Introduction
Asthma is a common chronic airway disease that is
characterized by airway inflammation, airway tissue remodeling and
airway hyperresponsiveness (AHR) (1,2).
Although the symptoms of asthma are largely controllable, the
disease is often not completely curable at present. This is
generally attributed to the absence of a complete understanding of
the mechanism(s) of asthma pathogenesis. Previously, complex
interactions between multiple genetic and environmental factors
contributing to the pathogenesis of asthma have been demonstrated
(3). Thus, a full investigation of
the genes associated with asthma is required to ascertain their
roles in mediating airway pathologies.
In the present study, one particular gene of
interest, a disintegrin and metalloproteinase 33 (ADAM33), was
observed for its correlation with the mechanics of airway smooth
muscle cells (ASMCs) in ovalbumin (OVA)-sensitized rat models of
asthma. In human studies, ADAM33 has been identified as an asthma
susceptibility gene (1,4–8).
Subsequently, the correlations between the ADAM33 gene and airway
inflammation and airway tissue remodeling have been demonstrated
(9,10). However, the correlation between the
ADAM33 protein and AHR that is equally, if not more important than
airway inflammation and tissue remodeling in asthma, remains to be
studied. AHR is determined by the mechanics of ASMCs and the ADAM33
protein is predominantly expressed in ASMCs (6,10,11).
Thus, it is feasible that in asthma, the protein level of ADAM33 is
altered, which may correlate with the mechanics of ASMCs and
contribute to the induction of asthma development.
To test this hypothesis, Sprague Dawley (SD) rats
were sensitized with OVA for up to 12 weeks to model chronic
asthma. The protein expression of ADAM33 was then measured, along
with the stiffness and contractility, traction force generation and
cytoskeletal structure of the ASMCs obtained from the animal models
at different time points of sensitization. The results demonstrated
that the protein expression of ADAM33 in the ASMCs of the
sensitized rats increased compared with that of the controls.
However, the increase peaked at 4 weeks of sensitization, and
gradually declined as sensitization continued. Notably, the
majority of mechanical properties appeared to change similarly
throughout the sensitization period, resulting in a positive
correlation between the protein expression of ADAM33 and the
stiffness, traction force generation, and expression of viculin and
F-actin. This implied that ADAM33 protein expression was correlated
with the mechanics of ASMCs, and that the two may be concurrently
involved in the pathogenesis and progression of asthma.
Materials and methods
Animal models and ASMC culture
Male SD rats (purchased from Chonqing Medical
School, Chonqing, China) were sensitized by OVA to simulate asthma
symptoms, following a standard protocol as described previously
(12,13). The study was approved by the Animal
Investigational Committee of Chongqing Medical School, Chongqing,
China. In brief, the rats were injected with 1% OVA and 10%
Al(OH)3 in NaCl solution on days 1 and 7. From day 14,
the rats were sensitized by OVA three times per week for 4, 6 or 12
weeks, to mimic chronic asthma at different stages (data not
shown). Rats recieving the same treatment schedule, but with saline
instead of OVA, were used as controls. At given time points of
sensitization (at 4, 6 and 12 weeks), primary ASMCs were isolated
from the SD rats and cultured in vitro according to the
method described previously (14).
In this study, all the cells used were between passages 2 to 5.
Immunoblotting for ADAM33 protein
Protein expression of ADAM33 was assessed by western
blot analysis as described previously (1,9).
Briefly, when the ASMCs grew to 90% confluence, they were lysed
with 1X loading buffer (Beyotime Biotech, Jiangsu, China) to
extract the total proteins. Subsequently, the proteins from each
group, together with β-tubulin as the reference marker, were loaded
in an equal volume of solution onto 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Western
blot analysis was performed using a specific primary antibody for
ADAM33 protein [AV49937; Sigma-Aldrich, St. Louis, MO, USA; diluted
to 1:200 in 1% bovine serum albumin (BSA)] and β-tubulin (Cell
Signaling Technology, Inc., Beverly, MA, USA) overnight at 4°C.
Subsequently, detection was performed using the corresponding
horseradish peroxidase-conjugated secondary antibodies (Wuhan
Boster, Biological Technology, Ltd., Wuhan, China). The protein
expression of ADAM33 and β-tubulin was detected using an enhanced
chemiluminescence kit (Beyotime Biotech, Jiangsu, China), and the
ratio of the protein expression of ADAM33 to that of β-tubulin was
used for quantitative assessment.
Assessment of ASMC stiffness and
contractility
To assess ASMC mechanical properties, the cell
stiffness and contractility were measured by optical magnetic
twisting cytometry (OMTC), as previously described (13,15).
