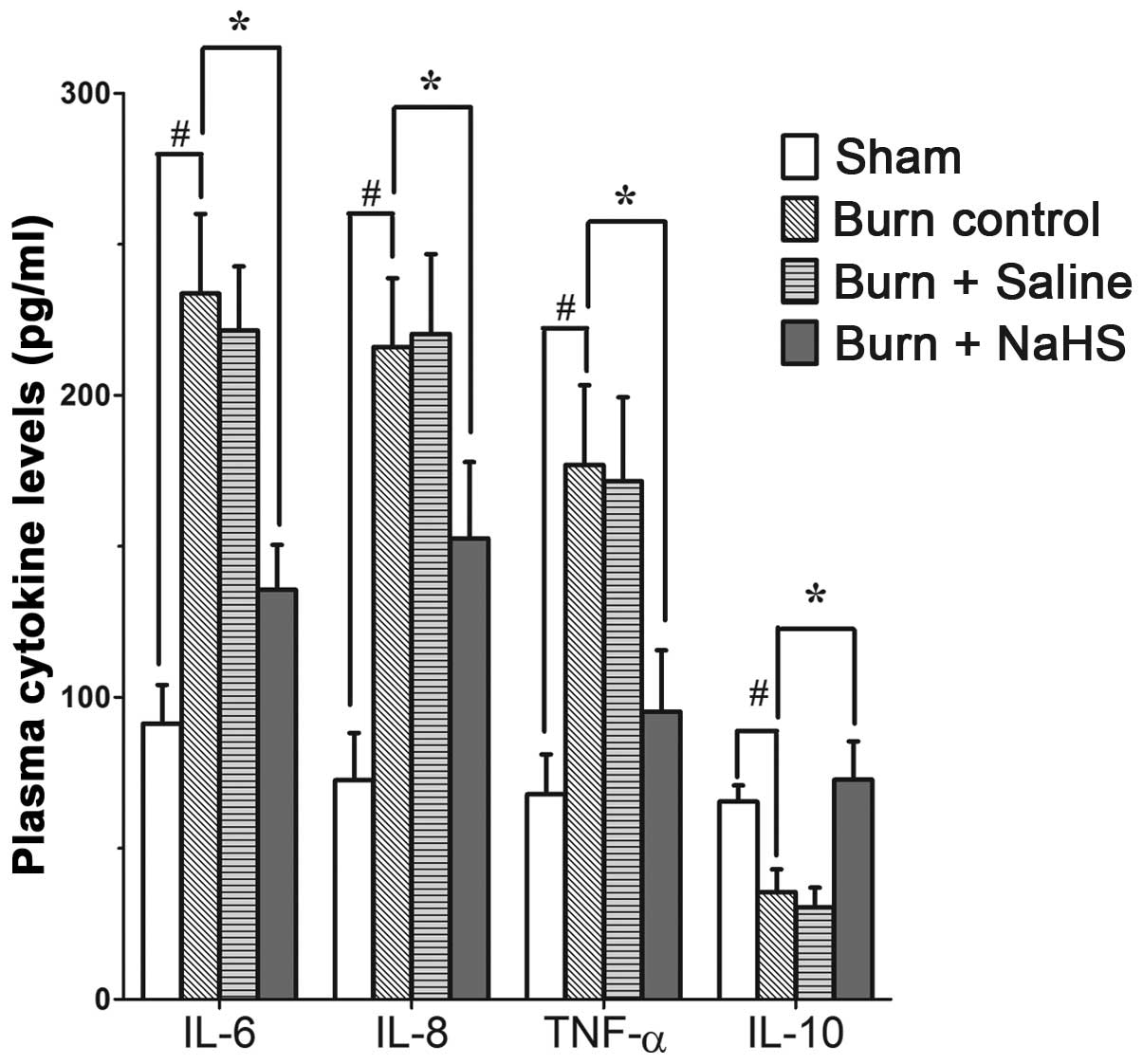 | Figure 2Effect of burn injury and NaHS
treatment on plasma cytokine levels. Following burn injuries, mice
were administered with the H2S donor, NaHS or the
vehicle (saline). After 10 h, the plasma cytokine levels of four
groups were measured by enzyme-linked immunosorbent assay and
results are presented as the mean ± SEM; n=7 for each group. Sham,
plasma H2S level in mice that received no burn injuries;
burn control, mice subjected to burn injury without treatments;
burn + saline, treatment with the vehicle (0.9% NaCl; 100 ml/kg
body weight; subcutaneously, s.c.) following burn injury; and burn
+ NaHS, treatment with NaHS (2 mg/kg body weight, s.c.) following
burn injury. #P<0.01 and *P<0.05. NaHS,
sodium hydrosulfide; H2S, hydrogen sulfide; NaCl, sodium
chloride. |
NaHS decreases the enhanced MPO activity
induced by burn injury
Tissue MPO activity, an indication of neutrophil
inflammatory activity, was markedly increased in the livers of mice
subjected to burn injury compared with that of the sham group,
indicating increased leukocyte infiltration in the mouse liver
(Fig. 3, P<0.01). However,
treatment with NaHS (2 mg/kg of body weight, s.c.) significantly
reduced the MPO activity (Fig. 3,
P<0.05).
