Introduction
Preeclampsia (PE) is a severe medical syndrome
affecting pregnant females. PE is characterized by new onset
hypertension and proteinuria following 20 weeks of pregnancy, and
threatens maternal and child health, as well as survival (1). The condition is subclassified into
early and late onset according to standards previously outlined by
von Dadelszen et al(2).
However, the pathogenesis of PE is unclear. Previously, a number of
studies have focused on the pathological changes involved in PE,
including infarct, fibrosis, arteriosclerosis and decidual
arterioles of the basal plate of the placenta, that affect
perfusion (3–5). However, these changes are not marked
in the basal plate of specific severe PE (SPE) patients,
particularly in the placenta of late onset SPE (LOSPE) and the SPE
accompanied by diabetes mellitus and multiple pregnancies (6).
The basal plate of the placenta is an important
region which controls nutrient-rich and oxygen-rich maternal blood
flowing into the intervillous space. It also regulates metabolic
products and carbon dioxide-rich fetal blood which flows from the
intervillous space into the maternal circulation (7). Pathological changes, including
fibrosis, arteriosclerosis and decidual arterioles, may cause the
obstruction of the vessels in the basal plate resulting in
metabolic accumulation and placental underperfusion, which may
induce hypoxia, oxidative stress and inflammation of the placenta
(8). These changes may also lead
to maternal vascular endothelial dysfunction and clinical
manifestations, such as hypertension and proteinuria (1).
It is well-known that extravillous trophoblasts
(EVTs) invade into the spiral arteries of the decidua and
superficial myometrium to establish flaccid low-resistance arteries
during pregnancy (9). If the EVTs
fail to remold the spiral arteries in the decidua and superficial
myometrium, the resistance of vessels in the basal plate may
increase and cause the pathogenesis of SPE (10). Previous studies on the pathological
changes of the placenta have also revealed that increased
contraction components exist in the regions of EVT shallow
implantation in the placental bed (11,12).
α-smooth muscle actin (α-SMA) is a component of microfilaments and
contributes to the cytoskeleton in specific contractile cells,
including smooth muscle cells (SMCs) and myofibroblasts of the
villous stroma in the basal plate (13).
To date, it has been hypothesized that specific
anchoring villi contain SMCs and villous stromal cells but not free
EVTs in the normomorph (normal structure) basal plate. In addition,
it has been reported that specific anchoring villi are involved in
infarct foci and/or other regions, which may contain cells with
contractile function (9,10). Hence, we hypothesized that
expression of α-SMA may be increased in an elevated number of
potentially contractile cells in the basal plate with pathological
changes, inducing the pathogenic process of SPE. However, few
studies have analyzed these pathological changes in the non-infarct
area of the villous clump. Therefore, the present study was
designed to explore the expression profile of α-SMA in the foci of
infarcts and villous clumps in the basal plate to determine the
correlation between α-SMA and SPE. An additional aim was to
identify the main placental pathological changes of patients with
LOSPE.
Materials and methods
Samples collection
This study was approved by the ethics committee of
Jilin University Bethune Second Hospital and all subjects provided
written informed consent. Females with normal or preeclamptic
pregnancies were defined according to criteria previously outlined
by Zhao et al(14). A total
of 78 placentas (38 from normal and 40 from preeclampsia) were used
in this study. The patient demographic data are summarized in
Table I.
 | Table IDemographic data of patients in all
groups. |
Table I
Demographic data of patients in all
groups.
| Group | Cases, n | Age, years | Pregnancy duration,
weeks |
|---|
| EOSPE | 20 | 28.66±6.33 | 28.86±3.64 |
| LOSPE | 20 | 29.05±7.01 | 37.63±1.49 |
| EC | 18 | 25.00±6.89 | 28.23±3.45 |
| LC | 20 | 28.20±5.75 | 37.44±1.56 |
Tissues explants
Tissues from the basal plate were selected with
infarct, villous clumps and normomorph. Infarct samples represented
three periods: early (purplish and soft), transitional (yellow and
stiff) and late (white and stiff) infarct (15). Villous clump is tissue that is a
little thicker and whiter than normomorph. All the tissues were
collected within 5 min after the placenta was delivered. One
section was stored at −80°C to prepare for the extraction of mRNA
and protein, and the other was fixed in 4% formaldehyde to prepare
the sample for immunohistochemistry.
Immunohistochemistry
Placental tissues were fixed in 4% formaldehyde and
then embedded into paraffin blocks. After deparaffinizing in xylene
and dehydrating in a gradient ethanol, the 2.5-μm thick sections
were heated for 20 min in the microwave to repair antigens. Next,
3% hydrogen peroxide was used to quench the activity of endogenous
peroxidase at room temperature for 10 min. The slides were
incubated with α-SMA monoclonal antibody obtained from mouse
(1:200; Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing,
China) for 60 min at 37°C in a humidified chamber. Poly-HRP
anti-mouse/rabbit IgG (PV-9000 2-step plus, Zhongshan Golden Bridge
Biotechnology Co., Ltd) was added to the slides for 50 min at 37°C
in a humidified chamber. Diaminobenzidine kit (Zhongshan Golden
Bridge Biotechnology Co., Ltd) was used to detect α-SMA staining in
the basal plate. Following counterstaining by hematoxylin,
redehydrating by graded ethanol and vitrification by
dimethylbenzene, the slides were mounted in neutral balsam. The
primary antibody was replaced by PBS for α-SMA-negative control.