Briefly, ASMCs were seeded into 96-well cell culture dishes
(Costar, Corning, Inc., NY, USA) coated with collagen (type I,
Sigma-Aldrich) at a density of 20,000 cells/well and cultured for
≥12 h in serum-free medium. Following this, ferrimagnetic beads
(diameter, 4.5 μm, provided by Dr. J. J. Fredberg of Harvard School
of Public Health, Boston, MA, USA) pre-coated with synthetic
Arginine-Glycine-Aspartic acid (RGD; 50 μg peptide/mg beads;
Integra Life Sciences, Plainsboro Township, NJ, USA) were added and
the cells were incubated for ~20 min to allow specific binding to
the integrin receptors on the cell surface (approximately one or
two beads per cell). The beads were then magnetized horizontally
and twisted in an oscillatory magnetic field at a frequency of
0.1–100 Hz. The cell stiffness (G′) was measured as the ratio
between the applied magnetic torque and bead displacement
(Pa/nm).
The change in cell stiffness in response to
stimulation by contractile agonist, such as KCl, was observed in
the ASMCs. ASMC stiffness was measured at a constant oscillation
frequency (0.3 Hz) for up to 60 sec. Following this, the
contractile agonist, KCl (80 mM; iso-osmotic in this case), was
immediately added to the cells. In response to KCl, the cells
contracted, and thus, the cell stiffness increased in ~1 min. The
ratio between the cell stiffness following the addition of KCl and
prior to the addition of KCl was defined as the contractility of
the ASMCs (16).
Assessment of traction force generation
by ASMCs
Traction force generated by ASMCs was measured using
an elastic polyacrylamide gel substrate embedded with 0.2-μm
diameter fluorescence beads (F8811; Invitrogen Life Technologies,
Carlsbad, CA, USA) as described previously (17,18).
Briefly, the polyacrylamide gel was prepared by adjusting the ratio
of 40% acrylamide and 2% bis-acrylamide to produce a gel with a
Young’s modulus of 4 kPa. The prepared gel was placed in a cell
culture dish with a glass bottom (to enable microscopy) and allowed
to polymerize, forming an elastic substrate with a diameter of ~18
mm and a thickness of 0.1 mm. Following gel polymerization, the
substrate was activated by sulfo-SANPAH (Pierce Biotechnology,
Inc., Rockford, IL, USA) and coated with 0.2 mg/ml collagen
solution (type I) overnight at 4°C. The following day, the
substrate was hydrated with 2 ml Dulbecco’s modified Eagle’s
medium/F-12 and incubated at 37°C and 5% CO2 for ≥24 h
prior to use.
Cells were seeded onto the polyacrylamide gel
substrate at a density of ~2000–5000 cells/well, incubated for 12 h
to allow the cells to attach to the substrate and cultured for ≥12
h in serum-free medium. Single ASMCs were imaged with phase
contrast by an inverted optical microscope (Leica DMI6000B; Leica
Microsystems CMS GmbH, Wetzlar, Germany) and then detached from the
substrate by trypsinization. Fluorescence imaging of the
fluorescent beads embedded in the gel substrate was conducted with
the inverted optical microscope prior to and following cell
detachment. The traction force generated by the cells was
subsequently computed by the displacement field of the elastic
substrate as measured by the positions of the embedded fluorescent
beads prior to and following cell detachment (18).
Assessment of the ASMC cytoskeletal
structure
To assess the cytoskeletal structure, ASMCs were
labeled with fluorescent probes for vinculin and F-actin, and
analyzed by confocal microscopy. Vinculin and F-actin are essential
components of the focal adhesions and the microfilament
cytoskeleton, respectively, and are therefore commonly used to
visualize and evaluate the structure of the cytoskeleton (17,19).
The ASMCs were fixed with 4% formaldehyde for ~20 min, washed with
phosphate-buffered saline (PBS) and permeabilized with 0.2% Triton
X-100 in PBS for 5 min at room temperature. Following washing with
PBS, cells were treated with a blocking solution (1% BSA) for 30
min at room temperature.
To label vinculin with a fluorescent probe, the
cells were incubated with monoclonal anti-vinculin antibody (Abcam,
Cambridge, UK; 1:200 dilution in 1% BSA) overnight at 4°C. The
cells were washed with PBS, and further incubated with the
secondary antibody, rhodamine labeled goat anti-mouse IgG
(ProteinTech, Chicago, IL, USA; 1:100 dilution in 1% BSA) for 2 h
at room temperature. To label F-actin with a fluorescent probe, the
cells were incubated with fluorescein isothiocyanate-phalloidin (5
μg/ml; cytoskeleton) for 30 min at room temperature. The
fluorescently labeled cells were subsequently observed and imaged
by confocal microscopy (Leica TCS SP5 II, Leica Microsystems CMS
GmbH). The fluorescence intensity of the labeled vinculin and
F-actin in each cell was quantified by analysis of the fluorescent
confocal microscopy images, using Image-Pro Plus software (Media
Cybernetics, Inc., Rockville, MD, USA).