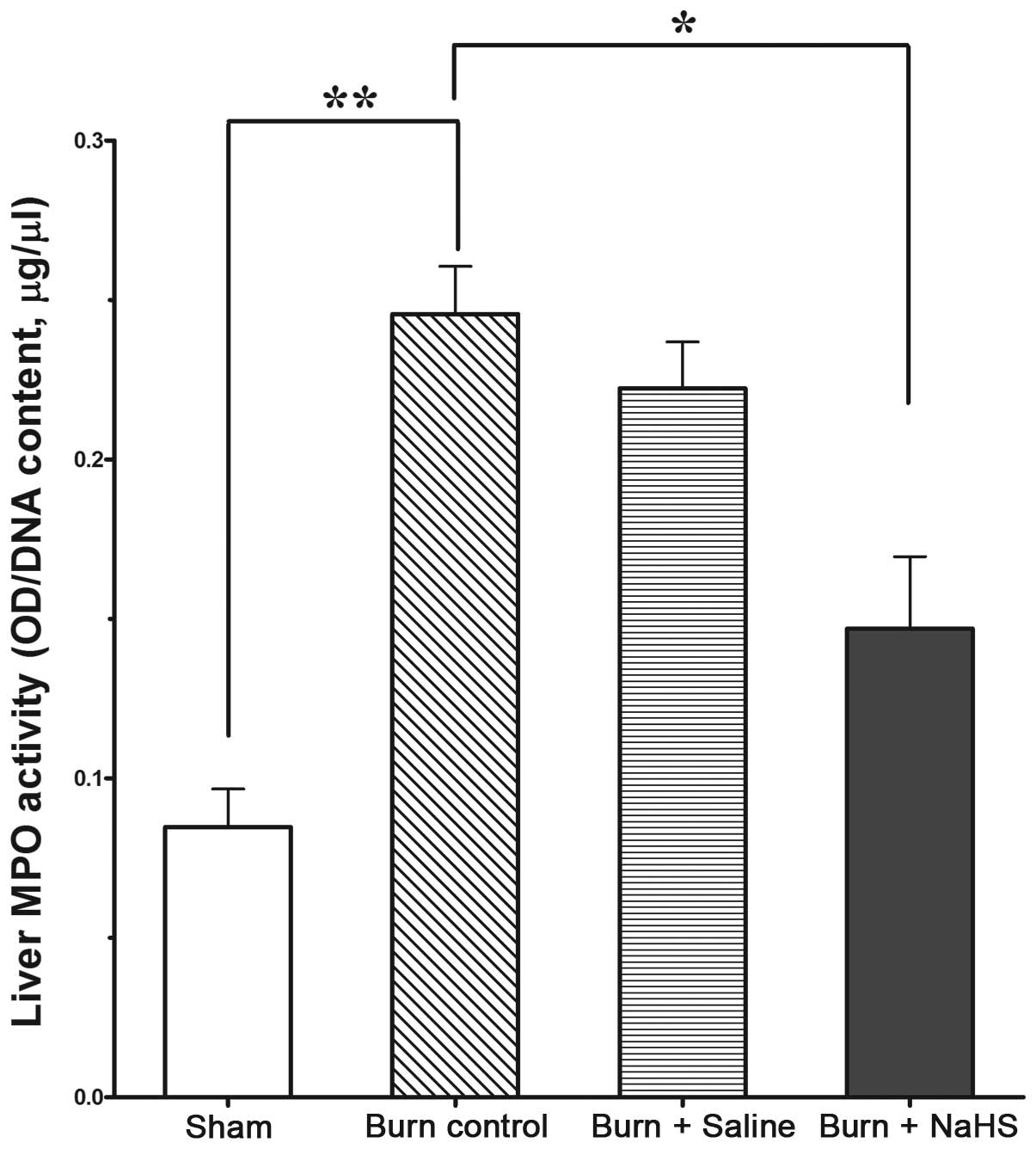 | Figure 3MPO activities in the livers of mice
in the sham group (mice that received no burn injuries), burn
control group (mice subjected to burn injury without treatment),
saline group [mice subjected to burn injury and treated with the
vehicle (0.9% NaCl, 100 ml/kg body weight; subcutaneously, s.c.)]
