Introduction
Osteosarcoma (OS) is the most common
nonhematological malignant bone tumor in children and adults, and
its peak incidence is during adolescence (1–4).
Currently, chemotherapeutic regimens for human OS treatment involve
the combination of multiple chemotherapeutic agents, including
high-dose methotrexate with leucovorin rescue, doxorubicin,
cisplatin (DDP) and ifosfamide either with or without etoposide
(5). Although these regimens have
remained the mainstay of OS chemotherapy for decades, none have
provided any major improvement in survival compared with the
original combination reported by Rosen et al(6,7).
Furthermore, these regimens have only been demonstrated to be
efficient in the treatment of localized OS, while they were shown
to perform poorly in the treatment of metastatic and recurrent OS
(5).
The process of carcinogenesis is driven by several
growth factors and pro-inflammatory cytokines, which are released
by the tumor microenvironment and neighboring cells of the innate
immune system. These cytokines, including tumor necrosis factor-α
(TNF-α), trigger the activation of the transcription factor nuclear
factor-κB (NF-κB), which in turn has been shown to induce the
expression of pro-inflammatory cytokine genes, to include
interleukin-6 (IL-6), IL-8 and TNF-α, and affect tumor cell
proliferation, apoptosis, angiogenesis and tumor invasion (8).
Among the novel antineoplastic drugs that are
currently under investigation, bioactive natural products have
emerged and gained considerable research attention.
Tetramethylpyrazine (TMP), an effective component of the
traditional Chinese medicine Chuanxiong, has been used in the
treatment of neurovascular and cardiovascular diseases. TMP has
been reported to have a beneficial effect in various types of
cancer, including glioma (9–10),
lung cancer (11), breast cancer
(12), ovarian cancer (13), hepatocellular cancer (14), leukemia (15) and melanoma (16). Additionally, TMP was found to have
anti-inflammatory actions in a rat model of spinal cord
ischemia-reperfusion injury (17).
However, the effects and underlying mechanism of action of TMP in
OS have not been elucidated to date.
The aim of the present study was to assess the
antitumor effects of TMP against OS and to investigate its
underlying biological mechanisms.
Materials and methods
Cell culture and regents
Human OS cell lines MG-63, SAOS-2 and U2OS were
obtained from the Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences, Shanghai, China). The OS cell lines
were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco)
in a humidified incubator at 37°C supplied with 5% CO2.
Only cells in the exponential growth phase were used in this study.
TMP and DDP were purchased from Sigma Chemical Co. (St. Louis, MO,
USA). Antibodies against p65, Bcl-2 and cyclin D1 were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
study was approved by the ethics committee of China Medical
University (Shenyang, China).
Cell viability assay
TMP cytotoxicity was assessed using an MTT assay
(Sigma Chemical Co.), which measured the metabolic activity of
viable cells. The cells were dislodged and suspended in medium, and
an appropriate number of cells were added to 96-well plates prior
to treatment. Following treatment with TMP for 48 h, MTT was
freshly prepared and added to each well at a final concentration of
0.5 mg/ml. After incubation for 4 h, formazan crystals were
dissolved in 100 μl DMSO, and the optical density was determined
using an ELISA reader (BioTek Instruments, Inc., Winooski, VT, USA)
at 570 nm. All the measurements were repeated in triplicate. The
inhibitory ratio was calculated using the following equation:
Inhibitory ratio (%) = (ODcontrol group −
ODdrug-treated group)/ODcontrol group.
Cell cycle analysis and apoptosis
assay
The cells were harvested following treatment with
TMP for 48 h. For cell cycle analysis, the cells were washed twice
with PBS, resuspended in 100 μl propidium iodide (PI) solution and
incubated for 30 min in the dark. The distribution of cells with
differing DNA content was analyzed on a FACSCalibur flow cytometer
using CellQuest software (BD Biosciences, San Jose, CA, USA) at an
excitation wavelength of 530 nm. Fluorescence emission was measured
using a 620-nm bandpass filter. Apoptosis was assessed by labeling
cells with annexin V-FITC and PI. Briefly, the cells were harvested
following treatment with TMP for 48 h, then washed twice with cold
PBS, resuspended in binding buffer and stained with 5 μl annexin
V-FITC and 5 μl PI solution (BD Pharmingen, San Diego, CA, USA) for
15 min at room temperature in the dark. Following incubation, 400
μl binding buffer was added and the cells were analyzed by flow
cytometry (BD Biosciences). All the measurements were repeated in
triplicate.