Correlation analysis and statistics
Pearson’s correlation was tested using the
Statistical Product and Service Solution v.17 (SPSS, Inc., Chicago,
IL, USA). Significant differences were analyzed using one-way
analysis of variance followed by Tukey’s test for multiple
comparisons between groups, using SigmaPlot 12.0 (SysStat Software,
San Jose, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
OVA sensitization induces ADAM33 protein
expression
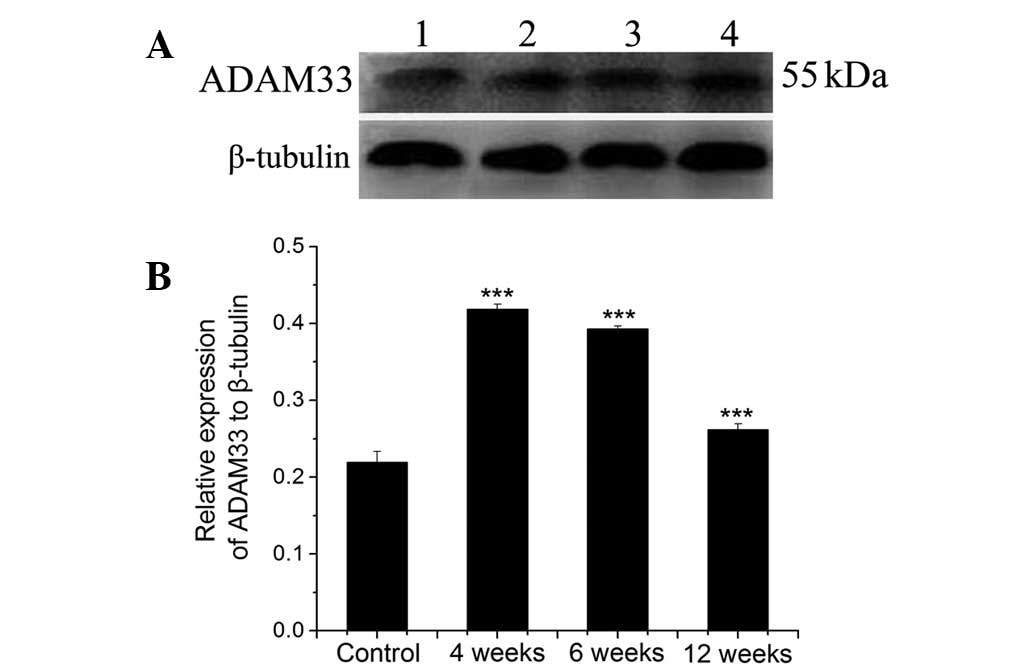
Fig. 1 demonstrates
the levels of the protein expression of ADAM33 in ASMCs from rats
recieving OVA sensitization for 4, 6 or 12 weeks, and from the
controls, as quantified by western blot analysis. Fig. 1A shows the western blot image in
which an intense band of ~55 kDa, corresponding to the molecular
weight of ADAM33 protein was observed, indicating ADAM33
expression. Lanes 1–4 in Fig. 1A
represent samples from the controls and the rats sensitized by OVA
for 4, 6 and 12 weeks, respectively. Fig. 1B shows the quantitative results of
the western blot analysis, as defined by the ratio of the
expression of ADAM33 protein to that of β-tubulin. Compared with
the controls, the protein expression of ADAM33 was increased in all
ASMCs from the OVA-sensitized rats, and this increase peaked when
the rats were sensitized for 4 weeks and then gradually declined as
OVA sensitization continued (P<0.001).
OVA sensitization leads to stiffening,
but no change in contractility, of the ASMCs
Fig. 2 demonstrates
the cell stiffness and contractility of ASMCs from rats with or
without OVA sensitization, as measured by OMTC. Fig. 2A shows the log-log plot of the cell
stiffness (G′) versus the frequency of measurement (0.1–100 Hz). In
all cases, the cell stiffness exhibited typical power-law behavior,
in which cell stiffness increased with frequency (f) as a
power-law response (G′~ƒα), where α is the power-law
exponent that is typically between 0.1 and 0.3 for living cells
(20). The magnitude of the cell
stiffness was observed to be in the ascending order of 12 weeks,
control, 6 weeks and 4 weeks, and α also increased with the same
trend. This trend was further demonstrated by the cell stiffness
measured at a single constant frequency (0.3 Hz), the G0
of ASMCs from rats with or without OVA sensitization. Compared with
the control rats, G0 in the OVA-sensitized rats
significantly increased, peaked when the rats were sensitized for 4
weeks (P<0.05) and gradually declined with continued
sensitization (Fig. 2B).