and NaHS group (mice with burn injury treated with NaHS, 2 mg/kg
body weight, s.c.). Data are presented as the mean ± SEM; n=7 for
each group. *P<0.05 and **P<0.01. MPO,
myeloperoxidase; NaCl, sodium chloride, NaHS, sodium
hydrosulfide. |
Discussion
Burn injuries remain one of the most widespread and
devastating forms of trauma and are ranked among the leading causes
of injury-related morbidity and mortality worldwide (20,21).
Although inflammation is important in the pathogenesis of burn
injuries, severe burns often lead to systemic inflammatory response
syndrome (SIRS), which may be responsible for the majority of the
associated morbidity and mortality (22,23).
H2S may be important in the regulation of the
inflammatory process, however, the effects of H2S on
inflammation are controversial (10,24,25).
In the present mouse model, a predominant anti-inflammatory effect
of H2S was observed. Burn injuries resulted in a
significant reduction in the H2S levels in the plasma
and an exaggerated inflammatory response was also observed, which
was identified by significantly increased levels of IL-6, IL-8 and
TNF-α, and a decreased level of IL-10. The MPO activity in the
liver tissue of injured mice was also markedly increased. However,
administration of NaHS, an H2S donor, alleviated the
immune response, as demonstrated by the upregulated levels of a
plasma anti-inflammatory cytokine (IL-10) and the reduced
generation of pro-inflammatory cytokines (IL-6, IL-8 and TNF-α). In
this model, H2S also decreased the elevated MPO activity
of the liver tissue induced by burn injury.
H2S has been demonstrated to be an
essential mediator of severe burn injury-induced inflammation in
mice (9). The results of the
present study in mice showed that a 40% TBSA full thickness burn
induced a significant decrease in plasma H2S levels.
Inflammatory cytokines exert well-characterized effects on the
pathogenesis of severe burn-induced injury (26). IL-6 is produced by numerous cell
types, including monocytes/macrophages, endothelial cells,
fibroblasts and smooth muscle cells, in response to stimulation by
endotoxin, IL-1β and TNF-α (27,28).
Circulating levels of IL-6 are strong predictors of the severity of
SIRS. The importance of IL-6 in the acute-phase response has been
demonstrated by its ability to stimulate the synthesis of
acute-phase proteins, including C reactive protein, from
hepatocytes in vitro and in vivo(29). Patients with systemic inflammatory
conditions, such as sepsis or SIRS, also exhibit increased
circulating levels of IL-8 (30).
In acute pancreatitis, increased IL-8 levels predicted the severity
of the disease (31). The
anti-inflammatory cytokine IL-10 is a central immunosuppressive
cytokine that regulates the innate and adaptive immune responses,
resulting in inhibition of the alveolar macrophage production of
pro-inflammatory mediators involved in SIRS (32). Increased IL-10 plasma levels in
animal models of endotoxemia may inhibit the release of
pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, from
monocytes and macrophages, thereby preventing subsequent tissue
damage (33). In the present
study, it was determined that the levels of TNF-α, IL-6 and IL-8 in
the plasma were significantly increased, while IL-10 secretion was
inhibited by burn injury. Administration of NaHS significantly
decreased the TNF-α, IL-6 and IL-8 levels in the plasma, but
elevated the IL-10 plasma levels. Inflammatory cytokines are
essential in systemic immune dysfunction. The results demonstrated
that NaHS decreased the levels of inflammatory cytokines and
increased those of the anti-inflammatory cytokines, which suggested
that H2S may provide protection by alleviating the
exaggerated inflammatory damage associated with burn injury.
However, further studies are required to determine the precise
mechanisms by which H2S regulates the inflammatory
response.
As leukocyte recruitment is pivotal in the
pathogenesis of organ injury caused by burn injury (19), it was investigated whether NaHS is
able to protect against organ injury by reducing leukocyte
recruitment. It was determined that burn injury significantly
enhanced the MPO activity in the liver, indicating increased
neutrophil infiltration in this organ. Moreover, NaHS treatment
reduced the MPO activity in the livers of mice subjected to burn
injury, indicating attenuated neutrophil infiltration. These
results were in accordance with those of a study demonstrating that
NaHS modulated leukocyte-mediated inflammation by decreasing
leukocyte adhesion and leukocyte infiltration (34).