The vessels in the placental parenchyma were used for a positive
control. The slides were assessed by light microscope (Bx51;
Olympus Corporation, Japan) by two independent pathologists. The
brown cytoplasm and membrane of SMCs and stromal cells stained by
α-SMA antibody were analyzed positively.
RNA preparation and semi-quantitative
RT-PCR
Total RNA was extracted from the samples by TRIzol
extraction (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA
concentration and purity was determined by absorbance at 260 and
280 nm (OD260/280, >1.8 and <2.1) using a
spectrophotometer (NanoDrop 2000; NanoDrop products, Wilmington,
DE, USA). RT-PCR (BioRT Two Step RT-PCR, China) was performed by
converting 1 μg RNA into cDNA. Reverse transcription was performed
according to the manufacturer’s instructions. The reaction system
of cDNA systhesis had a total volume of 10 μl, consisting of 5X RT
buffer, dNTP mixture (10 mM), oligo-dT, RNase-inhibitor, AMV
reverse transcriptase, RNA sample and RNase free H2O.
The total PCR volume was 25 μl, containing: 10X PCR buffer, dNTP
mixture (10 mM), primer, Taq mix DNA polymerase, cDNA and
double distilled H2O. The amplification of nucleic acids
was performed using 30 cycles of denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec, extension at 72°C for 1 min and
re-extension at 72°C for 5 min. The primers for α-SMA were
(forward) 5′-GCGTGGCTATTCCTTCGTTAC-3′ and (reverse)
5′-CATAGTGGTGCCCCCTGATAG-3′, amplified to a 331-bp fragment. The
housekeeping gene, GAPDH, served as an internal control. The
primers for GAPDH were (forward) 5′-GAAGGTGAAGGTCGGAGT-3′ and
(reverse) 5′-GAAGATGGTGATGGGATTTC-3′, amplified to a 226-bp
fragment. Primers were designed by Primer software and synthesized
by Sangon (Shanghai, China). PCR products were subjected to gel
electrophoresis in 1.5% agarose (Invitrogen Life Technologies)
followed by staining using bromophenol and were detected by
MultiImage Light Cabinet filter positions (Alpha Innotech, San
Leandro, CA, USA) and analyzed by Image J software.
Protein isolation and western
blotting
Basal plate tissues were homogenized in lysis buffer
[50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton-X100, 0.5% NaDC,
1% NP-40 and 1% SDS] on ice and were centrifuged at 9,180 × g for 8
min at 40°C. Western blotting was performed as described previously
(16,17). Membranes were immunoblotted by
mouse monoclonal α-SMA antibody (1:1,000; Beyotime Institute of
Biotechnology, Shanghai, China) overnight at 4°C. Membranes were
then washed three times with TBS-T and incubated with
peroxidase-conjugated Affinipure goat anti-mouse IgG (1:1,000;
Zhongshan Golden Bridge Biotechnology Co., Ltd) at room temperature
for 60 min. Expression of α-SMA protein was detected using an
enhanced chemiluminescence system (Millipore, Billerica, MA, USA)
and was exposed on film (Eastman Kodak Company, Rochester, NY,
USA). GAPDH expression was used as the control.
Statistical analysis
SPSS 17.0 software was used to analyze the data
(SPSS, Inc., Chicago, IL, USA). The data obtained obey the normal
distribution presented as the mean ± SD. One-way ANOVA was applied
and significant differences were observed between the groups.
χ2 test was used to provide evidence whether the
prevalence among all the groups was significantly different.
P<0.05 was considered to indicate a statistically significant
difference. Semiquantitative RT-PCR and western blotting were
performed in triplicate.
Results
Prevalence of multifocal infarct and
villous clumps in all groups
Firstly, the prevalence of multifocal infarct and
villous clumps in all groups was determined and results revealed
that the prevalence of multifocal infarct was 90.00% in 20 EOSPE
patients, 15.00% in 20 LOSPE patients, 5.56% in 18 EC patients and
10.00% in 20 LC patients, respectively (χ2=44.39;
P<0.05). The prevalence of villous clumps was EOSPE >LOSPE
>LC >EC (75.00, 60.00, 35.00 and 11.11%, respectively;
χ2=18.14; P<0.05; Table
II).
 | Table IIPrevalence of multifocal infarct and
villous clumps in the basal plate in all groups. |
Table II
Prevalence of multifocal infarct and
villous clumps in the basal plate in all groups.
| Group | Cases, n | Multifocal infarct, n
(%) | Multifocal villous
clump, n (%) |
|---|
| EOSPE | 20 | 18 (90.00) | 15 (75.00) |
| LOSPE | 20 | 3 (15.00) | 12 (60.00) |
| EC | 18 | 1 (5.56) | 2 (11.11) |
| LC | 20 | 2 (10.00) | 3 (35.00) |
The prevalence of multifocal infarct in EOSPE was
markedly higher than EC (P=0.000) and LOSPE (P=0.000). In addition,
the prevalence of multifocal villous clumps was significantly
higher in EOSPE compared with EC (P=0.000) and LOSPE compared with
LC (P=0.003; Table II).
