Introduction
Hypoxic pulmonary vasoconstriction (HPV) is the
rapid, reversible increase in pulmonary vascular resistance which
occurs when alveolar oxygen tension falls below a threshold level
(1). HPV is restricted to segments
of the vasculature serving the hypoxic lobe, thereby achieving
ventilation/perfusion matching and optimizing systemic
PO2 without significantly elevating pulmonary artery
pressure. However, a previous study (2) observed that in isolated
intrapulmonary arterial rings, HPV is biphasic, consisting of an
immediate, endothelium-independent constriction, which peaks in ~10
min (phase I) and a second, slowly developing endothelium-dependent
sustained contraction that peaks at ~40 min (phase II). Pulmonary
arteries receive the most attention in respect to HPV. Sustained
HPV eventually leads to pulmonary hypertension, which is a
progressive disease of pulmonary vasculature, characterized by
elevation of pulmonary artery pressure and resistance. The disease
has a short life expectancy and severely affects life quality.
Among the variety of signal transduction pathways in
HPV, arachidonic acid (AA) and its metabolites are involved in the
maintenance of vascular homeostasis, including vascular tone and
vessel wall tension (3). In
addition to classic prostanoids derived from cyclooxygenase (COX)
and lipoxygenase (LOX), prostanoids derived from the monooxygenase
pathway have been receiving increasing attention. Under
physiological conditions, epoxyeicosatrienoic acids (EETs), which
are produced by the cytochrome P450 (CYP) epoxygenase pathway of AA
metabolism and hydroxyeicosatetraenoic acid (HETE), which is
derived from hydroxygenase, have been suggested to mediate
pulmonary circulation and play a key role in pulmonary vascular
remodelling specifically under chronic hypoxic stimulation
(4,5). Selective epoxygenase inhibitors
significantly reduce acute hypoxic pulmonary vasoconstriction and
chronic hypoxia-induced pulmonary vascular remodelling (4). The soluble epoxide hydrolase
inhibitor enhances the hypoxic vasoconstriction. However,
experiments in isolated perfused lungs and in vivo animal
models have generated conflicting results. The aforementioned
complex systems contain blood cells, connective tissue and
interstitial cells, all of which may produce extra and
unpredictable prostanoids. Therefore, the results are likely to be
complex and difficult to interpret.
In the current study, isolated rat intrapulmonary
arteries (IPAs) were used to investigate the role of AA and its
metabolites via CYP on basal vascular tone and hypoxic
vasoconstriction. The study aimed to understand the contribution of
various prostanoids and the activation of their receptors in an
acute hypoxic response in rat IPAs.
Materials and methods
Ethics statement
Animal experiments conformed to the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health (DHWE publication no. 96-01, revised in 2002)
and were approved by the Ethics Review Board for Animal Studies of
the Institute of Molecular Medicine, Peking University (Beijing,
China).
Isolated rat IPAs
Male Wistar rats (250–350 g) were anesthetized with
sodium pentobarbital and sacrificed by cervical dislocation. All
preparations were performed in ice-cold physiological salt solution
(PSS) containing: NaCl, 118 mM; NaHCO3, 24 mM;
MgSO4, 1 mM; NaH2PO4, 0.435 mM;
glucose, 5.56 mM; CaCl2, 1.8 mM and KCl, 4 mM. Small
IPAs (150–350 μm) were isolated from surrounding tissue, cut as
rings and mounted in a temperature-controlled myograph system
(model: 610M; Danish Myo Technology, Gainesville, FL, USA).
Briefly, a segment of rat small IPAs ring, 2-mm long, corresponding
to a third or fourth order branch from the IPA, was mounted in a
small vessel myograph. Two 40-μm wires were threaded through the
lumen of the vessel segment. One wire was attached to a stationary
support driven by a micrometer, while the other was attached to an
isometric force transducer. Vessels were allowed to equilibrate
under zero force for 30 min. Prior to experiments, mounted rings
were gassed with 95% air/5% CO2 (pH 7.4) at 37ºC for 1 h
and were maintained with continuous bubbling with this gas during
experiments unless otherwise stated. Each ring was initially
stretched to give an optimal pressure of 30 mmHg. Denuded arteries
were obtained by passing through the artery with surgical thread.
The condition of endothelium cells was examined by relaxation in
response to 1 μM acetylcholine (Ach).
Hypoxic instructions
The hypoxic instructions employed in the present
study have been described previously (6). Briefly, IPAs were exposed to high
KPSS 3 times, which contained: NaCl, 42 mM; NaHCO3, 24
mM; MgSO4, 1 mM; NaH2PO4, 0.435
mM; glucose, 5.56 mM; CaCl2, 1.8 mM and KCl, 80 mM, with
a 2 min duration and a 10 min interval. PGF2α (5 μM) (a higher
concentration was required when specific chemicals were used) was
added to the solution to achieve a pretone equivalent to ~15% of
KPSS response. When the pretone was stable, IPAs were exposed to a
continuous gas of 95% N2/5% CO2 for 40 min,
washed with PSS and returned to normoxic conditions. Oxygen levels
were monitored using a dissolved oxygen meter in all experiments:
at hypoxic conditions, ~2% O2 and normoxic, ~19%
O2. Reproducible results were obtained following 60–90
min recovery time following exposure to hypoxia, so a standard 80
min recovery was observed between control and testing
experiments.
Chemicals
All chemicals were purchased from Biomol
International (Plymouth Meeting, PA, USA), with the exception of
verapamil (Sigma-Aldrich, St. Louis, MO, USA) and NS398
(Calbiochem, San Diego, CA, USA). They were dissolved in ethanol,
dimethyl sulfoxide (DMSO) or distilled water as a stock solution,
according to the manufacturer’s instructions. Chemicals were
diluted to determined concentrations on the date of experiments.
Ethanol and DMSO, at the same dilution concentration, were tested
and neither vasoconstriction or vasodilation was observed.
Calculations and statistical
analysis
All results were normalized using max
vasoconstriction induced by 80 mM high K in the control group. Mean
data presented in the histogram is expressed in terms of percentage
change in comparison with corresponding parts in the control group.
Results are shown as mean ± SEM and comparisons between groups were
performed by paired Student’s t-test. All data analysis was
performed using SPSS version 13.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
Exogenous application of AA causes
vasoconstriction and attenuates hypoxic vasoconstriction in
isolated rat IPAs
IPAs were equilibrated with 2–4 exposures to 80 mM
KPSS for a 5 min duration. Thereafter, IPA was pre-contracted with
3 μM PGF2α or 30 mM KPSS to facilitate the hypoxic response.
