Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease, which is prevalent in 0.5–1% of the world’s population
(1). RA is chronic and may lead to
joint damage, synovial membrane destruction, and cartilage and bone
damage (2,3). RA is an incurable disease that
seriously impacts human physical and mental health (4). RA and the side effects of RA
therapeutic agents may induce complications, including
cardiovascular, communicable, blood, gastrointestinal and lung
diseases (5). In addition, RA is
accompanied by long duration, high treatment costs and high
disability or mortality rate, which affects the quality of life of
patients (6). At present, the
etiology and pathogenesis of RA remains poorly understood; however,
numerous pathways have been observed to contribute. The toll-like
receptor (TLR) signal transduction pathway is considered to be an
important pathway in the pathogenesis of RA. TLR family members are
receptors involved in the immune system and microbial
identification; they mediate the innate immune reaction and
activate the acquired immune response. In addition, TLR4 has been
shown to be important in autoimmune diseases, particularly in RA
(7,8). The correlation between TLR4 and
nonbiological inflammatory injury has previously been shown
(9–11). It is hypothesized that injured
tissue and necrotic cells release endogenous activators, termed
adjuvants. These activators bind to TLR4 on the cell membrane.
MyD88, an adapter protein of TLR, is located in the cell and is the
key signaling molecule in the signal transduction pathway. When
cells are stimulated by an extracellular signal, nuclear
transcription factor-κB (NF-κB) is activated, which promotes the
transcription of a number of genes and the release of series a
cytokines, resulting in inflammation (12–15)
through the signaling cascade. Activated cells may also induce
specific immunity to increase the self-protection ability of the
organism. An excessive inflammatory reaction may also damage target
cells and tissue. Thus, the effects of NF-κB in the incidence and
treatment of RA has been an area of interest in studies concerning
rheumatism.
Traditional Chinese medicines (TCM) have been
observed to be clinically effective and are the most prevalent
treatment for RA in a number of Asian countries. RA is similar to
Bi Zheng in TCM theory, which is defined as a characteristic
syndrome of numbness and weakness, bone pain, joint stiffness and
deformation, and dyskinesia (16).
In TCM, it is generally considered that Bi Zheng occurs due to
attack of the meridians of the limbs by exogenous wind, dampness
and heat or cold pathogens (16,17).
Traditional Chinese herbal prescriptions have long been employed
for the treatment of RA, pain-relief, anti-inflammation and/or as
immunomodulatory therapies (18,19).
Traditional Chinese herbal prescriptions contain a number of herbs
and collectively exert therapeutic and modulatory effects (20). These formulae have been
demonstrated in numerous basic and clinical studies (3,21,22).
STBT (Min drug system approval no. Z20111008; patent
no. 201110347820.4) has been used as a clinical prescription for
~10 years and has become a standard hospital prescription at Fujian
University of TCM Affiliated People’s Hospital (Fuzhou, China).
However, pharmacological studies have not yet been conducted and
the underlying mechanisms of STBT remain to be fully elucidated. In
the present study, the rat CIA model, a widely used experimental
model of human RA, was used to investigate the effects of STBT on
acute arthritis and chronic joint damage, and the potential
mechanisms associated with the TLR signal transduction pathway.
Materials and methods
Materials and animals
A Dionex Ultimate 3000 liquid chromatography
equipped with DAD detector was purchased from Dionex Ltd.
(Sunnyvale, CA, USA); a KQ-500DE ultrasonic clearing machine was
purchased from Kunshan Ultrasonic Instruments Co., Ltd. (Kunshan,
China); a XS105 electronic analytical balance was obtained from
Mettler-Toledo Instruments (Shanghai) Co., Ltd. (Shanghai, China);
and the YLS-7B toe volume measuring instrument was purchased from
Yi Yan Technology Development Co., Ltd. (Jinan, China).
Acetonitrile was of chromatographic grade and was
used for high-performance liquid chromatography (HPLC). Reverse
osmosis Milli-Q water (18 ΩM; Millipore, Bedford, MA, USA) was used
throughout the study. All other chemical solvents in the study were
at least of analytical reagent grade. Complete Freund’s adjuvant
(CFA) and type II collagen were purchased from Sigma-Alrdich (St.