Xenograft tumor model
A xenograft tumor model was established as
previously described (18). Female
BALB/c nude mice (4 to 6 weeks old) were obtained from China
Medical University and all the procedures were performed in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals (19). Cells
(5×106) resuspended in 0.2 ml PBS were subcutaneously
inoculated into the lower right flank of nude mice. When the
developing tumors reached 100 mm3 in size, treatment was
initiated by intraperitoneal injection of vehicle (0.9% saline),
TMP (100 mg/kg) or DDP (1 mg/kg) every other day for 28 days. Tumor
size and body weight were measured every 3 days with calipers, and
the tumor volume (V) was calculated using the following formula: V
= length × width × height × 0.5236 (20). Following the last dose of TMP or
DDP, all the mice were sacrificed and the tumors were weighed. One
section of the tissue was fixed in formalin and another section was
frozen using liquid nitrogen.
Western blot analysis
Nuclear and cytoplasmic proteins were extracted
using the Nuclear-Cytosol Extraction kit (Applygen Technologies,
Inc., Beijing, China) according to the manufacturer’s instructions.
Protein concentration was quantified using the BCA protein assay
kit (Santa Cruz Biotechnology, Inc.). For western blot analysis,
equal amounts of total protein were boiled and then separated by
SDS-PAGE. Following electrophoresis, proteins were blotted onto
PVDF membranes and blocked for 1 h at room temperature. Each
membrane was incubated with appropriate primary antibodies at 4°C
overnight. The blots were incubated with horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. Protein bands were detected on X-ray films using an
enhanced chemiluminescence detection system.
Statistical analysis
The Statistical Package for the Social Sciences
(SPSS) version 13.0 was used to analyze the data. The data are
expressed as the mean ± SD, and the means of different groups were
compared using a one-way analysis of variance (ANOVA) test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TMP inhibits the proliferation of OS
cells in vitro
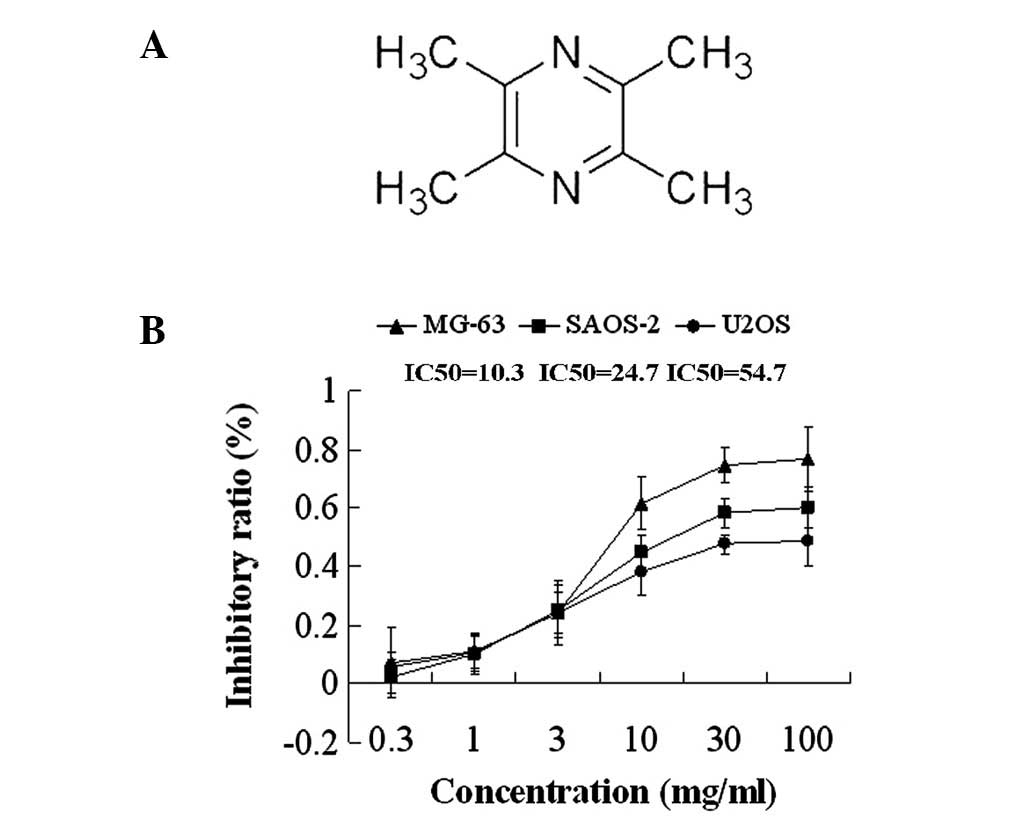
To investigate the effects of TMP, a cell
proliferation assay using MTT was performed on OS cells (MG-63,
SAOS-2 and U2OS). All the OS cell lines were treated with various
concentrations of TMP (0.3, 1, 3, 10, 30 and 100 mg/ml) for 48 h.