Subsequently, a hypoxic challenge for 45 min was applied and
thereafter washed off. An interval of 60–90 min was employed
between 2 hypoxic stimulations to allow producible hypoxic
vasoconstriction. Tested chemicals were applied during the 2nd
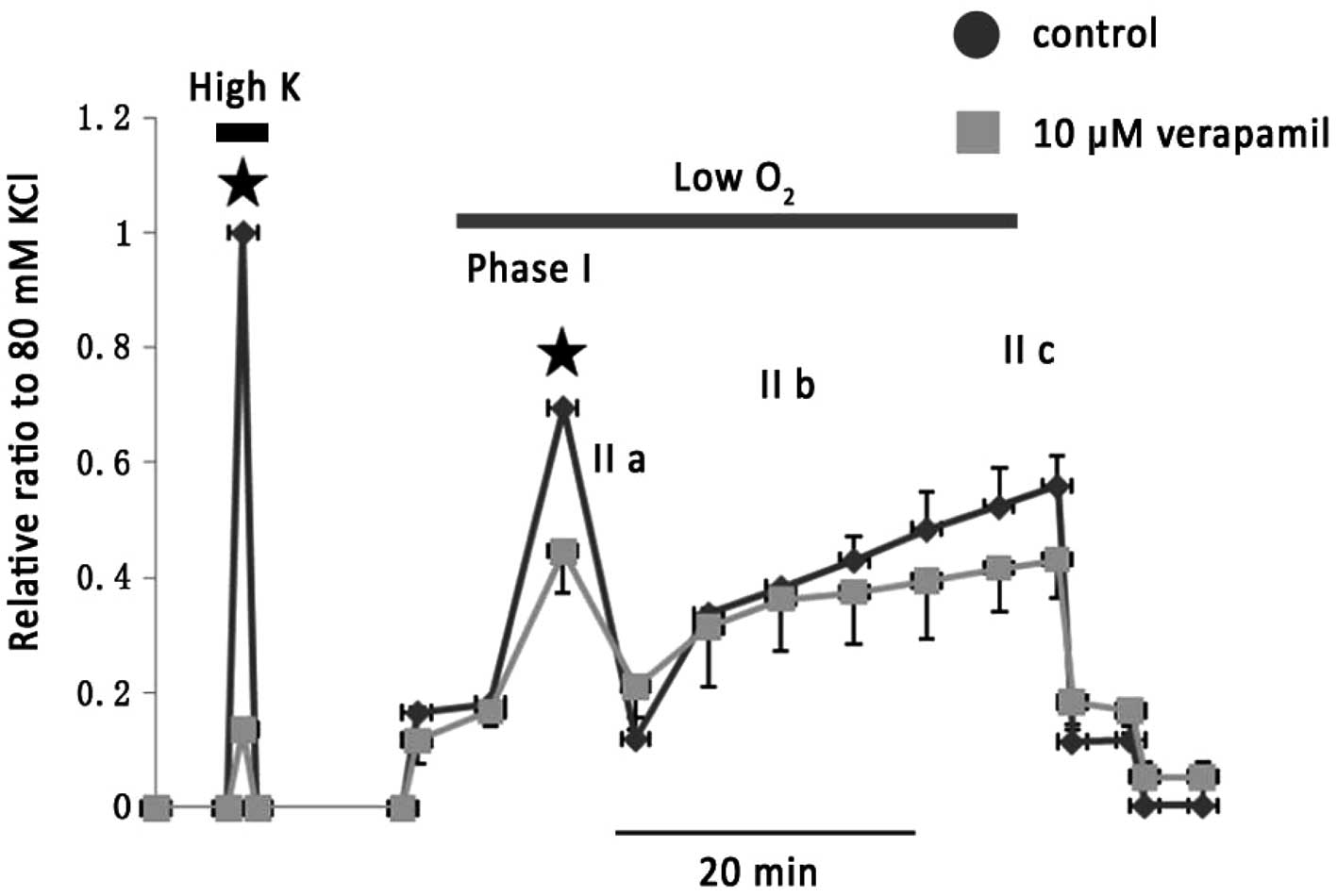
hypoxic challenge. As shown in Fig.
1, 10 μM verapamil greatly attenuated 80 mM KPSS-induced vessel
contraction and caused an inhibition on phase I of the hypoxic
vasoconstriction by 36.7±5.9% (n=5).
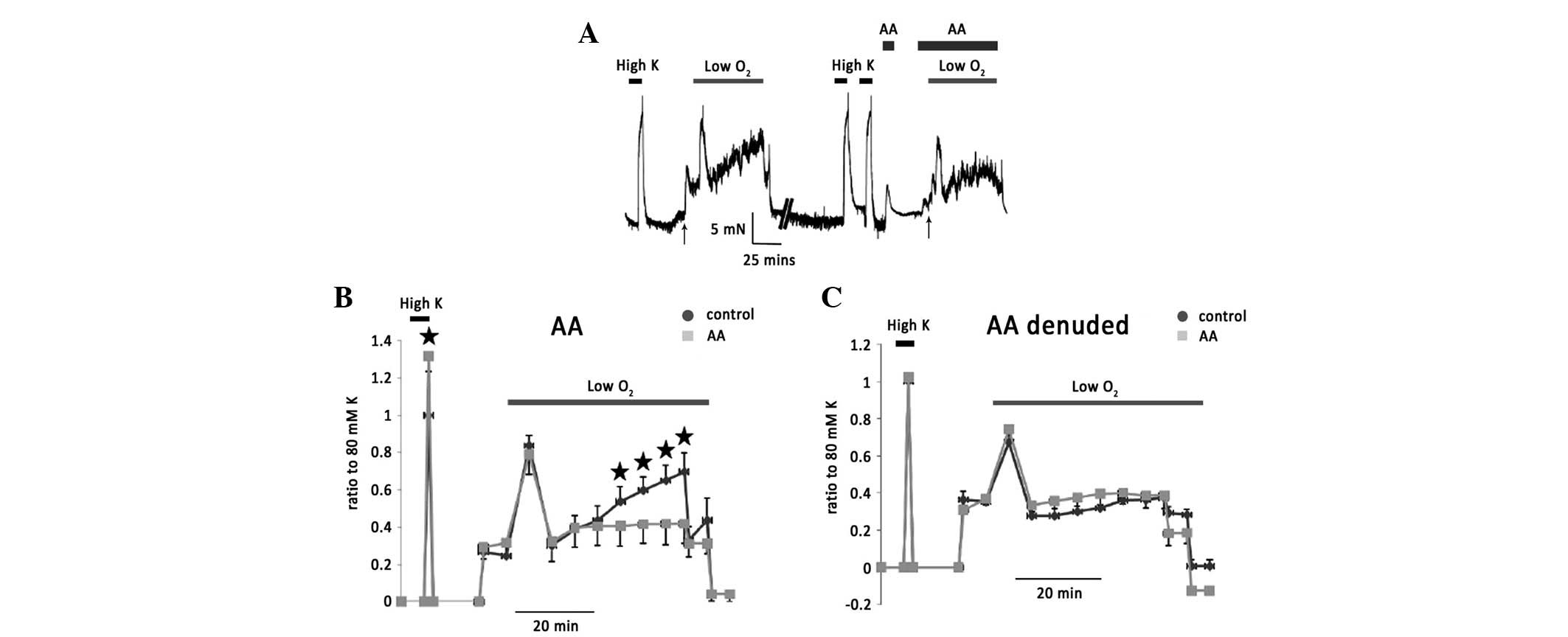
Following the same procedure, the exogenous
application of AA (20 μM) caused a significant transient
vasoconstriction. The example trace is shown in Fig. 2A. The basal tension returned to
control level when AA was washed off. Phase II of hypoxic
vasoconstriction was significantly attenuated by the application of
AA (Fig. 2B). In the denuded
isolated rat IPAs, 20 μM exerted no effect on basal tension or
hypoxic vasoconstriction (Fig.
2C), suggesting that endothelium factors are involved in these
AA effects.
It is well established that Ca2+ entry
via voltage-gated and voltage-independent Ca2+ channels
mediates hypoxic vasoconstriction. AA and its metabolites have been
observed to directly regulate these Ca2+ channels in an
enhancement or inhibition manner (7–9).