Louis, MO, USA). TRIzol reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA) and SuperScript II reverse
transcriptase was provided by Promega Corporation (Madison, WI,
USA). TLR4, MyD88 and NF-κB primary antibodies and horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Cell Signaling Technology Inc. (Beverly, MA, USA). All other
chemicals used, unless otherwise stated, were obtained from
commercial sources.
Specific pathogen-free (SPF) Wistar male rats (n=50;
weight, 180–220 g) were provided by the Animal Care and Use
Committee of Fujian University of TCM and were purchased from
Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China; license
no. 200700518360). Animals were housed under controlled temperature
(21–23°C), relative humidity 55±5%, and a 12-h light/dark cycle,
and had access to standard rat chow and tap water ad
libitum. All animal experiments were conducted in accordance
with international Ethical Guidelines and the National Institutes
of Health Guide concerning the Care and Use of Laboratory
Animals.
Methods
Preparation of STBT
STBT is a mixture of four traditional drugs. The
drugs were provided by Fujian University of TCM Affiliated People’s
Hospital and verified by Wei Lu (Pharmacy College of Fujian
University of TCM). The voucher specimens were deposited from the
Pharmacy College of Fujian University of TCM. STBT was prepared
according to the preparation of the STBT hospital prescription of
Fujian University of TCM Affiliated People’s Hospital.
Tripterygium wilfordii Hook.f., Sinomenium acutum
Rehd. et Wils, Dioscorea ipponica Makino and Glycyrrhiza
uralensis Fisch. were crushed into a coarse powder, mixed and
steeped in 10X 80% ethanol. Following 48 h of slow percolation of
the extract, the fluid from the percolation was collected and
filtrated. Finally, the fluid was adjusted to 10 g/ml (original
medicinal materials/volume).
The prepared STBT was processed as previously
described (23) and was filtrated
through a 0.45 μm microporous membrane and injected into the liquid
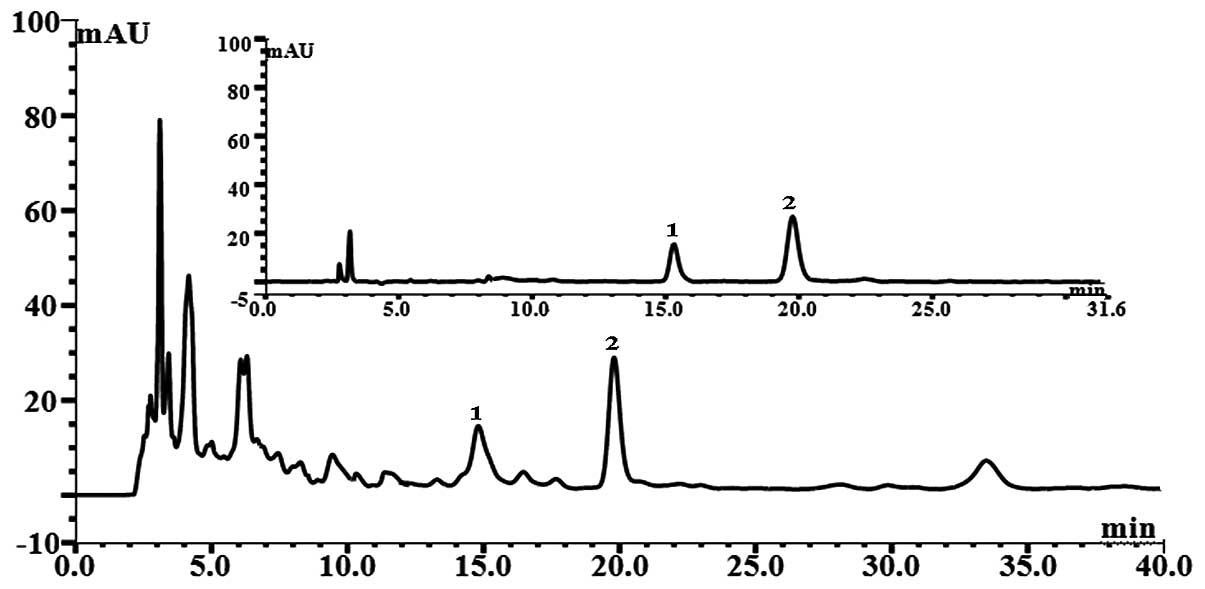
chromatograph. Major peaks were identified as the marker compounds
(Fig. 1).