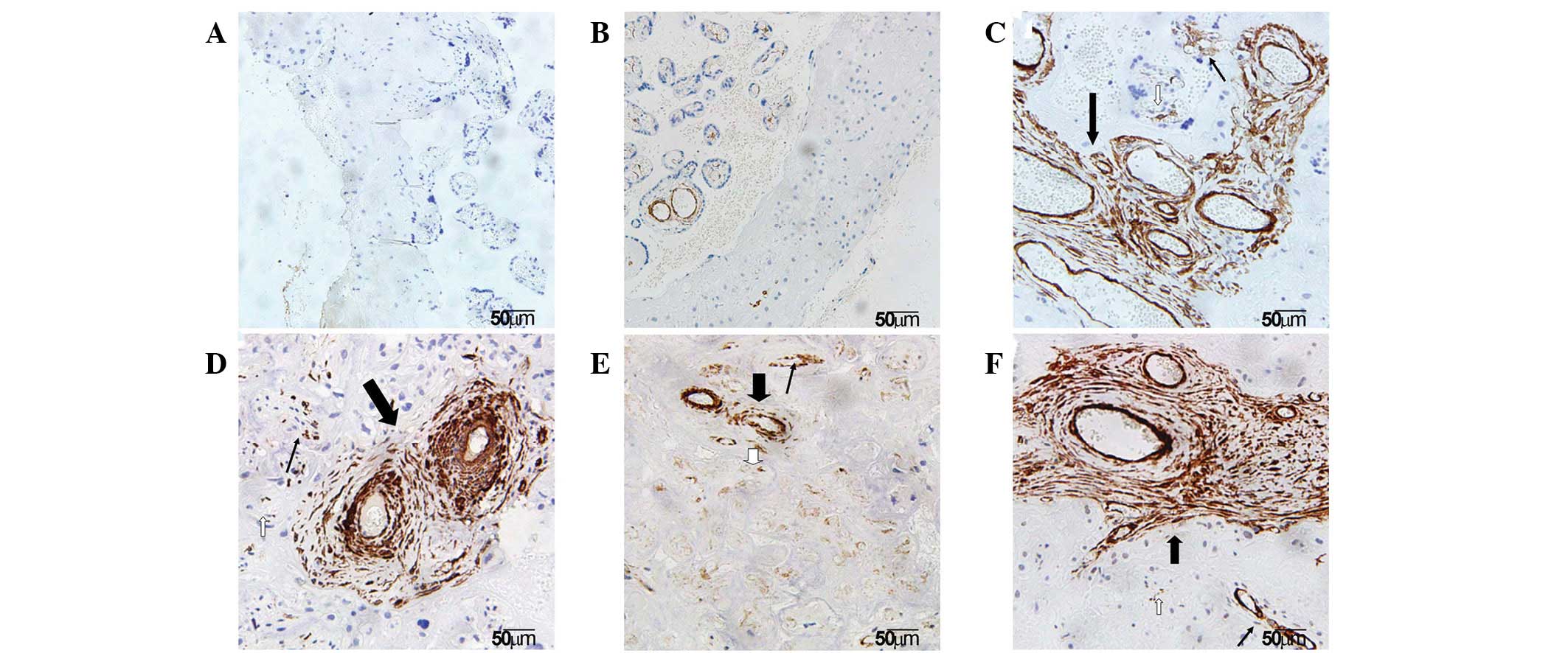
Localization of α-SMA in the basal
plate
It is well established that the placenta plays a key
role in the pathogenesis of SPE. To understand the pathological
changes in SPE placenta, placentas obtained from normal pregnancy
and SPE females were compared. To examine the localization of α-SMA
in various regions of the basal plate, including the infarct
(early, transitional and late infarct area), villous clumps and
normal areas, anti-α-SMA immunohistochemistry was performed.
Immunohistochemical staining results showed that α-SMA was
expressed in the placenta of all groups and was largely localized
to the SMCs of villous vessels and myofibroblasts of the villous
stroma in the basal plate. In addition, α-SMA was observed in the
cytomembrane and cytoplasm of these contractile cells under the
microscope (Fig. 1).
Identification of villi in infarct and
villous clumps
To characterize the morphological changes in various
regions of the basal plate, villi in infarct and villous clumps
were observed. α-SMA staining demonstrated that the morphology of
villi varied in early, transitional and late infarct. Typical early
infarct of the basal plate exhibited characteristics of collapsed
intervillous space. Features of denaturation and necrosis of
trophoblast cells and ghost villi were observed in the typical
transitional infarct. Late infarct is the final stage of infarct
and exhibits the characteristics of extensive fibrosis. The basal
plate tissues appeared to be a little thicker and whiter than
normomorph tissues. However, under the microscope, different
numbers, sizes and stages of villous clumps were observed (Fig. 1).