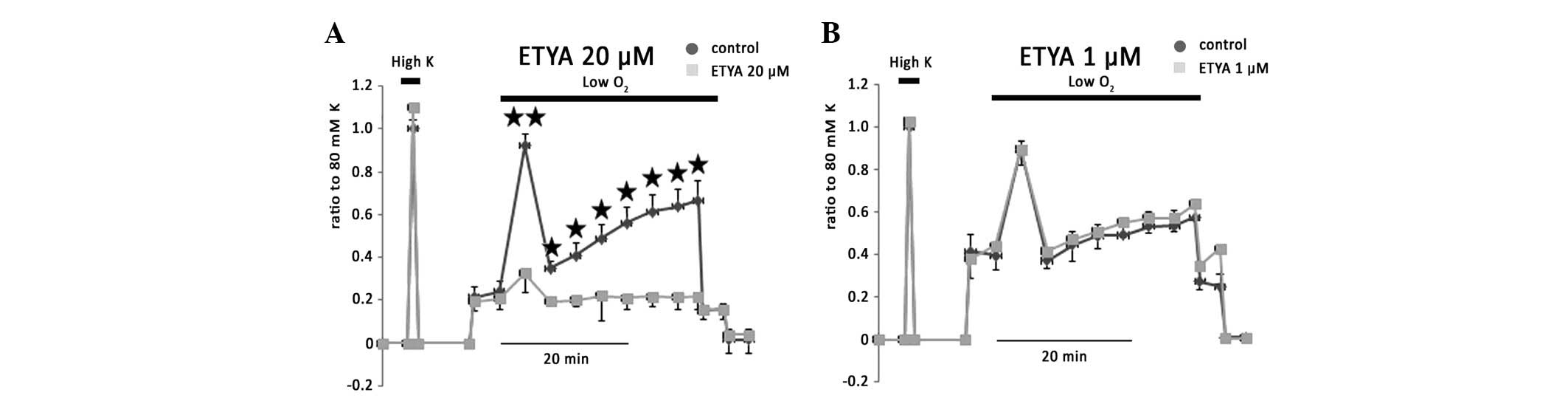
5,8,11,14-Eicosatetraynoic acid (ETYA) is a COX and LOX pathway
inhibitor. ETYA (20 μM), which is resistant to AA degenerative
enzymes, had no effect on basal tension and 80 mM KPSS-induced
vessel contraction, but greatly inhibited phases I, IIa, IIb and
IIc of hypoxic vasoconstriction to 13.1±7.1, 9.3±14.5, 4.9±3.7 and
4.3±4.8% of controls, respectively (Fig. 3A). This inhibition by ETYA may be
due to its blockage effects on COX and CYP. Accordingly, 1 μM ETYA,
which is hypothesized to inhibit 12-LOX and 15-LOX, exerted no
effect on hypoxic vasoconstriction (Fig. 3B), implying the key role of COX in
the regulation of rat IPA vascular wall tension.
Effect of EET and HETE on
vasoconstriction of rat IPAs
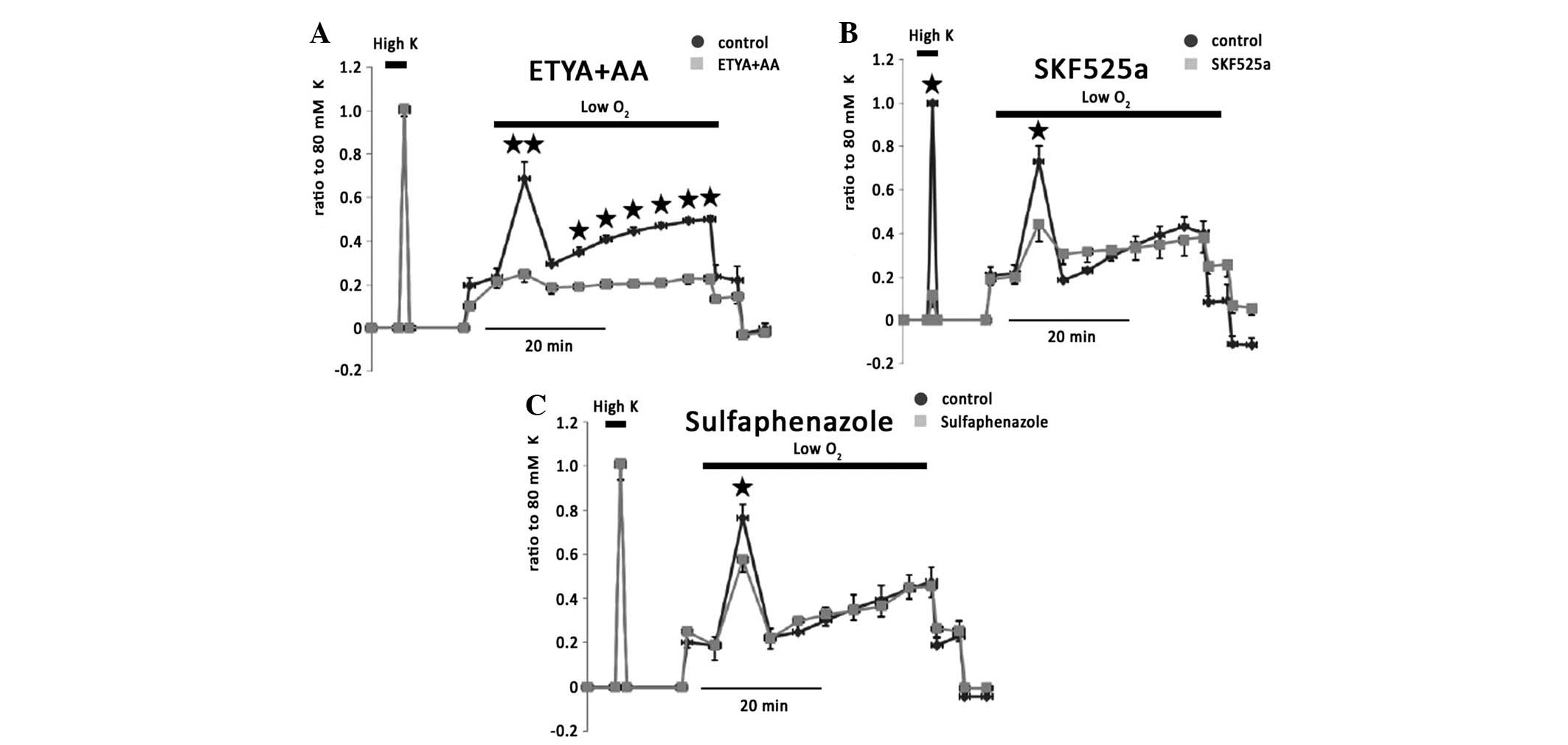
ETYA (20 μM) and AA (20 μM) eliminated hypoxic
vasoconstriction (Fig. 4A).
However, 20 μM ETYA reversed AA induced vasoconstriction (data not
shown), suggesting that AA metabolites generated from endothelium
may alter basal tension (n=5). EET, which is an endothelium-derived
relaxing substance and generated via epoxygenase (10) and HETE, which is generated via
epoxide hydrolase and contributes vasoconstriction and hypertension
(11), have been previously shown
to be associated with hypoxic pulmonary hypertension. CYP
epoxygenase derived EET is considered to elicit pulmonary
vasoconstriction. Therefore, the effect of AA metabolites via CYP
on vasoconstriction of IPAs were examined. SKF525a, a general
inhibitor of CYP, attenuated 80 mM KPSS-induced vessel contraction
to 11.5±5.4% of controls and inhibited phase I hypoxic
vasoconstriction to 36.4±5.6% of controls (n=6; Fig. 4B). A possible explanation for the
effect of SKF525a may be due to the blockage of Ca2+
channels. To further confirm the role of CYP, sulfaphenazole,
another antagonist of CYP, significantly reduced phase I of hypoxic
vasoconstriction to 64.9±25.9% of controls (n=6), but had no effect
on 80 mM KPSS-induced vessel contraction (Fig. 4C).