The MTT assay demonstrated that treatment with TMP inhibited the
proliferation of OS cells in a dose-dependent manner; the
IC50 for 48 h was 10.3, 24.7 and 54.7 mg/ml in MG-63,
SAOS-2 and U2OS cells, respectively (Fig. 1).
TMP induces apoptosis and G1/G0 arrest in
MG-63 OS cells in vitro
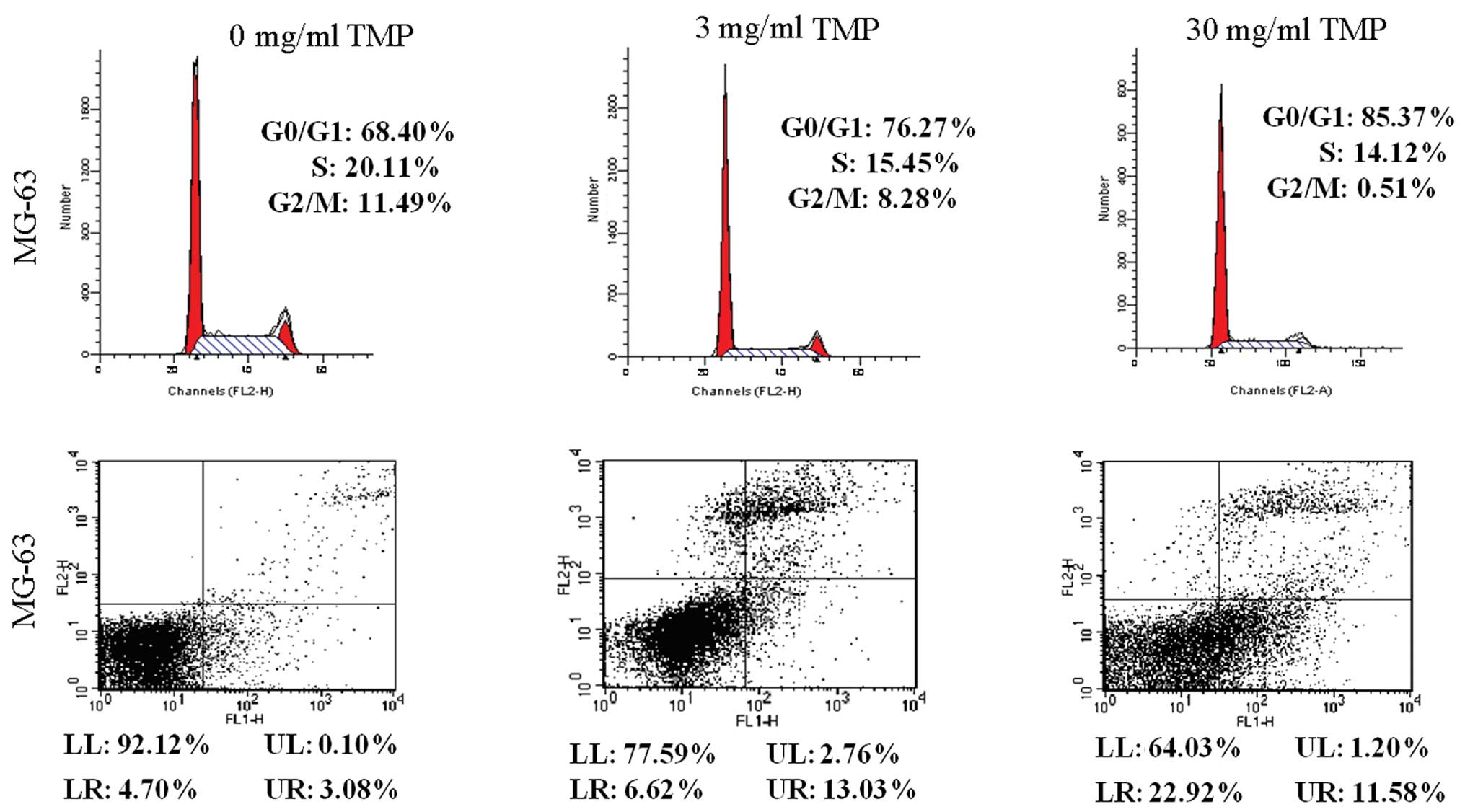
As shown in Fig. 2,
MG-63 OS cells were treated with TMP (3 and 30 mg/ml) for 48 h.