Proportion of infarct and villous clumps
of various stages in EOSPE and LOSPE
As stated, histological observations of the infarct
and villous clumps usually varied based on the stages under the
microscope, we moved forward a single step to make it clear that
the proportion of early, transitional and late stage in multifocal
infarct and multifocal villous clump. Results showed that infarct
in transitional and late stages was markedly more frequent than
that in early stage in EOSPE. In LOSPE, the infarct in the early
stage was significantly increased compared with transitional and
late stages (Table III). The
area of multifocal villous clump showed the same trend with
multifocal infarct.
 | Table IIIProportion of infarct in various
stages in SPE. |
Table III
Proportion of infarct in various
stages in SPE.
| Group | Cases, n | Multifocal infarct, n
(%) | Early infarct, n
(%) | Transitional-late
infarct, n (%) |
|---|
| EOSPE | 20 | 18 (90.00) | 6 (33.33) | 12 (66.67) |
| LOSPE | 20 | 3 (15.00) | 2 (10.00) | 1 (5.00) |
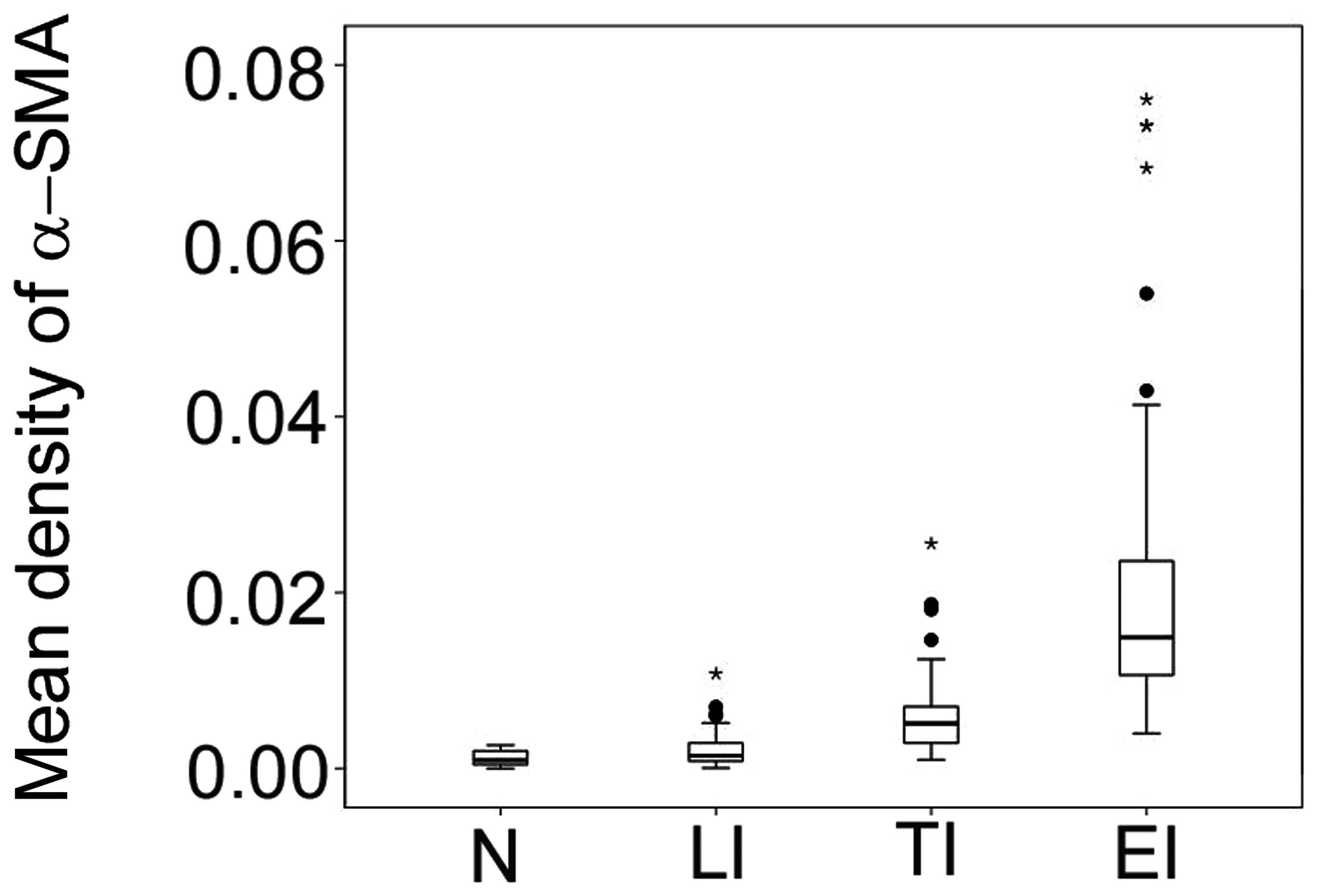
Expression levels of α-SMA in multifocal
infarct
To determine the expression levels of α-SMA in
multifocal infarct and villous clumps, mean density of α-SMA was
determined in multifocal infarct in early, transitional and late
stages. Results demonstrate that the expression levels of α-SMA
were lowest in the normomorph, followed by late, transitional and
early infarct in all groups (P<0.05; Fig. 2).