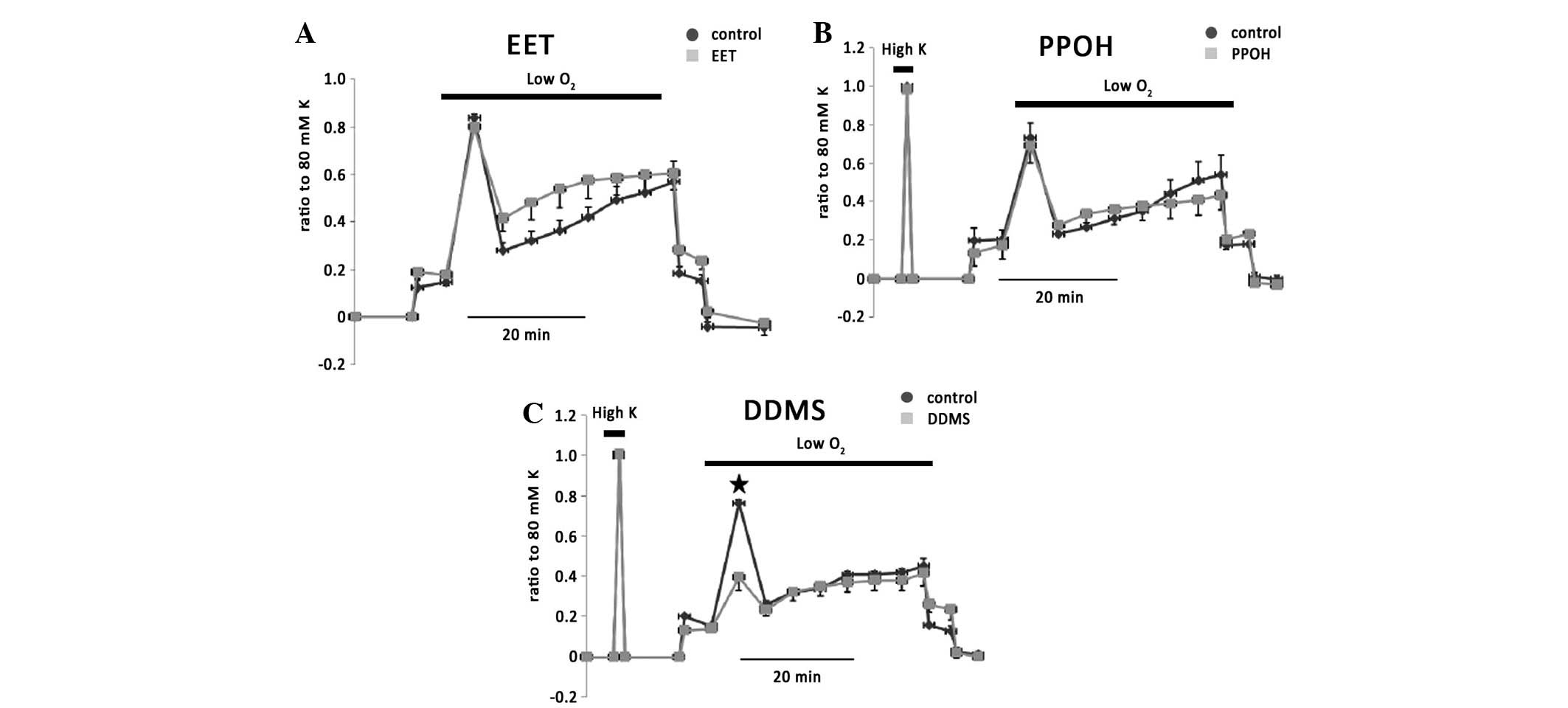
11,12-EET (200 nM), a major product from CYP 2C9
(12), exerted no effect on
hypoxic vasoconstriction (Fig. 5A;
n=3). 6-(2-proparglyloxyphenyl)hexanoic acid (PPOH), an antagonist
of CYP epoxygenase, exerted no effect on hypoxic vasoconstriction
and 80 mM KPSS-induced vessel contraction (Fig. 5B; n=3).
12,12-dibromo-N-(methylsulfonyl)-11-dodecenamide (DDMS), an
antagonist of CYP hydroxygenase, significantly blocked phase I of
hypoxic vasoconstriction by 58±11% (n=3; Fig. 5C), suggesting an involvement of
20-HETE in the hypoxic response in isolated rat IPAs and
independence to endothelium.
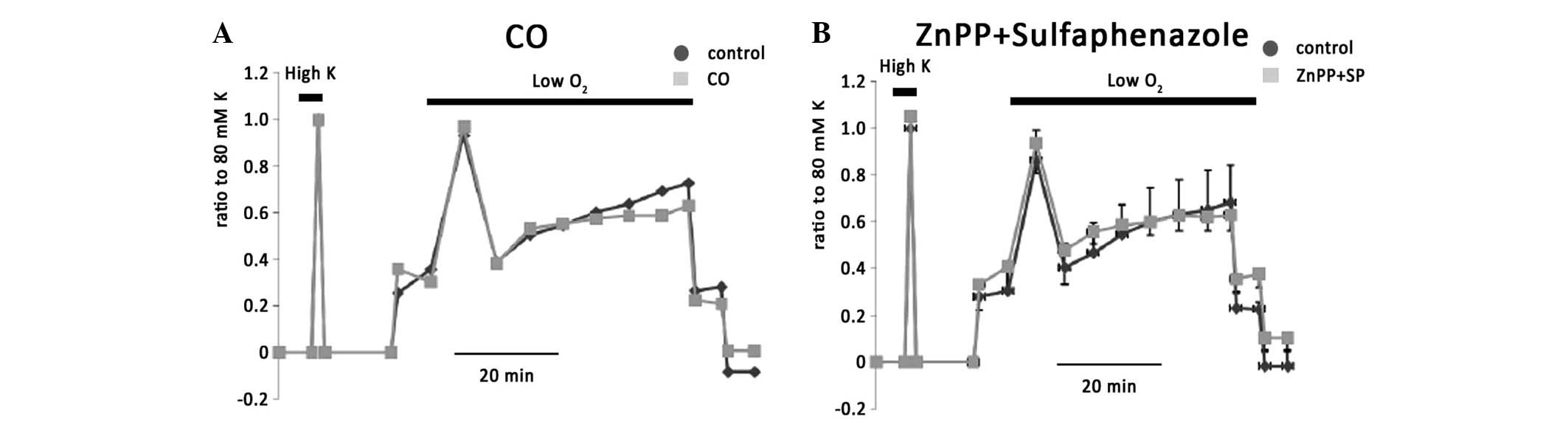
In addition, previous studies have suggested that
products of heme oxygenase and CYP are required to attenuate
vasoconstriction reactivity (13).
Carbon monoxide (CO) has been shown to cause vasoconstriction in
pulmonary arteries. CO and biliverdin are the general products of
heme oxygenase. A CO donor was therefore employed in the current
study. CO alone (n=6), had no effect on KPSS-induced vessel
contraction or hypoxic vasoconstriction (Fig. 6A). The combination of ZnPP, which
blocks heme oxygenase and sulfaphenazole (n=5) also had no effect
(Fig. 6B).
It has been observed that EET may upregulate nitric
oxide (NO) synthase and release (14), resulting in vasodilatation
(15). The NO effect on HPV was
investigated. Ach alone, which induces NO release from endothelium,
did not inhibit HPV but exerted a slight facilitate HPV (Fig. 7A). L-NAME, which is a synthase
inhibitor of NO, significantly inhibited phase I of HPV (Fig. 7B). Sodium nitroprusside (SNP; 20
nM), as a NO donor, significantly inhibited phase I and II of HPV
as predicted (Fig. 7C and D).