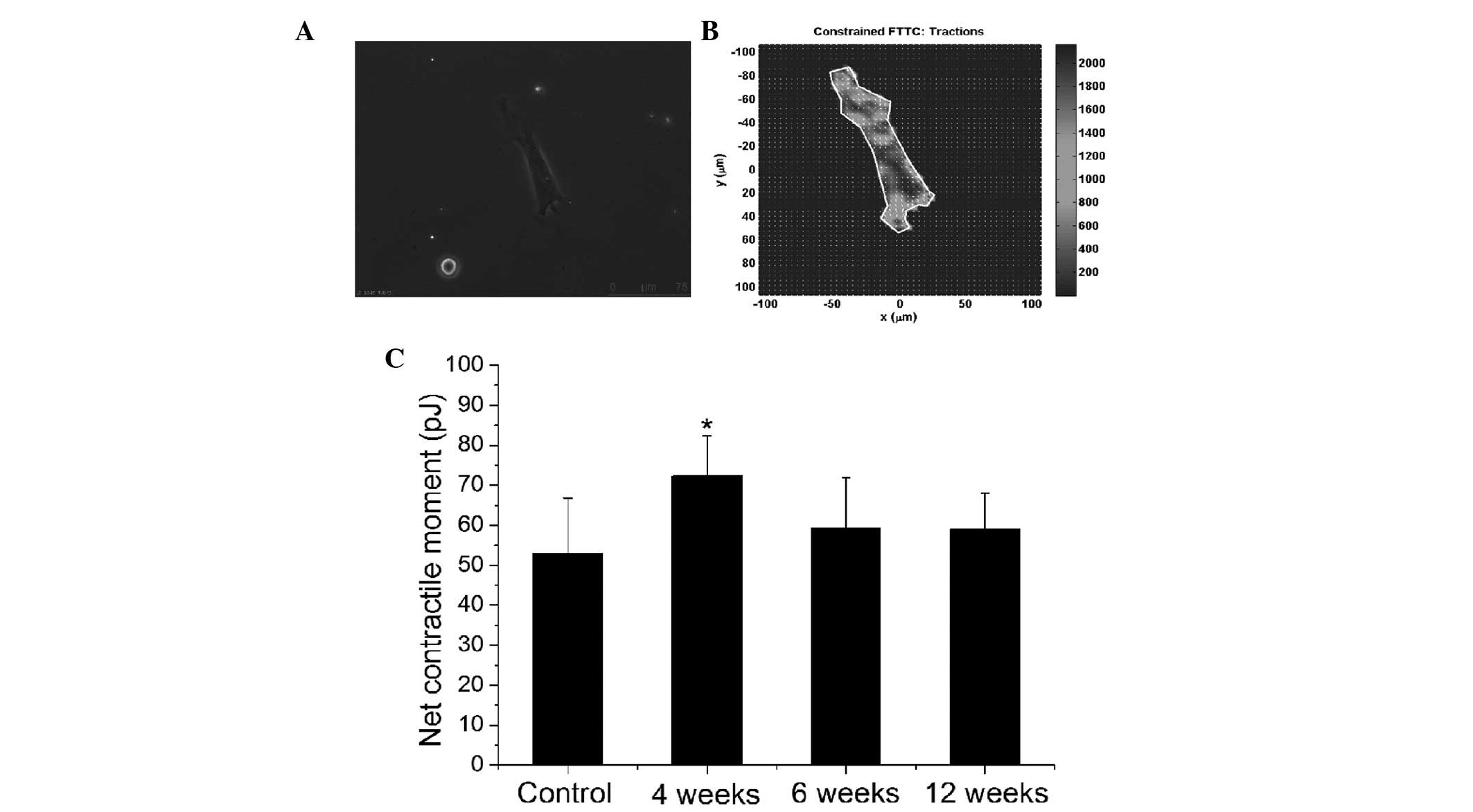
 | Figure 2Stiffness and contractility of the
ASMCs from either non-sensitized controls or OVA-sensitized rats.