G1/G0 arrest and apoptosis induced by TMP were evaluated by flow
cytometry. Results showed that treatment with TMP (3 and 30 mg/ml)
significantly increased G1/G0 arrest (76.27 and 85.37%,
respectively, vs. 68.40%) and apoptosis (19.65 and 34.50%,
respectively, vs. 7.78%) in MG-63 cells, when compared with control
cells (P<0.05).
TMP inhibits protein expression of
cytosolic and nuclear NF-κB p65, BCL-2 and cyclin D1 in MG-63 OS
cells in vitro
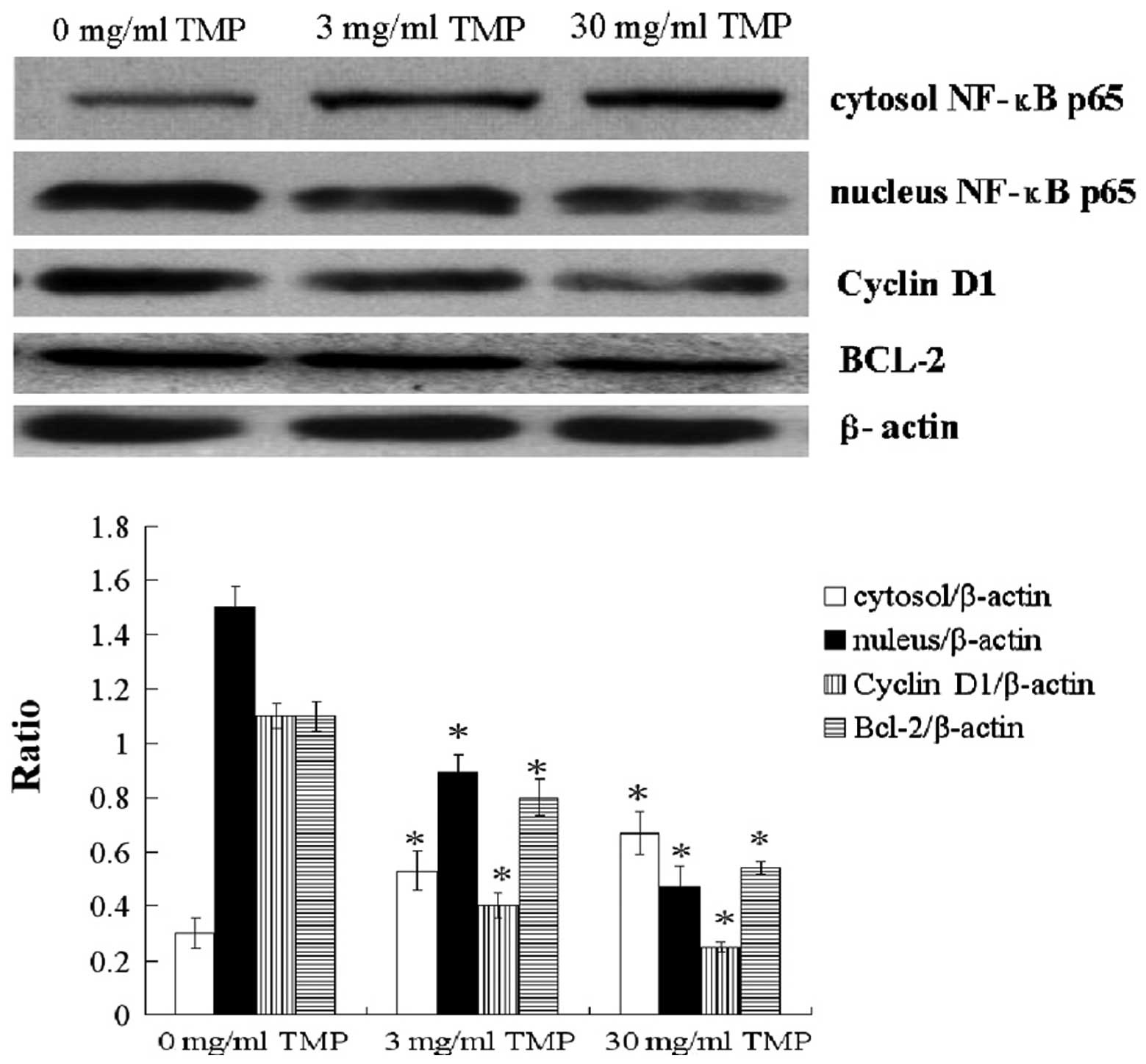
To investigate the mechanism by which TMP acts on
MG-63 OS cells, the protein expression of cytosolic and nuclear
NF-κB p65, BCL-2 and cyclin D1 was determined by western blot
analysis. Results showed that TMP treatment (3 and 30 mg/ml)
upregulated the protein expression of cytosolic NF-κB p65, while
downregulating the protein expression of nuclear NF-κB p65, cyclin
D1 and BCL-2 in MG-63 OS cells (P<0.05; Fig. 3).