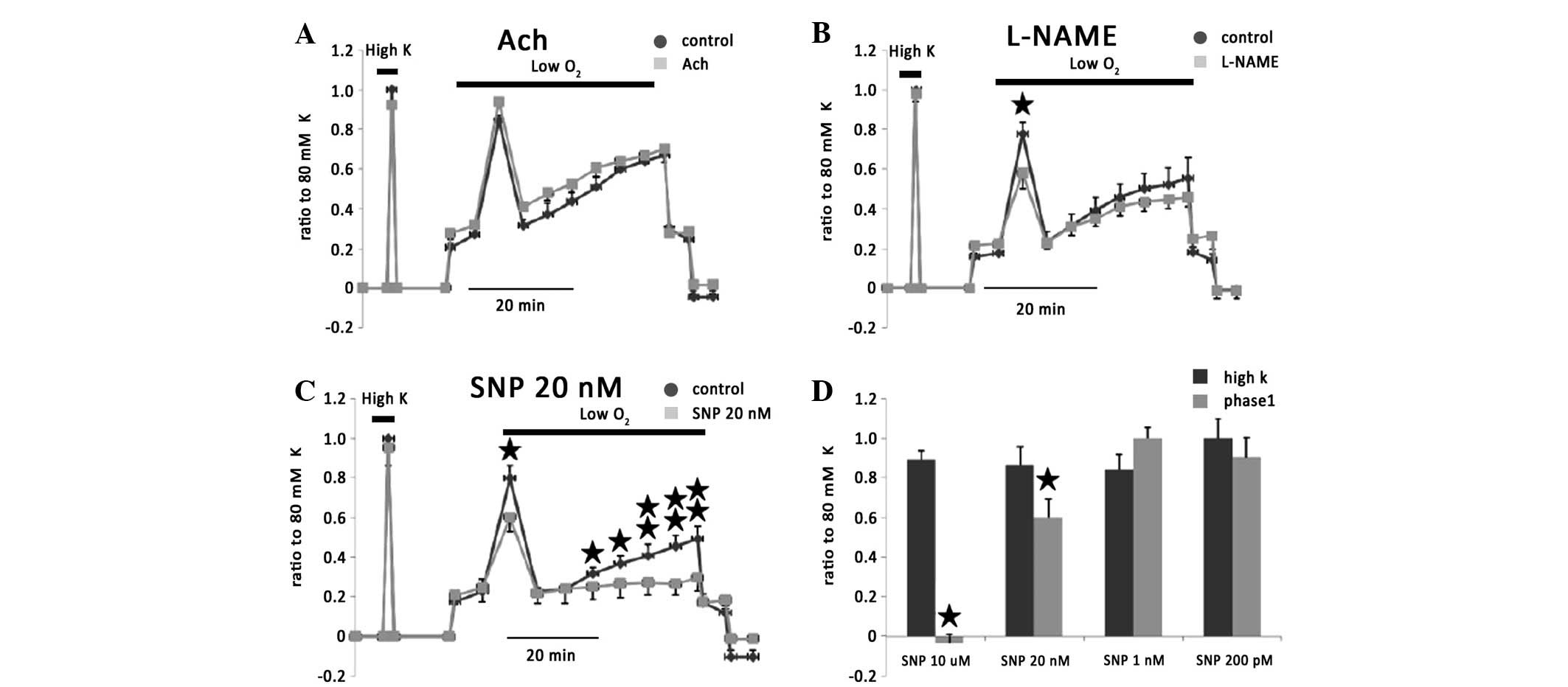 | Figure 7Effect of NO on vasoconstriction of
rat IPAs. (A) Ach alone, which induces NO release from endothelium,
did not inhibit HPV but exerted a slight facilitate HPV. (B)
L-NAME, significantly inhibited phase I of HPV. (C) SNP (20 nM), as
a NO donor, significantly inhibited phase I and II of HPV. (D) A
variety of concentrations of SNP affect phase I of hypoxia
vasoconstriction. *P<0.05, vs. respective control
group. NO, nitric oxide; IPAs, intrapulmonary arteries; HPV,
hypoxic pulmonary vasoconstriction; SNP, sodium nitroprusside; Ach,
acetylcholine. |
Effects of prostacyclin and thromboxane
(TX) on the vasoconstriction of IPAs
The smaller effect of EET on HPV suggested
predominant contraction during the hypoxic condition. Thus, another
potent vasodilator, PGI2, which is also metabolized from
AA was investigated. PGI2, which is biosynthesized in
endothelial cells, exerted no effect on 80 mM KPSS or hypoxic
vasoconstriction in isolated rat IPAs (n=8; Fig. 8A). Since its half life under
physiological conditions is 2 min, PGI2 was added to the
bath every 2 min to maintain the concentration during the entire
hypoxic challenge. Carbacyclin, which is a stable PGI2,
had no effect (Fig. 8B).
15(s)-HPETE, which inhibits prostacyclin synthase, exerted no
effect on hypoxic vasoconstriction and 80 mM KPSS-induced vessel
contraction (Fig. 8C). However,
Forskolin, a potent stimulator of platelet aggregation and release
of TX, significantly eliminated hypoxic vasoconstriction in phases
I and II (Fig. 8D).
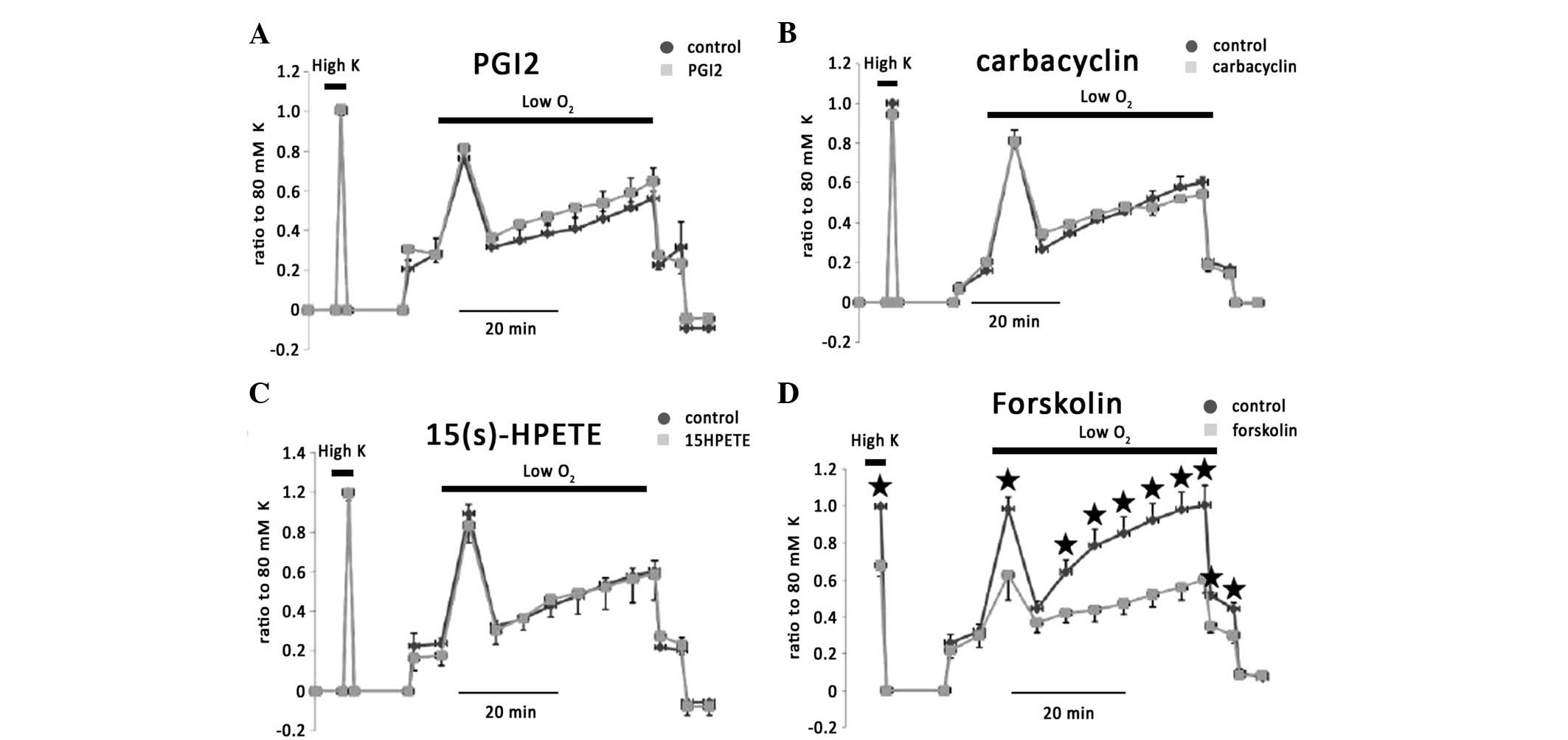 | Figure 8Effect of prostacyclin on
vasoconstriction of rat IPAs. (A) Exogenous PGI2 exerted
no effect on 80 mM KPSS- or hypoxia-induced vessel contraction. (B)
Carbacyclin, which is a stable PGI, also had no effect. (C)
15(s)-HPETE, an inhibitor of prostacyclin synthase, exerted no
effect on the vasoconstriction of rat IPAs. (D) Forskolin, a potent
stimulator of platelet aggregation and release of TX, significantly
eliminated hypoxic vasoconstriction in phases I and II.