However, Zhang et al demonstrated that mice
subjected to 30% TBSA burn injury (10) exhibited significantly elevated
plasma and hepatic H2S levels, with a concomitant
increase in liver and lung expression of cystathionine-β-synthase
and cystathionine-γ-lyase (CSE), 8 h after injury. Prophylactic and
therapeutic administration of PAG reduced burn-associated
neutrophil accumulation and histological changes in the liver and
lung tissues. Injection of NaSH (10 mg/kg; i.p.) at the same time
as burn injury aggravated the burn-associated tissue damage and
inflammation. These results are not concordant with those observed
in the present study, and thus may be due to the different animal
models and doses of H2S donors used. Severe
full-thickness burn injury initially produces a large systemic
inflammatory reaction characterized by leukocyte activation and
plasma leakage in the microvasculature of tissues and organs remote
from the wound. The degree of the inflammatory response correlates
directly with the percentage of TBSA burnt (35). In our study, mice received a 40%
TBSA burn injury, which was different from the previous animal
model. Moreover, the dose and administration routes were different.
In this study, the injected dose of NaHS was 2 mg/kg (s.c.), which
was markedly lower than that in the study by Zhang et al (10
mg/kg; i.p.). It has been demonstrated that a high dose of
H2S donor may aggravate inflammation and injury while a
low dose of H2S decreases inflammation.
In conclusion, the present study demonstrated that
in a murine model of inflammation induced by burn injury,
administration of NaHS alleviated the immune response, as
determined by the reversed MPO activity of the liver tissue,
increased levels of an anti-inflammatory cytokine (IL-10) and
reduced levels of pro-inflammatory cytokines (IL-6, IL-8 and
TNF-α). This study provides novel insights into the involvement of
H2S in attenuating the burn-induced systemic immune
response. Modulation of endogenous H2S or exogenous
administration of H2S may be a novel therapeutic
strategy for immune dysfunction induced by burn injury.
Abbreviations:
|
H2S
|
hydrogen sulfide
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
TNF-α
|
tumor necrosis factor-α
|
|
MPO
|
myeloperoxidase
|
|
NaHS
|
sodium hydrogen sulfide
|
|
TBSA
|
total body surface area
|
References
|
1
|
Shakespeare P: Burn wound healing and skin
substitutes. Burns. 27:517–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evers LH, Bhavsar D and Mailänder P: The
biology of burn injury. Exp Dermatol. 19:777–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller CL and Baker CC: Changes in
lymphocyte activity after thermal injury. The role of suppressor
cells. J Clin Invest. 63:202–210. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hansbrough JF, Zapata-Sirvent R, Peterson
V, et al: Characterization of the immunosuppressive effect of
burned tissue in an animal model. J Surg Res. 37:383–393. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pae HO, Lee YC, Jo EK and Chung HT: Subtle
interplay of endogenous bioactive gases (NO, CO and H(2)S) in
inflammation. Arch Pharm Res. 32:1155–1162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wallace JL: Physiological and
pathophysiological roles of hydrogen sulfide in the
gastrointestinal tract. Antioxid Redox Signal. 12:1125–1133. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bian JS, Yong QC, Pan TT, et al: Role of
hydrogen sulfide in the cardioprotection caused by ischemic
preconditioning in the rat heart and cardiac myocytes. J Pharmacol
Exp Ther. 316:670–678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fiorucci S, Antonelli E, Mencarelli A, et
al: The third gas: H2S regulates perfusion pressure in both the
isolated and perfused normal rat liver and in cirrhosis.
Hepatology. 42:539–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whiteman M and Winyard PG: Hydrogen
sulfide and inflammation: the good, the bad, the ugly and the
promising. Expert Rev Clin Pharmacol. 4:13–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Sio SW, Moochhala S and Bhatia M:
Role of hydrogen sulfide in severe burn injury-induced inflammation
in mice. Mol Med. 16:417–424. 2010.PubMed/NCBI
|
|
11
|
Bhatia M, Wong FL, Fu D, Lau HY, Moochhala
SM and Moore PK: Role of hydrogen sulfide in acute pancreatitis and
associated lung injury. FASEB J. 19:623–625. 2005.PubMed/NCBI
|
|
12
|
Collin M, Anuar FB, Murch O, Bhatia M,
Moore PK and Thiemermann C: Inhibition of endogenous hydrogen
sulfide formation reduces the organ injury caused by endotoxemia.