Correlation between expression levels of
α-SMA and number of cells stained in basal plate infarcts
To investigate the correlation between expression
levels of α-SMA and the number of stained cells, integrated optical
density values in normomorph and late, transitional and early
multifocal infarct samples were obtained and stained cells were
quantified. The results revealed that α-SMA expression levels
increased in normomorph, late, transitional and early multifocal
infarct progressively (P<0.05), and positively correlated with
the number of stained cells in the basal plate (r=0.48, P=0.07;
r=0.652, P=0.000; r=0.544, P=0.002; and r=0.472, P=0.008,
respectively; Fig. 3).
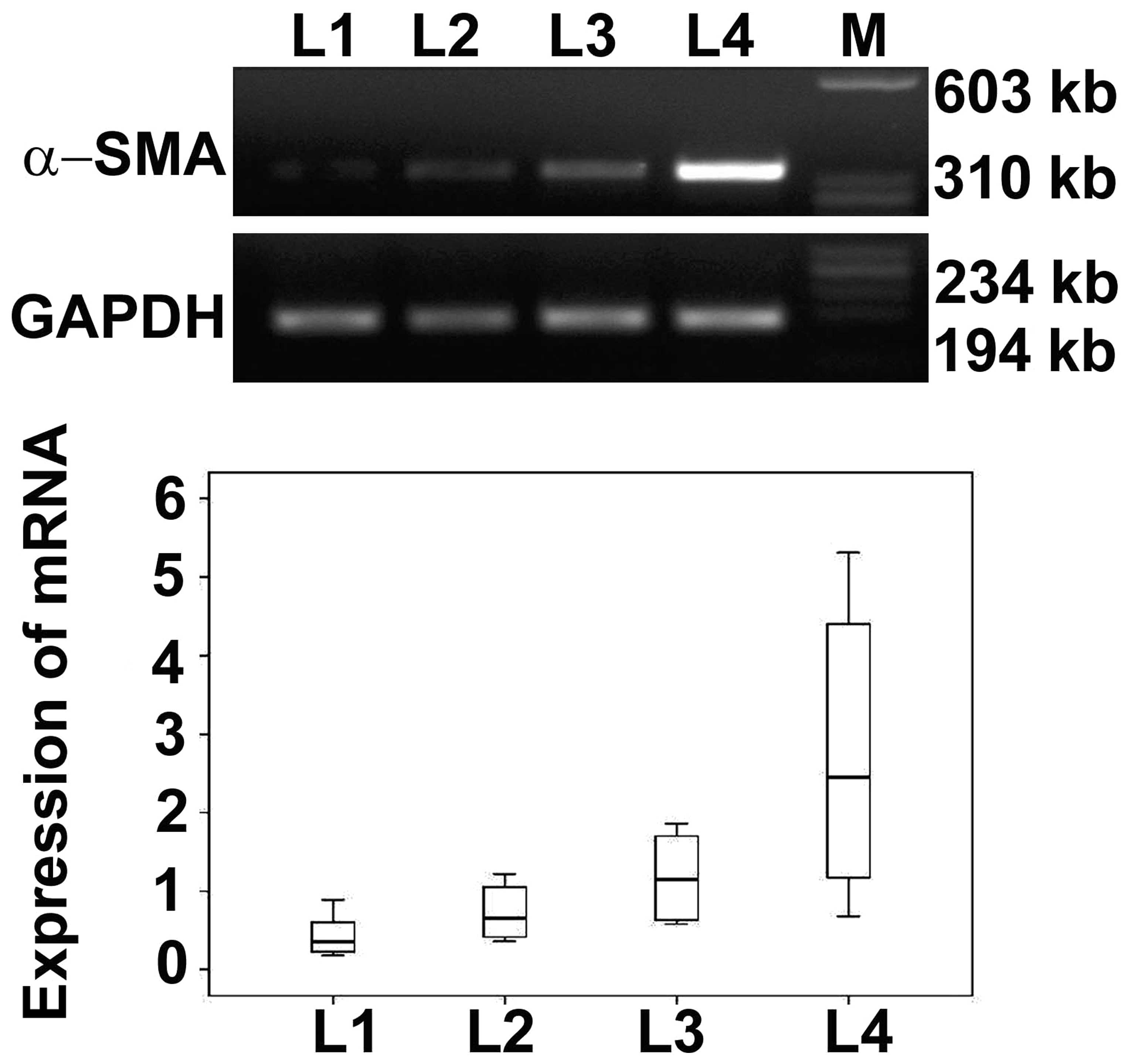
Expression of α-SMA mRNA in basal plate
infarcts
Immunohistochemical results demonstrated that the
expression levels of α-SMA were highest in early infarct, followed
by transitional infarct, late infarct and normomorph. To determine
the expression levels of α-SMA mRNA, RT-PCR was performed. Results
of RT-PCR showed that the expression levels of α-SMA mRNA increased
in normomorph, late, transitional and early infarct samples
progressively. The α-SMA mRNA expression levels were determined by
optical density (Fig. 4) and the
results were consistent with immunohistochemical results.