(A) The stiffness of the ASMCs measured by OMTC across the spectrum
of frequencies (0.1–100 Hz). Data on the ASMCs from rats that were
either non-sensitized (controls, □) or OVA-sensitized for 4 (Δ), 6
(▽) or 12 weeks (⋄). The straight lines represent the
linear-fitting for the log-log plots of the cell stiffness (G′)
versus the frequency, displaying power-law relationships with
corresponding slopes (α). (B) Baseline stiffness (G0)
measured at a constant 0.3 Hz and averaged over ~50 sec for ASMC
from either non-sensitized (controls) or OVA-sensitized rats (from
left to right, 4, 6 and 12 weeks, respectively). (C) A typical time
course of ASMC measured by OMTC at a constant 0.3 Hz prior to and
following the addition of KCl (80 mM; isotonic). Prior to the
addition of KCl, the stiffness fluctuated about the baseline level
(G0). Upon addition of KCl (~60 sec), ASMCs contracted
and thus the stiffness rapidly increased from the baseline level
(G0) and stabilized at a higher level (GKCl)
in ~1 min. (D) ASMC contractility quantified as the ratio of the
stiffness measured following the addition of KCl to the baseline
stiffness measured prior to the addition of KCl
(GKCl/G0). The data bars from left to right
represent the quantified contractility of ASMC from rats that were
either non-sensitized (controls), or OVA-sensitized for 4, 6 and 12
weeks, respectively. (*P<0.05 and
###P<0.001, n=6). OVA, ovalbumin; ASMCs, airway
smooth muscle cells; OMTC, optical magnetic twisting cytometry. |
Fig. 2C shows a
typical time course plot of cell stiffness in response to KCl
stimulation. Prior to the addition of KCl, the cell stiffness
fluctuated with an averaged baseline level (G0).
Following the addition of KCl to the ASMCs, the cells responded by
contraction that rapidly increased the cell stiffness to a higher
level (GKCl) in ~1 min. Thus, the contractility of ASMCs
with KCl stimulation was quantified as the ratio between the cell
stiffness following KCl activation (GKCl) and the
baseline cell stiffness (G0), i.e.
GKCl/G0. As demonstrated in Fig. 2D, OVA sensitization did not result
in a significant change in the contractility of ASMCs from SD
rats.
OVA-sensitization results in an acute
increase in the traction force of ASMCs
Fig. 3 demonstrates
the effect of OVA sensitization on the traction force generated by
ASMCs. Fig. 3A shows the
phase-contrast image of a single ASMC cultured on the
polyacrylamide substrate, and Fig.
3B demonstrates the field of the traction force generated in
this cell. From the traction force field, the net contractile
moment of each ASMC was quantified. In Fig. 3C, it was observed that compared
with the controls, the traction force generated by ASMCs was
significantly greater in the rats that had been sensitized by OVA
for 4 weeks (P<0.05), and marginally (but not significantly)
enhanced for the longer duration of OVA sensitization.
OVA sensitization enhances vinculin and
F-actin expression in ASMCs
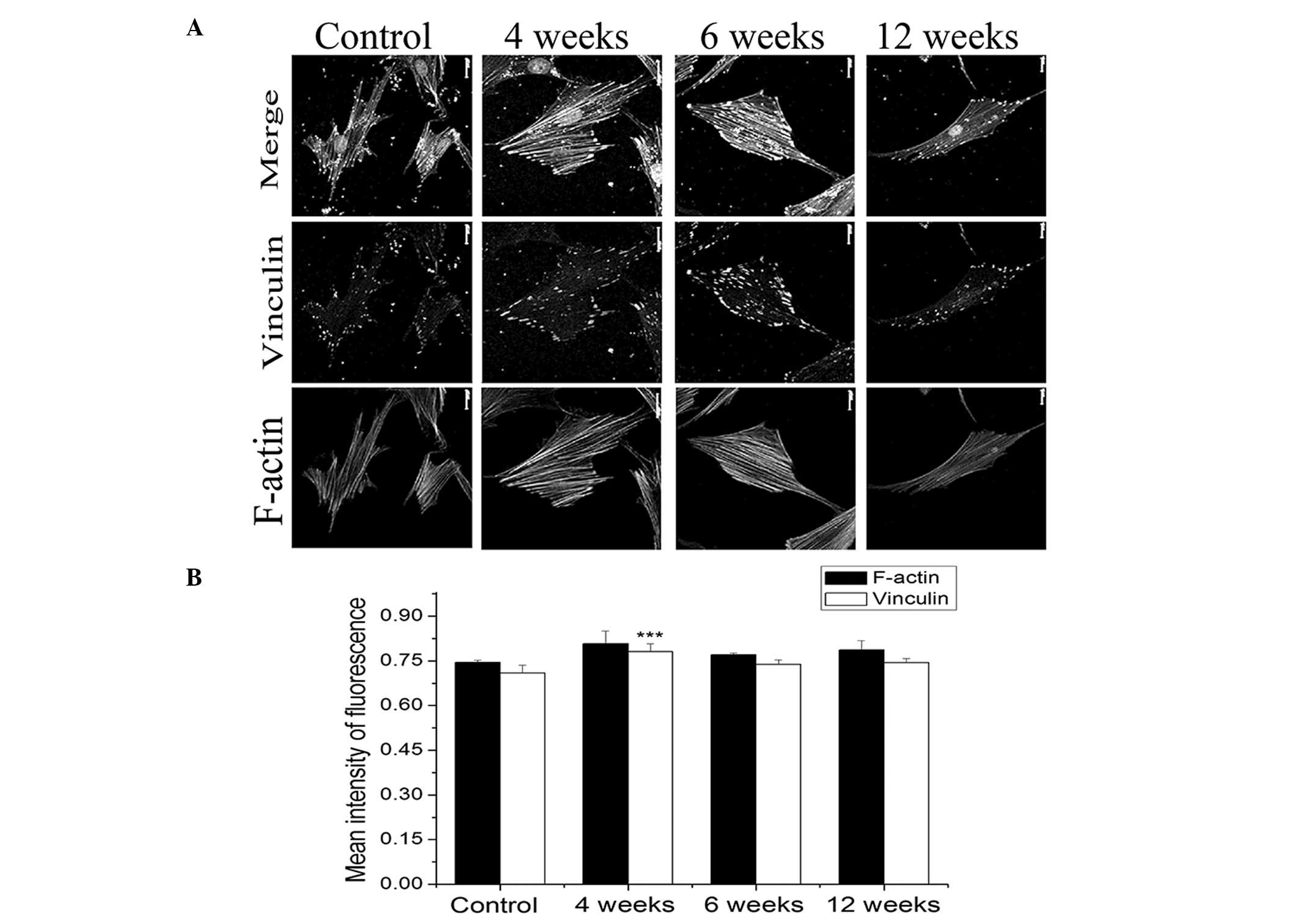
Fig. 4 demonstrates
the effect of OVA-sensitization on the expression of vinculin and
F-actin in the ASMCs of the SD rats. Representative images of
vinculin and F-actin, labeled by respective fluorescent probes and