TMP exerts antitumor effects against OS
in a xenograft nude mouse model
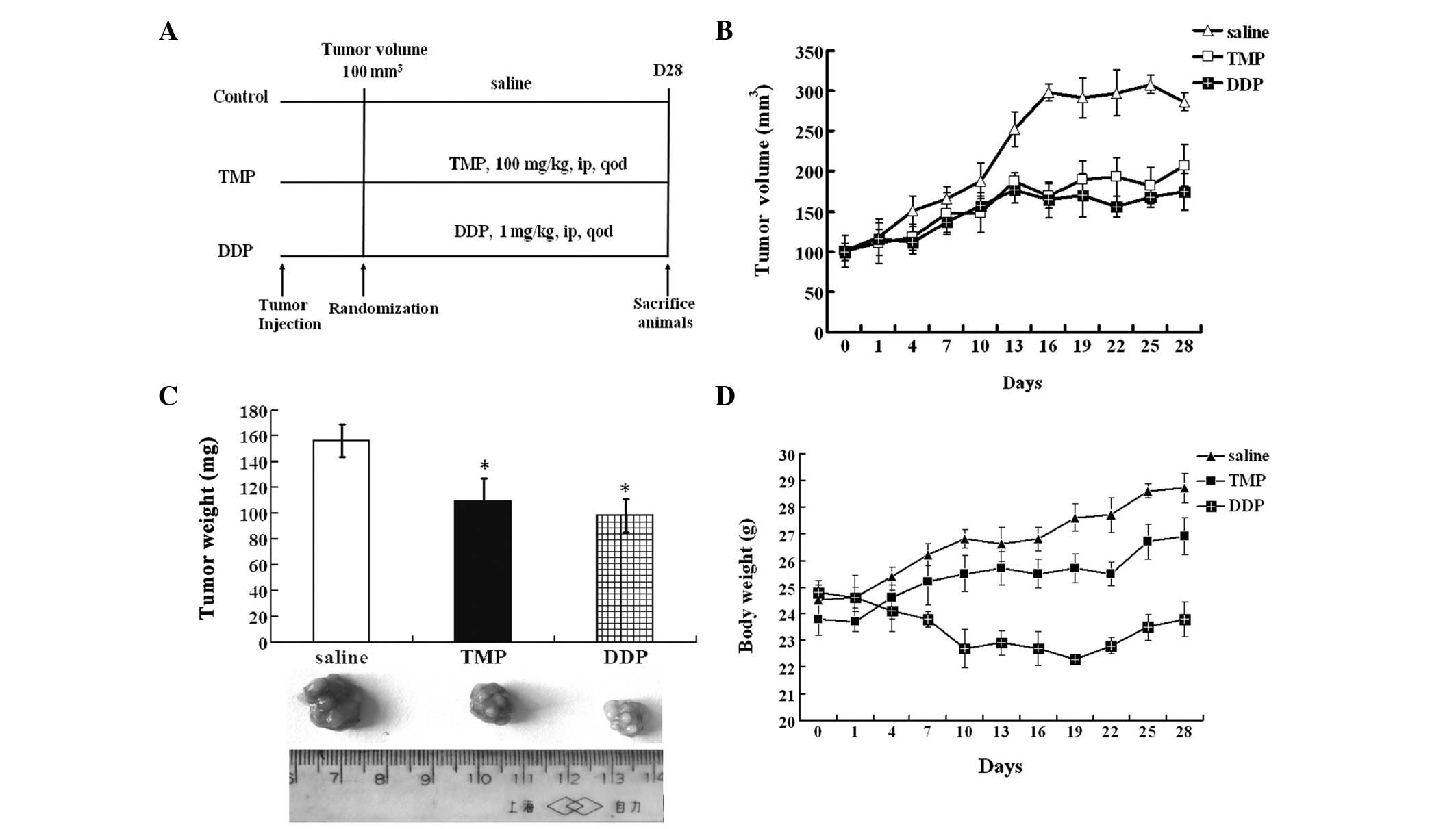
The inhibitory potential of TMP on the growth of
subcutaneously implanted MG-63 OS cells in nude mice was
investigated. The experimental protocol is shown in Fig. 4A. TMP administered at a dose of 100
mg/kg every other day for 28 days was found to significantly
inhibit the growth of tumors compared with that in the vehicle
group (P<0.05). DDP administered at a dose of 1 mg/kg every
other day for 28 days also effectively inhibited tumor growth
compared with that of vehicle-treated tumors (P<0.05; Fig. 4B and C). However, DDP significantly
reduced the body weight of mice (P<0.05), while TMP had a
smaller effect on the body weight of mice (Fig. 4D).