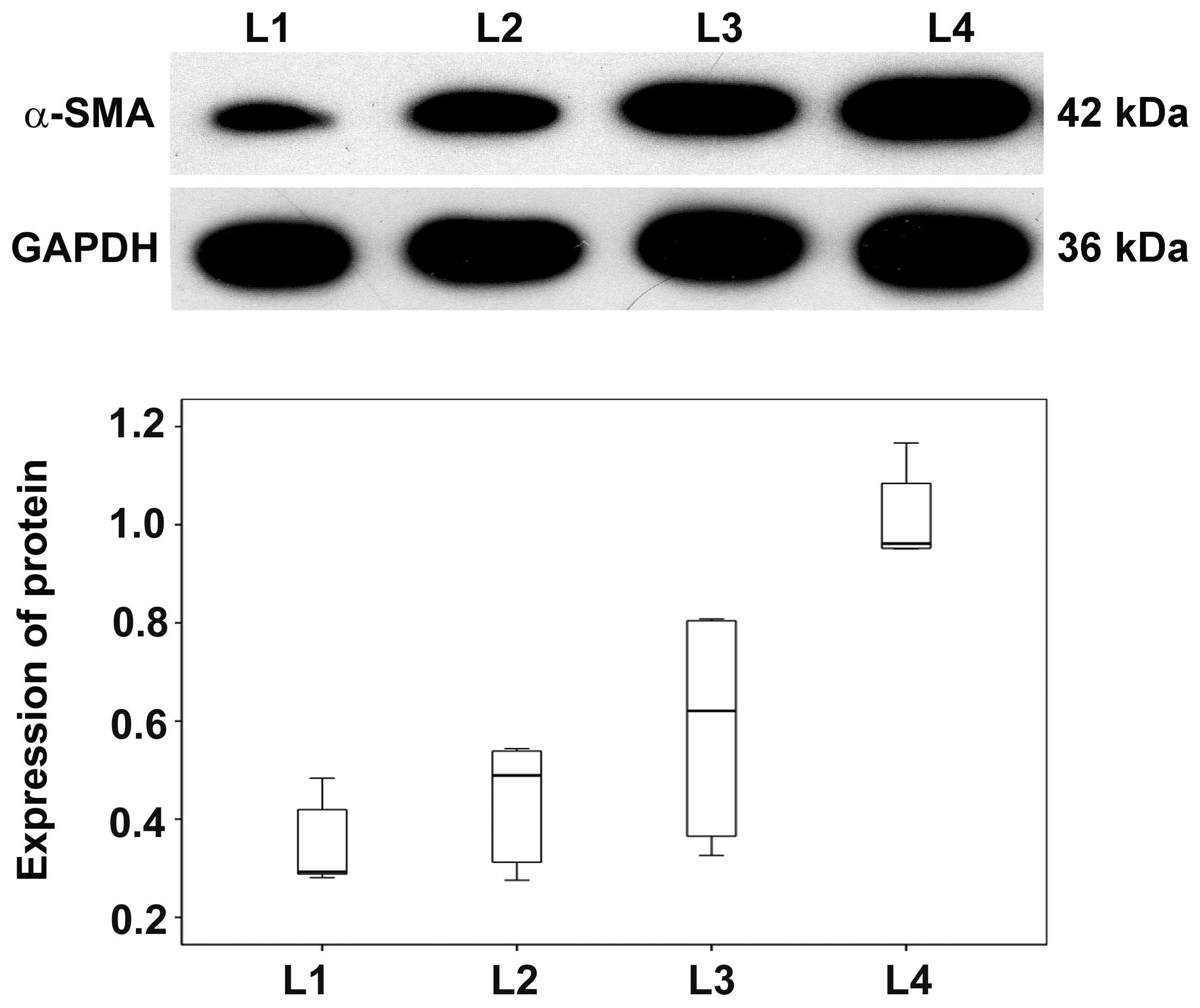
Expression of α-SMA protein in basal
plate infarcts
As our previous results showed that α-SMA mRNA was
upregulated in basal plate infarcts, α-SMA protein levels were
analyzed in SPE patients by western blotting. Expression levels of
α-SMA protein were progressively higher in early, transitional and
late infarct samples compared with normomorph placental tissues. In
addition, levels in early infarct were higher than late infarct and
transitional infarct among the SPE patients while levels in
transitional infarct were higher than late infarct (Fig. 5). These results indicate that, in
addition to α-SMA mRNA levels, α-SMA proteins levels are also
altered in SPE patients.
Discussion
SPE is a complication of pregnancy associated with a
number of organ systems, including renal, retinal, cerebral and
cardiovascular, and correlates with significant maternal and fetal
morbidity and mortality worldwide (18). It is generally accepted that the
placenta is a causative organ to SPE patients and when it is
removed from the uterus, clinical manifestations, including
hypertension and proteinuria are easily controlled (19).
The main hypothesis of shallow implantation of the
placenta was the abnormal villous cytotrophoblast caused by immune
factors, genetic factors and so on, invading into the spiral
arteries of maternal decidua and myometrium, which failed to
establish flaccid low-resistance uteroplacental arteries (20). Remodeling of uterine spiral
arteries is important for the growth and development of the fetus.
The failure of arterial remolding is associated with relatively
hypoxic trophoblast tissue and a state of oxidative stress in the
placenta. The hypoxia/oxidative stress may then result in clinical
manifestation of SPE, including hypertension and proteinuria.