visualized by laser scanning confocal microscopy, are shown in the
middle and lower panels of Fig.
4A. The merged results of vinculin and F-actin images are shown
in the top panel of Fig. 4A.
Images from the left to the right of Fig. 4A represent the cytoskeletal
structure of the ASMCs in control rats or in rats sensitized by OVA
for 4, 6 and 12 weeks, respectively. Through the analysis of the
fluorescence intensity in the images, it appears that ASMCs from
the OVA-sensitized rats exhibited marginally higher levels of
fluorescence intensity for vinculin and F-actin compared with those
of the controls. In particular, ASMCs from the rats sensitized by
OVA for 4 weeks exhibited the greatest level of vinculin expression
(P<0.001).
Correlation between ADAM33 protein
expression and the mechanics of ASMCs
The similarity between the OVA-induced ADAM33
protein expression and the altered mechanical properties of ASMCs
in response to OVA sensitization suggested that the protein
expression of ADAM33 was correlated with the mechanics of the
ASMCs. Further analysis of these results with respect to Pearson’s
correlation coefficient resulted in largely positive coefficients
between the tested results of ADAM33 protein expression and the
measured mechanical properties, including the cell stiffness,
traction force, and levels of vinculin and F-actin (Table I).
 | Table ICp between ADAM33 protein expression
and the G′, traction force, vinculin expression and F-actin
expression of ASMC. |
Table I
Cp between ADAM33 protein expression
and the G′, traction force, vinculin expression and F-actin
expression of ASMC.
| Cp | G′ | Traction force | Vinculin | F-actin |
|---|
| ADAM33 | 0.864 | 0.716 | 0.774 | 0.662 |
Discussion
The present study demonstrated that OVA
sensitization of SD rats resulted in increased protein expression
levels of ADAM33 in the ASMCs, in a time-dependent manner. The
protein expression levels of ADAM33 in the ASMCs reached a maximum
at ~4 weeks of OVA sensitization, and then gradually subsided as
OVA sensitization continued. Notably, the mechanical properties of
the ASMCs, including cell stiffness and traction force, were
altered during the course of OVA sensitization in the same manner
as that of the ADAM33 protein. The OVA-induced alteration of the
ASMC mechanics and ADAM33 protein expression were positively
correlated. These results implied that ADAM33 protein expression
was correlated with the mechanics of the ASMCs during the
pathogenesis and progression of asthma.
The protein expression of ADAM33 was increased in
the ASMCs of the OVA-sensitized rats, which was consistent with a
previous study that demonstrated that the expression of ADAM33 was
increased in asthmatic patients (1). However, in this study, ASMCs were
studied in vitro, and the protein expression of ADAM33 was
likely to be variable as the primary cells were passaged over time
(21). To control for this
passage-dependent variability, only the cells between passages 2 to
5 were used in the present study, and the relative expression of
the ADAM33 protein was obtained from cells of the same passage.
ASMC mechanics are generally recognized as the final
pathway to AHR that characterizes asthma (11,13).