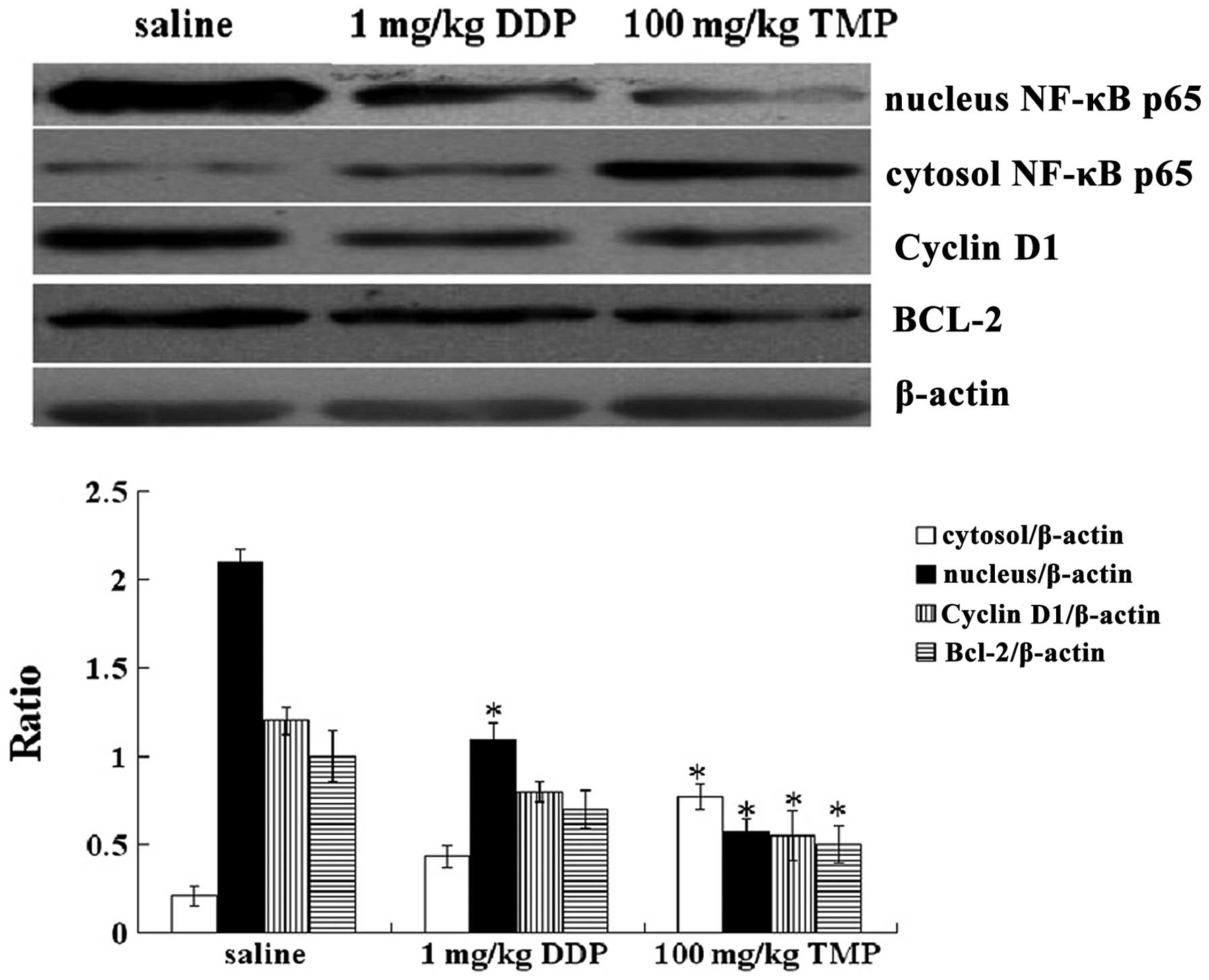
TMP inhibits protein expression of
cytosolic and nuclear NF-κB p65, BCL-2 and cyclin D1 in OS
tissues
We also evaluated the effect of TMP on the protein
expression of cytosolic and nuclear NF-κB p65, BCL-2 and cyclin D1
in OS tissues. As shown in Fig. 5,
TMP administered at a dose of 100 mg/kg every other day for 28 days
was effective in suppressing the protein expression of nuclear
NF-κB p65, cyclin D1 and BCL-2, while increasing the protein
expression of cytosolic NF-κB p65 in OS tissues (P<0.05).