However, this hypothesis is based on previous observations in which
the basal plate was misidentified as the placental bed (21). As it is difficult to collect
specimens from the maternal decidua and myometrium of SPE patients
and the control group (22), these
results do not provide convincing evidence to confirm that shallow
implantation is responsible for the pathogenesis of SPE.
Infarct foci and villous clumps may lead to
malperfusion and hypoxia of the placenta, and the dysfunction of
villi involved in these areas. To date, multifocal infarct, as well
as multifocal infarct of the placental basal plate, has not been
described. The term multifocal infarct of the placental basal plate
was defined by reference to the definition of pervasive infarct of
the placenta (23). The multifocal
infarct of the placental basal plate is the infarct area covering
15% of the total placental basal plate. It has been widely accepted
that pathological changes in the basal plate infarct may cause
underperfusion of the placenta in EOSPE with or without fetal
growth restriction (24). However,
few studies have analyzed the microchanges of placental pathology
affecting placental perfusion in the non-infarct area. In the
present study, villous clumps exhibiting a normomorph appearance
were observed in the basal plate without infarct foci with the
naked eye. Similarly, the multifocal villous clumps of the basal
plate was the area of villous clump which covered 15% of the total
basal plate. In various prevalences between these groups, the
multifocal infarct of the basal plate was the main pathological
change of LOSPE, while multifocal villous clumps of the basal plate
was the common pathway in the pathogenic process of EOSPE and
LOSPE. In addition, these observations are not consistent with the
traditional hypothesis that placental lesions associated with
maternal underperfusion are less frequently observed in LOSPE than
in EOSPE (24).
The increased number of contractile proteins in the
basal plate may represent an additional pathological change which
affects placental perfusion and aggravates uterine tension. To
date, few studies have discussed this hypothesis. Actin is a
cytoskeleton protein with contractile function and has at least six
distinct isoforms in the vertebrate tissues (25). The actin proteins include
α-skeletal muscle actin, α-cardiac muscle actin, α-smooth muscle
actin, β-actin, γ-smooth muscle actin and γ-nonmuscle actin
(26). Matsumura et
al(13) reported that the
human placenta expresses three isoforms of actin, β-actin, α-SMA
and γ-SMA, which account for 60, 30 and 10%, respectively. β-actin
largely localizes within the extravascular stroma while α-SMA
localizes in endovascular tissues. In addition, α-SMA is a
biomarker of myofibroblasts (27).
Results of the present study indicate that α-SMA is largely
localized to the cytomembrane and cytoplasm of the SMCs of villi
and myofibroblasts of villous stroma in the basal plate. These
villi mainly spread over infarct foci and villous clumps. These
results are consistent with previous studies indicating that α-SMA
is expressed in various cells, including mesenchyme, reticulum,
smooth muscle, hofbauer, filamented and vacuolated cells, and
fibroblasts and myofibroblasts in the stroma (28). The villi components labeled by
α-SMA mainly exist in infarcts and villous clumps of the basal
plate. Current staining results indicate that the contraction of
these α-SMA-labeled cells may play a role in the pathogenesis of
SPE by affecting maternal circulation and inducing the
contractility of uterine.
Tannheimer et al(29) previously showed that myofibroblasts
participate in injury repair by secreting collagen, extracellular
matrix and proinflammatory mediators. During oxidative stress, the
structure of actin may be damaged and the cells stained by α-SMA
may be undergoing cytoclasis and fibrosis (30). These concepts provide a good
explanation for the altered expression profile of α-SMA in
normomorph, late, transitional and early infarct samples in the
basal plate. The expression and contraction of α-SMA may vary in a
time-dependent manner and a longer time under conditions of
hypoxia, and a higher rate of cell degeneration and fibrosis
enables lower expression of α-SMA and weaker contraction of
α-SMA.
More importantly, results of the present study may
be useful for clinical application. To date, the only method to
control SPE is termination of pregnancy, which may increase the
rate of preterm births and neonatal mortalities of SPE. The early
infarct and villous clumps of basal plate may be the target of
minimally invasive interventional treatment through ultrasonic
Doppler.
In conclusion, the present study shows that
multifocal infarcts and multifocal villous clumps may participate
in the pathogenic progress of SPE by blocking blood vessels,
inducing vessel contraction and aggravating uterine tension by
α-SMA.
Acknowledgements
This study was funded by grants from the Science and
Technology Department of Jilin Province (no. 20090464) and the
Science and Technology Agency of Changchun (no. 08SF44). The
authors would like to thank Mei Sun and Yang Xia (Department of
Pathology, Jilin University Bethune Second Hospital) for their
assistance in observing placental pathology.
References
|
1
|
George EM and Granger JP: Recent insights
into the pathophysiology of preeclampsia. Expert Rev Obstet
Gynecol. 5:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
von Dadelszen P, Magee LA and Roberts JM:
Subclassification of preeclampsia. Hypertens Pregnancy. 22:143–148.
2003.
|
|
3
|
Hargitai B, Marton T and Cox PM: Best
practice no 178. Examination of the human placenta. J Clin Pathol.