*P<0.05 represents the significant difference. IPAs,
intrapulmonary arteries; 15(s)-HPETE,
15S-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid; TX,
thromboxane. |
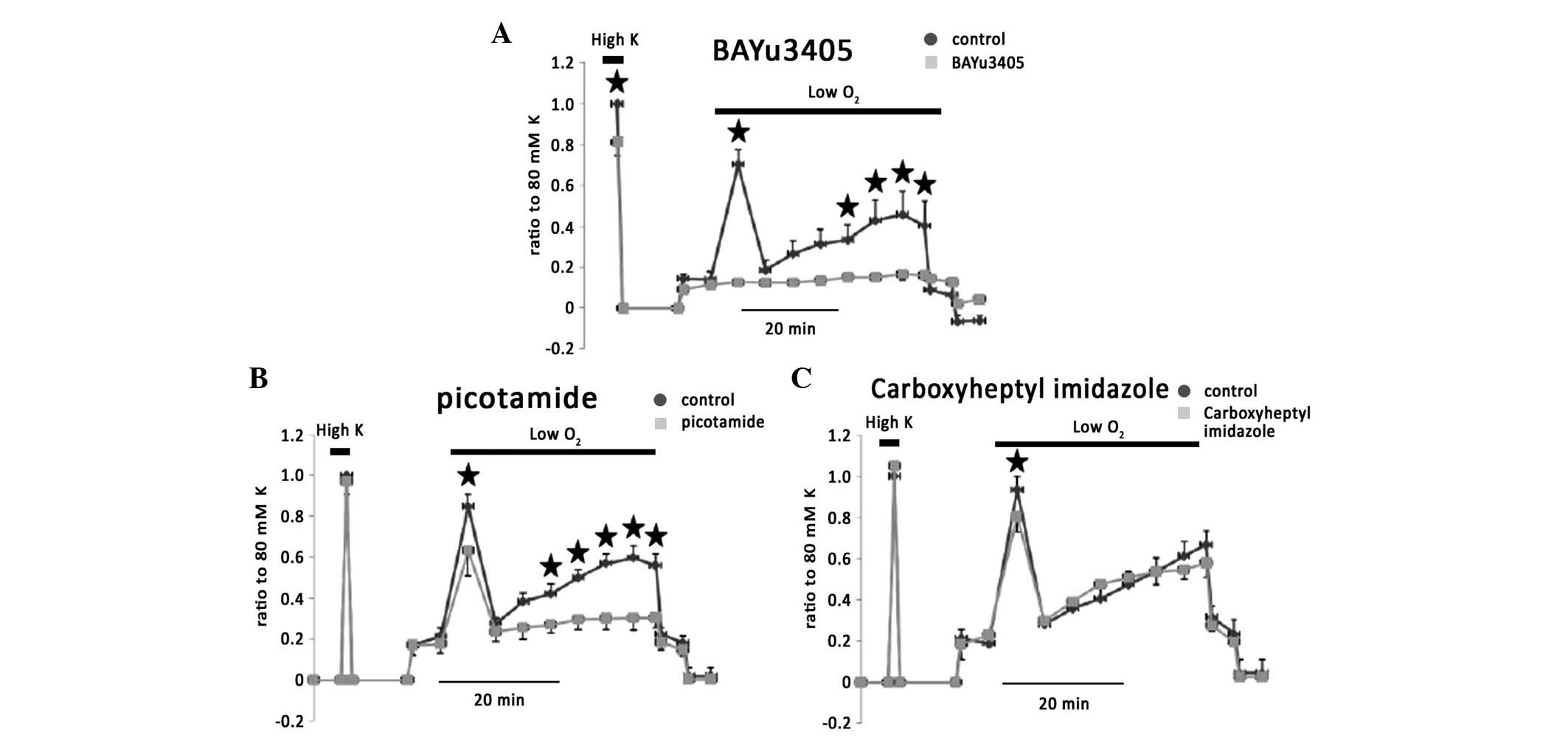
To confirm the hypothesis that vasoconstriction
during hypoxia overrides the dilatators, blockage of
vasoconstriction was employed during a period of hypoxic
stimulation. TX is considered as a major vasoconstrictor. Blockage
of the TX receptor by BAYu345 significantly inhibited phase I, IIb
and IIc of hypoxic vasoconstriction by 78.7±3.3, 79.3±5.1 and
74.9±10%, respectively (n=5; Fig.
9A). Picotamide (100 μM), which inhibits TXA2
activity and synthase, also inhibited phase I, IIb and IIc of
hypoxic vasoconstriction by 18.5±7.4, 57±8.3 and 50±10%,
respectively (Fig. 9B; n=5).
Carboxyheptyl imidazole, which blocks TX synthase reduced phase I
of hypoxic vasoconstriction (Fig.
9C).
Discussion
The present study showed that exogenous AA is
capable of eliciting vasoconstriction to enhance the vascular tone
via an endothelium-dependent mechanism. In the acute hypoxic
stimulation, AA metabolites via COX and CYP but not LOX, played a
primary role in the formation and regulation of hypoxic
vasoconstriction. Although metabolites through CYP only encompass a
part of hypoxic vasoconstriction, the pathway via CYP hydroxylase,
but not via epoxygenase, is hypothesized to be involved in
initiating transient vasoconstriction. In addition, activation of
TP receptors is predominant during acute hypoxic challenges. Other
prostanoids initiate hypoxic vasoconstriction via TP receptors,
since blockage of TX synthesis removes part of hypoxic
vasoconstriction. These results determined a principal role of TP
receptors in acute hypoxia response and also suggested that
vasoconstrictors are significant in forming hypoxic
vasoconstriction and that vasodilatators are potent in forming
vascular tone.
A previous study on AA metabolites via CYP in
pulmonary blood vessels was performed in whole animal (16) or perfused lungs (17). Although the aforementioned
experimental conditions are consistent with the physiological
environment, it is difficult to determine the contributions between
endothelium and smooth muscle. In addition, inflammatory factors
and the diverse prostanoids released and generated from blood
cells, which are in abundance in an organ, are likely to exert more
profound effects on pulmonary blood vessels, resulting in
difficulties in interpreting the results. Therefore, isolated IPAs
were used in the present study to examine the role of AA and its
metabolites on acute hypoxic vasoconstriction. The advantage of the
system is to contain simple and minimum tissue for investigation.