In the present study, it was demonstrated that in the
OVA-sensitized SD rat models of asthma, the magnitude of ASMC
stiffness increased during OVA sensitization. Notably, the time
dependence of the cell stiffness of the ASMCs appeared to be
similar to that of the protein expression of ADAM33. Statistical
analysis verified that the stiffness was positively correlated with
the ADAM33 protein expression in the ASMCs of the OVA-sensitized
rats (Pearson’s correlation coefficient, 0.864). ASMC stiffness
also exhibited characteristic power-law behavior when measured over
a range of frequencies, which was consistent with previous studies
(15,22). However, it was noted that the
contractility of the ASMCs as determined by the stiffness increase
in response to KCl stimulation was not affected by OVA
sensitization. The absence of an effect of OVA-sensitization on the
KCl-induced contractility in the ASMCs was somewhat but not
completely surprising, due to the nature of KCl stimulation. As a
non-specific contractile agonist, it is possible that the OVA
sensitization was only effective on certain structure(s) of the
contractile machinery of the ASMCs, which KCl was unable to target
or activate. Specific agonists, such as histamine, that target the
G-protein coupled receptors may be used to further investigate
this.
Fourier transform traction microscopy was utilized
to assess the ASMCs for the ability to generate traction force at
the single-cell level. The results demonstrated that compared with
the non-sensitized rats, ASMCs from the OVA-sensitized rats
generated greater traction force, and the extent of traction force
enhancement was also positively correlated with the increase in
ADAM33 protein expression during OVA sensitization (Pearson’s
correlation coefficient, 0.716). The enhancement of traction force
generation by the ASMCs may be attributed to various factors,
including a strengthened cytoskeletal structure, the interaction of
actin and myosin filaments (19)
and enhanced focal adhesions due to an increased quantity of
structural and signaling proteins, such as vinculin (23). These factors, separately or
combined, may enhance the physical links between the cytoskeleton
and the extracellular matrix, exerting a greater traction force to
the extracellular matrix (24). In
the present study, the results of the confocal microscopy of the
ASMCs that were fluorescently labeled for vinculin and F-actin
demonstrated that the ASMCs from the OVA-sensitized rats exhibited
a greater level of fluorescence intensity of vinculin and F-actin
compared with those from the non-sensitized rats. The increases in
vinculin and F-actin were also positively correlated with the
increased protein expression of ADAM33 during OVA-sensitization
(Pearson’s correlation coefficients, 0.774 and 0.662 for vinculin
and F-actin, respectively). This suggests a possible signaling
pathway of ASMCs in which ADAM33 protein modulates cell mechanics
via the regulation of cytoskeletal proteins, such as vinculin and
F-actin.
Furthermore, the results demonstrated that the
effects of OVA sensitization on the ASMCs were greatest when the
rats were sensitized for 4 weeks, and then they gradually decreased
regardless of the continuation of OVA sensitization. This time
dependence of the efficacy of OVA sensitization may be largely
attributed to the phenomenon of desensitization, which has been
observed in repeatedly challenged rats and guinea pigs (25,26).
The rate of desensitization was faster for ASMC mechanics compared
with that of the expression of the ADAM33 protein. This difference
implied that the ASMC mechanics that characterize airway function
may be more sensitive and adaptable to the presence of an allergen
compared with biological targets, such as ADAM33 protein (27); however, further studies are
required.
To the best of our knowledge, this study
demonstrated for the first time in vivo that OVA
sensitization of SD rats led to the increased expression of ADAM33
protein in ASMCs. OVA sensitization also resulted in increased
stiffness, traction force and expression of vinculin and F-actin in
ASMCs. The extent of these changes were time-dependent during OVA
sensitization. Notably, the change in the expression of ADAM33
protein in the ASMCs was correlated with the changes in ASMC
mechanics, including cell stiffness, traction force and the
expression of vinculin and F-actin. This suggested that the ADAM33
protein and the ASMC mechanics may be concordantly involved in the
pathogenesis and progression of asthma. As ADAM33 is an asthma
susceptibility gene and ASMC mechanics are the common pathway to
AHR that is the hallmark of asthma, these results may have
identified a potential mechanism through which ADAM33 contributes
to the pathogenesis of asthma, and thus may aid in the development
of novel ADAM33-based therapeutics to treat asthma.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 11172340); the Training
Program for Hundreds of Distinguished Leading Scientists of
Chongqing, Chongqing Natural Science Foundation (project nos.
CSTC2010BA5001 and CSTC2012jjA0588); the Fundamental Research Funds
for the Central Universities (grant no. CQDXWL-2012-123); the
Specialized Research Fund for the Doctoral Program of Higher
Education of China (grant no. 20120191120032); and the Fundamental
Research Funds for the Central Universities (project nos.
CDJXS11230038 and CDJXS11230027).
References
|
1
|
Lee JY, Park SW, Chang HK, et al: A
disintegrin and metalloproteinase 33 protein in patients with
asthma: Relevance to airflow limitation. Am J Respir Crit Care Med.