Br J Pharmacol. 146:498–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zanardo RC, Brancaleone V, Distrutti E,
Fiorucci S, Cirino G and Wallace JL: Hydrogen sulfide is an
endogenous modulator of leukocyte-mediated inflammation. FASEB J.
20:2118–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elrod JW, Calvert JW, Morrison J, et al:
Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury
by preservation of mitochondrial function. Proc Natl Acad Sci USA.
104:15560–15565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sidhapuriwala J, Li L, Sparatore A, Bhatia
M and Moore PK: Effect of S-diclofenac, a novel hydrogen sulfide
releasing derivative, on carrageenan-induced hindpaw oedema
formation in the rat. Eur J Pharmacol. 569:149–154. 2007.
View Article : Google Scholar
|
|
16
|
Hu LF, Wong PT, Moore PK and Bian JS:
Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation
by inhibition of p38 mitogen-activated protein kinase in microglia.
J Neurochem. 100:1121–1128. 2007. View Article : Google Scholar
|
|
17
|
Stevenson JM, Gamelli RL and Shankar R: A
mouse model of burn wounding and sepsis. Methods Mol Med.
78:95–105. 2003.PubMed/NCBI
|
|
18
|
Geng B, Cui Y, Zhao J, et al: Hydrogen
sulfide downregulates the aortic L-arginine/nitric oxide pathway in
rats. Am J Physiol Regul Integr Comp Physiol. 293:R1608–R1618.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhatia M, Saluja AK, Hofbauer B, et al:
Role of substance P and the neurokinin 1 receptor in acute
pancreatitis and pancreatitis-associated lung injury. Proc Natl
Acad Sci USA. 95:4760–4765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Church D, Elsayed S, Reid O, Winston B and
Lindsay R: Burn wound infections. Clin Microbiol Rev. 19:403–434.
2006. View Article : Google Scholar
|
|
21
|
Endorf FW and Ahrenholz D: Burn
management. Curr Opin Crit Care. 17:601–605. 2011. View Article : Google Scholar
|
|
22
|
Dahiya P: Burns as a model of SIRS. Front
Biosci. 14:4962–4967. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenhalgh DG, Saffle JR, Holmes JH IV, et
al: American Burn Association consensus conference to define sepsis
and infection in burns. J Burn Care Res. 28:776–790. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oh GS, Pae HO, Lee BS, et al: Hydrogen
sulfide inhibits nitric oxide production and nuclear factor-kappaB
via heme oxygenase-1 expression in RAW264.7 macrophages stimulated
with lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar
|
|
25
|
Kubo S, Doe I, Kurokawa Y, Nishikawa H and
Kawabata A: Direct inhibition of endothelial nitric oxide synthase
by hydrogen sulfide: contribution to dual modulation of vascular
tension. Toxicology. 232:138–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cone JB, Wallace BH, Lubansky HJ and
Caldwell FT: Manipulation of the inflammatory response to burn
injury. J Trauma. 43:41–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhatia M: Novel therapeutic targets for
acute pancreatitis and associated multiple organ dysfunction
syndrome. Curr Drug Targets Inflamm Allergy. 1:343–351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nijsten MW, Hack CE, Helle M, ten Duis HJ,
Klasen HJ and Aarden LA: Interleukin-6 and its relation to the
humoral immune response and clinical parameters in burned patients.
Surgery. 109:761–767. 1991.PubMed/NCBI
|
|
30
|
Hack CE, Hart M, van Schijndel RJ, et al:
Interleukin-8 in sepsis: relation to shock and inflammatory
mediators. Infect Immun. 60:2835–2842. 1992.PubMed/NCBI
|
|
31
|
Makhija R and Kingsnorth AN: Levels of the
chemokines growth-related oncogene alpha and epithelial
neutrophil-activating protein 78 are raised in patients with severe
acute pancreatitis. Br J Surg. 89:566–572. 2002. View Article : Google Scholar
|
|
32
|
Torre D, Tambini R, Aristodemo S, et al:
Anti-inflammatory response of IL-4, IL-10 and TGF-beta in patients
with systemic inflammatory response syndrome. Mediators Inflamm.
9:193–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamayo E, Alvarez FJ, Alonso O, et al:
Effects of simvastatin on systemic inflammatory responses after
cardiopulmonary bypass. J Cardiovasc Surg (Torino). 50:687–694.
2009.PubMed/NCBI
|
|
34
|
Volpato GP, Searles R, Yu B, et al:
Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac
and metabolic function in the mouse. Anesthesiology. 108:659–668.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barber RC, Maass DL, White DJ and Horton
JW: Increasing percent burn is correlated with increasing
inflammation in an adult rodent model. Shock. 30:388–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|