Although blood vessel rings are stretched by mechanical force and
equilibrated with high K+ medium to initiate a basal
tone, significant relaxation caused by vasodilatators e.g., Ach and
NO are more evident when vessels are markedly contracted by
norepinephrine. However, this relaxation has been investigated in
the current study. If the relaxation caused by relaxants in basal
tension level was too small to be observed, inhibition on 80 mM
KPSS-induced vessel contraction or hypoxic vasoconstriction should
aid in understanding the relaxation observed. Therefore, KPSS
stimulation was repeated prior to and following application of
chemicals. The statistical results on high K and hypoxia induced
vessel contraction were also presented.
Inconsistent with a previous study, AA exerted
vasoconstriction in pulmonary artery but not vasodilatation
(18). This may be simply due to
species and tissue difference. For example, AA causes significant
vessel contraction in human pulmonary vein and AA infusion triggers
marked, sustained pulmonary vasoconstriction and pulmonary
hypertension in broilers (19).
The vasoconstriction effect of AA is endothelium dependent, since
AA exerts no effect on denuded vessel rings. In addition, this
contraction effect is unlikely due to the effect of AA and is
mediated by AA metabolites, since 20 μM ETYA may reverse this
effect. The possible candidates include a number of EETs (20) and 20-HETE (21), which are produced in the
endothelium and cause constriction in isolated pulmonary blood
vessel rings. In renal and mesenteric arteries, AA induces
relaxation through the LOX pathway (22). However, in isolated IPAs, LOX
appears to have no effect on basal tone or hypoxic
vasoconstriction.
Although ETYA is primarily regarded as a
pharmacological analog of AA and resistant to AA degenerative
enzymes, a number of other aspects of ETYA were also considered in
the present study. ETYA is hypothesized to inhibit AA uptake, COX,
all LOXs and CYP. This led to a rational explanation that 20 μM
ETYA almost eliminated hypoxic vasoconstriction but 1 μM ETYA had
no effect. This result was further confirmed by blockage of TP
receptors, suggesting that prostanoids, via COX and CYP, play a
permissive role in mediating the hypoxic response in rat IPAs. The
profound effects of ETYA on AA degenerative enzymes have also been
observed in the canine femoral artery, in which formation of
prostanoids in endothelium was blocked by ETYA, resulting in
reversing the AA and Ach relaxant effect.
Prostanoids derived from CYP have been markedly
addressed in the accumulating literature as playing a key role in
mediating pulmonary vascular tone and wall tension (4,10,23).
The results that 11,12-EET activates Ca2+-activated
K+ channels (BK) (24)
and apamin-sensitive K+ channels (25) in vascular smooth muscle cells and
20-HETE attenuates BK (26),
further highlight the mechanism of degenerative products via CYP in
the regulation of blood vessel tone. The current results from
sulfaphenazole and DDMS, demonstrate that 20-HETE, via CYP epoxide
hydrolase, is involved in hypoxic vasoconstriction and its effect
is limited only in part of vessel contraction, which is independent
to the endothelium. By comparison with the effect of blockage of
the TP receptor, prostanoids via CYP are capable of facilitating a
hypoxic response. These results indicate that voltage-gated
Ca2+ entry relies on a part of hypoxic vasoconstriction
in IPAs and Ca2+ elevation in pulmonary artery smooth
muscle cells (7,27,28).
Modulation of membrane potential by BK, EET and HETE (29) may therefore, affect a part of
endothelium-independent hypoxic vasoconstriction and this is
demonstrated in the present study. Activation of TP receptors,
which are G-protein coupled receptors, potently modulates
voltage-independent Ca2+ entry, including receptor
operated Ca2+ entry and store operated Ca2+
entry, resulting in modulation of hypoxic vasoconstriction.
Ca2+ entry via voltage-independent pathways appears to
play a primary role in the hypoxic response in rat IPAs. The
current observations are inconsistent to the previous studies in
Webster mice (4), in which
selective epoxygenase inhibition significantly reduces hypoxic
pulmonary vasoconstriction and chronic hypoxia-induced pulmonary
vascular remodeling. The difference, in conclusion, may be due to
the difference in the experimental system and observation period.
In the previous experiment, the mouse was intermittently exposed to
chronic hypoxia. The significant elevations of CYP 2C9 and EET have
been revealed by chronic hypoxic stimulation. In the long term, EET
modulates membrane potential resulting in alternation in vascular
tone and eventual pulmonary hypertension. In the current study, the
responses of IPAs were examined. Hypoxic challenge was <50
min.
The majority of studies on vascular tone describe
contractions produced by TX analogs and relaxations induced by
PGI2 analogs (3). A
number of vascular smooth muscles express TP and prostacyclin
receptors (IP) accordingly. PGI have been found to exhibit potent
dilatation in pulmonary arteries (30) via an increase of cellular cAMP.
Forsklin markedly inhibits HPV but not PGI2, suggesting
a lower density of IP receptors in the pulmonary artery or an
unknown mechanism which is required to activate IP. In the present
study, the TP receptor has been implicated to mediate hypoxic
vasoconstriction in isolate rat IPAs. This observation is
consistent with previous studies in human pulmonary artery rings.
Although BAYu3405 exhibits a strong effect on the prostaglandin D
(DP) receptor, a similar blockage effect from picotamide, which is
an antagonist of TXA2, confirms the role of TP receptors
in hypoxic vasoconstriction. In addition, DP receptors also
contribute to part of endothelium independent hypoxic
vasoconstriction. There appears to be a minimum of nine G proteins
coupled to TP receptors (31).
Although the significance of this promiscuity remains unknown,
stimulation of TP receptors leads to activation of phospholipase C,
generation of inositol trisphosphate and dystrophin-associated
glycoprotein, activation of PKC and myosin light chain kinase, etc.
(3), resulting in the regulation
of the voltage-independent Ca2+ channel.
Decreased production of NO has been proposed as
being significant in the development of pulmonary arterial
hypertension. Increased NO formation in the pulmonary circulation
exerts a compensatory mechanism to offset the degree of pulmonary
vasoconstriction (32). The
current results are consistent with these previous observations.
SNP blocks hypoxic vasoconstriction. However, controversial
negative results have been observed (33), by administration of NO to reduce
pulmonary hypertension. A constitutive hyperactivation of NO in
caveolin-1-deficient mice causes severe lung fibrosis with marked
pulmonary hypertension. Administration of L-NAME attenuates the
hypoxic vasoconstriction and relieves pulmonary hypertension
(34). L-NAME attenuates NO
release and should augment hypoxic vasoconstriction. No effect of
L-NAME on HPV from the current results markedly suggested
vasoconstrictors as being predominant in HPV. It may also be
reflected by the evidence that administration of L-NAME causes a
slight increase in tonic pulmonary vascular tone (35) or no alteration in baseline
pulmonary arterial pressure during physiological hypoxic response
(36).
The present data revealed that exogenous AA exerts
vasoconstriction on the basal tone of isolated rat IPAs. This
effect is endothelium dependent and may be blocked by antagonists
of COX and CYP. The prostanoids derived from CYP hydroygenase, but
not from epoxygenase, contribute to hypoxic vasoconstriction in rat
IPAs. The activation of TP receptors plays a permissive role in the
regulation of the hypoxic response in rat IPAs.
Acknowledgements
This study was partly supported by a grant from the
Natural Science Foundation of China (grant no. 81170105) to
Chengchun Tang (Department of Cardiology, Zhongda Hospital of
Southeast University Medical School).
References
|
1
|
Sommer N, Dietrich A, Schermuly RT, et al:
Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms.
Eur Respir J. 32:1639–1651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moudgil R, Michelakis ED and Archer SL:
Hypoxic pulmonary vasoconstriction. J Appl Physiol. 98:390–403.
2005. View Article : Google Scholar
|
|
3
|
Norel X: Prostanoid receptors in the human
vascular wall. Scientific World Journal. 7:1359–1374. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pokreisz P, Fleming I, Kiss L, et al:
Cytochrome P450 epoxygenase gene function in hypoxic pulmonary
vasoconstriction and pulmonary vascular remodeling. Hypertension.