Discussion
OS is a heterogeneous group of malignancies
characterized by varying degrees of mesenchymal differentiation.
The unifying histological feature of OS is the presence of
malignant osteoid produced by neoplastic cells (21). Successful clinical management of OS
faces two major challenges. Firstly, the toxic and adverse effects
associated with chemotherapy may significantly reduce patient
quality of life, although preoperative and postoperative
chemotherapy regimens have improved the 5-year survival rate
(22). Secondly, OS has a high
rate of recurrence and metastasis, which causes the majority of
OS-related mortalities (22).
Thus, there is an urgent requirement to identify less toxic and
more efficacious treatment alternatives. As a result, increasing
attention has been focused on the application of natural products
in the treatment of OS.
TMP (molecular weight, 136.19), one of the major
bioactive components purified from the Chinese herb Ligusticum
wallichii Franch., has been widely used in the treatment of
cardiovascular and cerebral diseases in China (23). However, a number of additional
pharmacological effects of TMP have been identified; TMP has been
found to act as an anti-inflammatory agent in a rat asthma model
(24). Fu et al(9) also reported that TMP affects the
growth and migration of a glioma cell line by inhibiting calcium
influx. Similarly, Chen et al(15) showed that TMP inhibits melanoma
metastasis in vivo partly through suppressing vascular
endothelial growth factor (VEGF) activity. Therefore, TMP may
constitute a potentially effective option for the treatment of
inflammation and tumors. Furthermore, TMP has been reported to have
a beneficial effect on various types of cancer (9–16).
However, the function and underlying mechanisms of TMP in OS have
not been elucidated to date.
The aim of the present study was to investigate
whether TMP had antitumor activity against human OS. TMP was found
to suppress the proliferation of OS cell lines in a dose-dependent
manner. These inhibitory effects of TMP were correlated with
TMP-induced cell apoptosis and cell cycle arrest at the G1/G0
phase. It was also shown for the first time that the
intraperitoneal administration of TMP effectively suppressed the
growth of OS in a xenograft mouse model, and had a smaller effect
on the body weight of mice compared with DDP treatment. These
results indicate that TMP possesses more effective antitumor
activities against OS and has a lower toxicity.
The NF-κB transcription factor is important in a
number of cellular processes, particularly in inflammation and
tumor development. NF-κB is normally sequestered in the cytoplasm
by a family of inhibitory proteins, known as inhibitors of κB
(IκB). A wide variety of stimuli cause the phosphorylation of IκBα,
which is followed by its ubiquitination and subsequent degradation.
The loss of IκBα results in the release of free NF-κB unit p65,
which translocates from the cytoplasm to the nucleus, where p65
activates the expression of target genes, including BCL-2, cyclin
D1 and MMP-9. To investigate the underlying mechanisms of TMP
against OS, the protein expression of cytosolic and nuclear NF-κB
p65 and the NF-κB-regulated target genes, BCL-2 and cyclin D1, were
detected. TMP was found to downregulate the expression of proteins
associated with cell proliferation, including cyclin D1 and BCL-2,
which may explain its potent antiproliferative effects on OS.
Notably, TMP suppressed the expression of nuclear NF-κB p65 and
increased the expression of cytosolic NF-κB p65. This may be
associated with the inhibition of the NF-κB p65 translocation from
the cytoplasm to the nucleus by TMP; however, additional evidence
is required.
In conclusion, the present study showed that TMP
exerted more effective antitumor activities against OS in
vitro and in vivo, and that TMP had a lower toxicity.
Furthermore, TMP induced cell apoptosis and cell cycle arrest at
the G1/G0 phase and upregulated the protein expression of cytosolic
NF-κB p65, while downregulating the protein expression of nuclear
NF-κB p65, BCL-2 and cyclin D1; this may be the mechanism via which
TMP exerts it effects in OS. However, further and more
comprehensive studies are required to confirm this. These data
strongly suggest for the first time that TMP may have a significant
potential for application in the treatment of OS.
Acknowledgements
This study was supported by the National Science
Foundation (grant no. 81070688/H0726).