173:729–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogers DF: Airway mucus hypersecretion in
asthma: an undervalued pathology? Curr Opin Pharmacol. 4:241–250.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Moffatt MF and Cookson WO:
Genetic and genomic approaches to asthma: new insights for the
origins. Curr Opin Pulm Med. 18:6–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JH, Park HS, Park SW, et al: ADAM33
polymorphism: association with bronchial hyper-responsiveness in
Korean asthmatics. Clin Exp Allergy. 34:860–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tripathi P, Awasthi S, Prasad R, et al:
Association of ADAM33 gene polymorphisms with adult-onset asthma
and its severity in an Indian adult population. J Genet.
90:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Eerdewegh P, Little RD, Dupuis J, et
al: Association of the ADAM33 gene with asthma and bronchial
hyperresponsiveness. Nature. 418:426–430. 2002.PubMed/NCBI
|
|
7
|
Holgate ST, Davies DE, Powell RM and
Holloway JW: ADAM33: a newly identified protease involved in airway
remodelling. Pulm Pharmacol Ther. 19:3–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werner M, Herbon N, Gohlke H, et al:
Asthma is associated with single-nucleotide polymorphisms in
ADAM33. Clin Exp Allergy. 34:26–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Powell RM, Wicks J, Holloway JW, et al:
The splicing and fate of ADAM33 transcripts in primary human
airways fibroblasts. Am J Respir Cell Mol Biol. 31:13–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umland SP, Garlisi CG, Shah H, et al:
Human ADAM33 messenger RNA expression profile and
post-transcriptional regulation. Am J Respir Cell Mol Biol.
29:571–582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
An SS, Bai TR, Bates JH, et al: Airway
smooth muscle dynamics: a common pathway of airway obstruction in
asthma. Eur Respir J. 29:834–860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jie Z, Jin M, Cai Y, et al: The effects of
Th2 cytokines on the expression of ADAM33 in allergen-induced
chronic airway inflammation. Respir Physiol Neurobiol. 168:289–294.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song A, Liao Q, Li J, et al: Chronic
exposure to sulfur dioxide enhances airway hyperresponsiveness only
in ovalbumin-sensitized rats. Toxicol Lett. 214:320–327. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirst SJ: Airway smooth muscle cell
culture: application to studies of airway wall remodelling and
phenotype plasticity in asthma. Eur Respir J. 9:808–820. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fabry B, Maksym GN, Shore SA, et al:
Selected contribution: time course and heterogeneity of contractile
responses in cultured human airway smooth muscle cells. J Appl
Physiol. 91:986–994. 2001.
|
|
16
|
Fairbank NJ, Connolly SC, Mackinnon JD, et
al: Airway smooth muscle cell tone amplifies contractile function
in the presence of chronic cyclic strain. Am J Physiol Lung Cell
Mol Physiol. 295:L479–L488. 2008. View Article : Google Scholar
|
|
17
|
Chen C, Krishnan R, Zhou E, et al:
Fluidization and resolidification of the human bladder smooth
muscle cell in response to transient stretch. PLoS One.
5:e120352010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butler JP, Tolić-Nørrelykke IM, Fabry B
and Fredberg JJ: Traction fields, moments, and strain energy that
cells exert on their surroundings. Am J Physiol Cell Physiol.
282:C595–C605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JH and Lin JS: Cell traction force
and measurement methods. Biomech Model Mechanobiol. 6:361–371.
2007. View Article : Google Scholar
|
|
20
|
Deng L, Trepat X, Butler JP, et al: Fast
and slow dynamics of the cytoskeleton. Nat Mater. 5:636–640. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tribius S, Pidel A and Casper D: ATM
protein expression correlates with radioresistance in primary
glioblastoma cells in culture. Int J Radiat Oncol Biol Phys.
50:511–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fabry B, Maksym GN, Butler JP, et al:
Scaling the microrheology of living cells. Phys Rev Lett.
87:1481022001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burton K, Park JH and Taylor DL:
Keratocytes generate traction forces in two phases. Mol Biol Cell.
10:3745–3769. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang N, Tolić-Nørrelykke IM, Chen J, et
al: Cell prestress. I. Stiffness and prestress are closely
associated in adherent contractile cells. Am J Physiol Cell
Physiol. 282:C606–C616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andrew DK, Schellenberg RR, Hogg JC, et
al: Physiological and immunological effects of chronic antigen
exposure in immunized guinea pigs. Int Arch Allergy Appl Immunol.
75:208–213. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szelenyi I: Animal models of bronchial
asthma. Inflamm Res. 49:639–654. 2000. View Article : Google Scholar
|
|
27
|
Trepat X, Deng L, An SS, et al: Universal
physical responses to stretch in the living cell. Nature.
447:592–595. 2007. View Article : Google Scholar : PubMed/NCBI
|