47:762–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhanasekaran A, Al-Saghir R, Lopez B, et
al: Protective effects of epoxyeicosatrienoic acids on human
endothelial cells from the pulmonary and coronary vasculature. Am J
Physiol Heart Circ Physiol. 291:H517–H531. 2006. View Article : Google Scholar
|
|
6
|
Frazziano G, Moreno L, Moral-Sanz J, et
al: Neutral sphingomyelinase, NADPH oxidase and reactive oxygen
species. Role in acute hypoxic pulmonary vasoconstriction. J
Cellular Physiol. 226:2633–2640. 2011. View Article : Google Scholar
|
|
7
|
Meng F, To WK and Gu Y: Inhibition effect
of arachidonic acid on hypoxia-induced [Ca(2+)](i) elevation in
PC12 cells and human pulmonary artery smooth muscle cells. Respir
Physiol Neurobiol. 162:18–23. 2008.
|
|
8
|
Harteneck C: Function and pharmacology of
TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol.
371:307–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grimm C, Kraft R, Schultz G and Harteneck
C: Activation of the melastatin-related cation channel TRPM3 by
D-erythro-sphingosine [corrected]. Mol Pharmacol. 67:798–805.
2005.PubMed/NCBI
|
|
10
|
Fleming I: Epoxyeicosatrienoic acids, cell
signaling and angiogenesis. Prostaglandins Other Lipid Mediat.
82:60–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llinás MT, Alexander BT, Capparelli MF,
Carroll MA and Granger JP: Cytochrome P-450 inhibition attenuates
hypertension induced by reductions in uterine perfusion pressure in
pregnant rats. Hypertension. 43:623–628. 2004.PubMed/NCBI
|
|
12
|
Larsen BT, Gutterman DD, Sato A, et al:
Hydrogen peroxide inhibits cytochrome p450 epoxygenases:
interaction between two endothelium-derived hyperpolarizing
factors. Circ Res. 102:59–67. 2008. View Article : Google Scholar
|
|
13
|
Naik JS and Walker BR: Role of vascular
heme oxygenase in reduced myogenic reactivity following chronic
hypoxia. Microcirculation. 13:81–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Cui Y, Geng B, Zeng X and Tang C:
11,12-Epoxyeicosatrienoic acid activates the L-arginine/nitric
oxide pathway in human platelets. Mol Cell Biochem. 308:51–56.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hercule HC, Schunck WH, Gross V, et al:
Interaction between P450 eicosanoids and nitric oxide in the
control of arterial tone in mice. Arterioscler Thromb Vasc Biol.
29:54–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y, Feng J, Ma K, et al:
8,9-Epoxyeicosatrienoic acid inhibits antibody production of B
lymphocytes in mice. PLoS One. 7:e402582012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kiss L, Schütte H, Padberg W, et al:
Epoxyeicosatrienoates are the dominant eicosanoids in human lungs
upon microbial challenge. Eur Respir J. 36:1088–1098. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Connolly M, Nagaraj C, et al:
Peroxisome proliferator-activated receptor-β/δ, the acute signaling
factor in prostacyclin-induced pulmonary vasodilation. Am J Respir
Cell Mol Biol. 46:372–379. 2012.
|
|
19
|
Alvarez-Medina DI, Hernandez A and Orozco
C: Endothelial hyperpolarizing factor increases
acetylcholine-induced vasodilatation in pulmonary hypertensive
broilers arterial rings. Res Vet Sci. 92:1–6. 2012. View Article : Google Scholar
|
|
20
|
Liu Y, Wang R, Li J, et al: Stable EET
urea agonist and soluble epoxide hydrolase inhibitor regulate rat
pulmonary arteries through TRPCs. Hypertens Res. 34:630–639. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacobs ER, Bodiga S, Ali I, et al: Tissue
protection and endothelial cell signaling by 20-HETE analogs in
intact ex vivo lung slices. Exp Cell Res. 318:2143–2152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chawengsub Y, Gauthier KM and Campbell WB:
Role of arachidonic acid lipoxygenase metabolites in the regulation
of vascular tone. Am J Physiol Heart Circ Physiol. 297:H495–H507.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michaelis UR, Xia N, Barbosa-Sicard E,
Falck JR and Fleming I: Role of cytochrome P450 2C epoxygenases in
hypoxia-induced cell migration and angiogenesis in retinal
endothelial cells. Invest Ophthalmol Vis Sci. 49:1242–1247. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higashimori H, Blanco VM, Tuniki VR, Falck
JR and Filosa JA: Role of epoxyeicosatrienoic acids as autocrine
metabolites in glutamate-mediated K+ signaling in
perivascular astrocytes. Am J Physiol Cell Physiol.
299:C1068–C1078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ayajiki K, Fujioka H, Toda N, et al:
Mediation of arachidonic acid metabolite(s) produced by endothelial
cytochrome P-450 3A4 in monkey arterial relaxation. Hypertens Res.
26:237–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gebremedhin D, Yamaura K and Harder DR:
Role of 20-HETE in the hypoxia-induced activation of
Ca2+-activated K+ channel currents in rat
cerebral arterial muscle cells. Am J Physiol Heart Circ Physiol.
294:H107–H120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robertson TP, Hague D, Aaronson PI and
Ward JP: Voltage-independent calcium entry in hypoxic pulmonary
vasoconstriction of intrapulmonary arteries of the rat. J Physiol.
525:669–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng F, To WK and Gu Y: Role of TRP
channels and NCX in mediating hypoxia-induced [Ca2+](i)
elevation in PC12 cells. Respir Physiol Neurobiol. 164:386–393.
2008.PubMed/NCBI
|
|
29
|
Fleming I and Busse R: Endothelium-derived
epoxyeicosatrienoic acids and vascular function. Hypertension.
47:629–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morales-Blanhir J, Santos S, de Jover L,
et al: Clinical value of vasodilator test with inhaled nitric oxide
for predicting long-term response to oral vasodilators in pulmonary
hypertension. Respir Med. 98:225–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cyphert JM, Allen IC, Church RJ, et al:
Allergic inflammation induces a persistent mechanistic switch in
thromboxane-mediated airway constriction in the mouse. Am J Physiol
Lung Cell Mol Physiol. 302:L140–L151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baliga RS, Milsom AB, Ghosh SM, et al:
Dietary nitrate ameliorates pulmonary hypertension: cytoprotective
role for endothelial nitric oxide synthase and xanthine
oxidoreductase. Circulation. 125:2922–2932. 2012. View Article : Google Scholar
|
|
33
|
Kästner SB, Kull S, Kutter AP, Boller J,
Bettschart-Wolfensberger R and Huhtinen MK: Cardiopulmonary effects
of dexmedetomidine in sevoflurane-anesthetized sheep with and
without nitric oxide inhalation. Am J Vet Res. 66:1496–1502.
2005.PubMed/NCBI
|
|
34
|
Wunderlich C, Schmeisser A, Heerwagen C,
et al: Chronic NOS inhibition prevents adverse lung remodeling and
pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm
Pharmacol Ther. 21:507–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwenke DO, Pearson JT, Tsuchimochi H,
Kangawa K and Shirai M: Pulmonary vascular reactivity of
spontaneously hypertensive rats is exacerbated in response to the
central administration of exogenous nitric oxide. Clin Exp
Pharmacol Physiol. 34:88–94. 2007. View Article : Google Scholar
|
|
36
|
Schwenke DO, Pearson JT, Mori H and Shirai
M: Does central nitric oxide elicit pulmonary hypertension in
conscious rats? Respir Physiol Neurobiol. 153:250–260. 2006.
View Article : Google Scholar : PubMed/NCBI
|