57:785–792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khong TY and Werger AC: Myometrial fibers
in the placental basal plate can confirm but do not necessarily
indicate clinical placenta accreta. Am J Clin Pathol. 116:703–708.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogler C, Petterchak J, Sotelo-Avila C and
Thorpe C: Placental pathology for the surgical pathologist. Adv
Anat Pathol. 7:214–229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramma W and Ahmed A: Is inflammation the
cause of pre-eclampsia? Biochem Soc Trans. 39:1619–1627. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von Versen-Hoeynck FM and Powers RW:
Maternal-fetal metabolism in normal pregnancy and preeclampsia.
Front Biosci. 12:2457–2470. 2000.PubMed/NCBI
|
|
8
|
Lash GE, McLaughlin BE,
MacDonald-Goodfellow SK, et al: Relationship between tissue damage
and heme oxygenase expression in chorionic villi of term human
placenta. Am J Physiol Heart Circ Physiol. 284:H160–H167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myatt L: Role of placenta in preeclampsia.
Endocrine. 19:103–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Whitley GS and Cartwright JE: Cellular and
molecular regulation of spiral artery remodelling: lessons from the
cardiovascular field. Placenta. 31:465–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furuya M, Ishida J, Aoki I and Fukamizu A:
Pathophysiology of placentation abnormalities in pregnancy-induced
hypertension. Vasc Health Risk Manag. 4:1301–1313. 2008.PubMed/NCBI
|
|
12
|
Lyall F: Priming and remodelling of human
placental bed spiral arteries during pregnancy - a review.
Placenta. 26(Suppl A): S31–S36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumura S, Sakurai K, Shinomiya T,
Fujitani N, Key K and Ohashi M: Biochemical and immunohistochemical
characterization of the isoforms of myosin and actin in human
placenta. Placenta. 32:347–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao S, Gu Y, Fan R, Groome LJ, Cooper D
and Wang Y: Proteases and sFlt-1 release in the human placenta.
Placenta. 31:512–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufmann P, Luckhardt M, Schweikhart G and
Cantle SJ: Cross-sectional features and three-dimensional structure
of human placental villi. Placenta. 8:235–247. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
17
|
Rasul A, Yu B, Khan M, Zhang K, Iqbal F,
Ma T and Yang H: Magnolol, a natural compound, induces apoptosis of
SGC-7901 human gastric adenocarcinoma cells via the mitochondrial
and PI3K/Akt signaling pathways. Int J Oncol. 40:1153–1161.
2012.PubMed/NCBI
|
|
18
|
Paruk F and Moodley J: Maternal and
neonatal outcome in early- and late-onset pre-eclampsia. Semin
Neonatol. 5:197–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redman CW and Sargent IL: Placental
debris, oxidative stress and pre-eclampsia. Placenta. 21:597–602.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hung TH and Burton GJ: Hypoxia and
reoxygenation: a possible mechanism for placental oxidative stress
in preeclampsia. Taiwan J Obstet Gynecol. 45:189–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaufmann P, Black S and Huppertz B:
Endovascular trophoblast invasion: implications for the
pathogenesis of intrauterine growth retardation and preeclampsia.
Biol Reprod. 69:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Craven CM, Morgan T and Ward K: Decidual
spiral artery remodelling begins before cellular interaction with
cytotrophoblasts. Placenta. 19:241–252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crum CP, Nucci MR and Lee KR: Diagnostic
Gynecologic and Obstetric Pathology. 2nd ed. Saunders/Elsevier;
Philadelphia, PA: pp. 12022011
|
|
24
|
Ogge G, Chaiworapongsa T, Romero R, et al:
Placental lesions associated with maternal underperfusion are more
frequent in early-onset than in late-onset preeclampsia. J Perinat
Med. 39:641–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vandekerckhove J and Weber K: At least six
different actins are expressed in a higher mammal: an analysis
based on the amino acid sequence of the amino-terminal tryptic
peptide. J Mol Biol. 126:783–802. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glukhova MA, Frid MG and Koteliansky VE:
Developmental changes in expression of contractile and cytoskeletal
proteins in human aortic smooth muscle. J Biol Chem.
265:13042–13046. 1990.PubMed/NCBI
|
|
27
|
Teraoka R, Shimada T and Aburada M: The
molecular mechanisms of the hepatoprotective effect of gomisin A
against oxidative stress and inflammatory response in rats with
carbon tetrachloride-induced acute liver injury. Biol Pharm Bull.
35:171–177. 2012. View Article : Google Scholar
|
|
28
|
Sati L, Seval Y, Yasemin Demir A, Kosanke
G, Kohnen G and Demir R: Cellular diversity of human placental stem
villi: an ultrastructural and immunohistochemical study. Acta
Histochem. 109:468–479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tannheimer SL, Wright CD and Salmon M:
Combination of roflumilast with a beta-2 adrenergic receptor
agonist inhibits proinflammatory and profibrotic mediator release
from human lung fibroblasts. Respir Res. 13:282012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rogers KR, Morris CJ and Blake DR: The
cytoskeleton and its importance as a mediator of inflammation. Ann
Rheum Dis. 51:565–571. 1992. View Article : Google Scholar : PubMed/NCBI
|