References
|
1
|
Sandberg AA and Bridge JA: Updates on the
cytogenetics and molecular genetics of bone and soft tissue tumors:
osteosarcoma and related tumors. Cancer Genet Cytogenet. 145:1–30.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helman LJ and Meltzer P: Mechanisms of
sarcoma development. Nat Rev Cancer. 3:685–694. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haydon RC, Luu HH and He TC: Osteosarcoma
and osteoblastic differentiation: a new perspective on oncogenesis.
Clin Orthop Relat Res. 454:237–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
6
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: the
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen G, Caparros B, Huvos AG, Kosloff C,
Nirenberg A, Cacavio A, et al: Preoperative chemotherapy for
osteogenic sarcoma: selection of postoperative adjuvant
chemotherapy based on the response of the primary tumor to
preoperative chemotherapy. Cancer. 49:1221–1230. 1982. View Article : Google Scholar
|
|
8
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu YS, Lin YY, Chou SC, Tsai TH, Kao LS,
Hsu SY, Cheng FC, Shih YH, Cheng H, Fu YY and Wang JY:
Tetramethylpyrazine inhibits activities of glioma cells and
glutamate neuroexcitotoxicity: potential therapeutic application
for treatment of gliomas. Neuro Oncol. 10:139–152. 2008. View Article : Google Scholar
|
|
10
|
Yu K, Chen Z, Pan X, Yang Y, Tian S, Zhang
J, Ge J, Ambati B and Zhuang J: Tetramethylpyrazine-mediated
suppression of C6 gliomas involves inhibition of chemokine receptor
CXCR4 expression. Oncol Rep. 28:955–960. 2012.PubMed/NCBI
|
|
11
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
12
|
Zhang Y, Liu X, Zuo T, Liu Y and Zhang JH:
Tetramethylpyrazine reverses multidrug resistance in breast cancer
cells through regulating the expression and function of
P-glycoprotein. Med Oncol. 29:534–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin J, Yu C, Yang Z, He JL, Chen WJ, Liu
HZ, Li WM, Liu HT and Wang YX: Tetramethylpyrazine inhibits
migration of SKOV3 human ovarian carcinoma cells and decreases the
expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling
pathways. Oncol Rep. 26:671–679. 2011.PubMed/NCBI
|
|
14
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.PubMed/NCBI
|
|
15
|
Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao
M, Zheng SZ, Wang AY, Zhang CB, Zhang WW and Lei N: Ligustrazine
inhibits B16F10 melanoma metastasis and suppresses angiogenesis
induced by Vascular Endothelial Growth Factor. Biochem Biophys Res
Commun. 386:374–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Xu Y, Gu X, Hu Y and Wang C:
Molecular mechanism of tetramethylpyrazine to induce human
promyelocytic HL-60 leukemia cells differentiation. Zhongguo Zhong
Yao Za Zhi. 36:3007–3011. 2011.(In Chinese).
|
|
17
|
Fan L, Wang K, Shi Z, Die J, Wang C and
Dang X: Tetramethylpyrazine protects spinal cord and reduces
inflammation in a rat model of spinal cord ischemia-reperfusion
injury. J Vasc Surg. 54:192–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X
and Dong W: Thymoquinone inhibits growth and augments
5-fluorouracil-induced apoptosis in gastric cancer cells both in
vitro and in vivo. Biochem Biophys Res Commun. 417:864–868. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Institute for Laboratory Animal Research.
Guide for the Care and Use of Laboratory Animals. Eighth Edition.
The National Acadamies Press; Washington, D.C., USA: 2011
|
|
20
|
Luong QT, O’Kelly J, Braunstein GD,
Hershman JM and Koeffler HP: Antitumor activity of suberoylanilide
hydroxamic acid against thyroid cancer cell lines in vitro and in
vivo. Clin Cancer Res. 12:5570–5577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorfman HD and Weiss SW: Borderline
osteoblastic tumors: problems in the differential diagnosis of
aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn
Pathol. 1:215–234. 1984.PubMed/NCBI
|
|
22
|
He BC, Chen L, Zuo GW, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li WM, Liu HT, Li XY, Wu JY, Xu G, et al:
The effect of tetramethylpyrazine on hydrogen peroxide-induced
oxidative damage in human umbilical vein endothelial cells. Basic
Clin Pharmacol Toxicol. 106:45–52. 2010.PubMed/NCBI
|
|
24
|
Xiong L, Fang ZY, Tao XN, Bai M and Feng
G: Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a
rat asthma model. Am J Chin Med. 35:1011–1020. 2007. View Article : Google Scholar : PubMed/NCBI
|