CIA modeling and drug
administration
Wistar rats (n=50) were randomly divided into five
groups (n=10 per group); control; model; votalin ointment, an
accepted control medicine clinically used to treat RA (~1 cm/time);
STBT low-dose (0.5 ml/time) and STBT high-dose groups (2 ml/time).
Collagen (2 mg/ml) was emulsified with an equal volume of CFA to a
final concentration of 0.1 mg/ml. In addition to the control group,
0.2 mg collagen emulsion was injected into the tail root of each
rat by intradermal injection. Provocation testing was performed
after 7 days using similar methods. Drugs were administered once
daily 1 day following primary immunization. In the STBT low- and
high-dose groups, STBT was sprayed onto the back of rats whose hair
had been shaved twice daily. The area of the back where the drug
was administered measured 3 × 3 cm. The votalin ointment group were
administered votalin ointment in the same method as STBT. The
control and model groups did not receive any treatment. All animals
were treated for 35 days continuously.
Evaluation of paw swelling and body
weight
A volume method was used to measure the paw volume
of rats (24). The paw volume
prior to modeling was measured initially and then 11 days following
the primary immunization, the paw volume of each rat was measured
every 4 days. Swelling was expressed by the volume difference (ml)
prior to and following modeling. The body weight of the rats was
measured using an SE402F electronic scale [Ohaus Instruments
(Shanghai) Co., Ltd., Shanghai, China] every 3 days from the
primary immunization.
Histopathological examination
Following the termination of the experiment, rats
were anesthetized and the right hind knee was removed and fixed in
4% neutral buffered formalin for 24 h. Following decalcification
with 12.5% EDTA (pH 7.0) for ~20 days, the right hind knee was
paraffin-embedded, sectioned at a 5-mm thickness and stained with
hematoxylin and eosin.
Western blot analysis
Synovial tissue was extracted from the hind paws of
the rats and were homogenized with nondenaturing lysis buffer and
centrifuged at 4°C at 12,000 × g for ~15 min. The supernatants were
denatured with protein loading buffer following the determination
of the protein concentration. Protein (45 μg) was resolved on 12%
sodium dodecyl sulfate-polyacrylamide gel, blotted onto a
polyvinylidene fluoride (PVDF) membrane and blocked for 2 h with 5%
skimmed milk in Tris-buffered saline with Tween 20. Membranes were
incubated with the desired primary antibody against TLR4, MyD88,
NF-κB or β-actin with a dilution of 1:1,000 overnight at 4°C and
then with the appropriate HRP-conjugated secondary antibody for 50
min. Following washing, the membranes were visualized by enhanced
chemiluminescence detection.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the synovial tissue of
rats with TRIzol reagent. Total RNA (2 μg) was reverse-transcribed
to cDNA and used to determine the mRNA levels of TLR4, MyD88 and
NF-κB by PCR with Taq DNA polymerase (Fermentas, Rockford,
IL, USA). PCR was performed under the following conditions: Initial
denaturation at 95°C for 3 min, denaturation for 30 sec at 95°C,
extension at 72°C for 45 sec and amplification for 35 cycles. The
annealing temperature was 62.8°C for TRL4, 65.5°C for MyD88 and
60°C for NF-κB. Primers for TLR4, MyD88 and NF-κB were as follows:
Forward: 5′-ATGCCAGGATGATGCCTCTCTTGCA-3′ and reverse:
5′-TTCACACCTGGATAAATCCAGCCAC-3′ for TLR4; forward:
5′-AGTTGCTAGCCTTGTTAGACC GTGAGG-3′ and reverse:
5′-AAACAACCACCACCATGCGACGACACC-3′ for MyD88; forward: 5′-GCGCAT
CCAGACCAACAATAAC-3′ and reverse: 5′-GCCGAA GCTGCATGGACACT-3′ for
NF-κB; and forward, 5′-GTCATCCATGACAACTTTGG-3′ and reverse,
5′-GAGCTTGACAAAGTGGTCGT-3′ for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). GAPDH served as an internal control. All
experiments were performed according to the manufacturer’s
instructions. Samples were analyzed by gel electrophoresis (1.5%
agarose) and the DNA bands were examined in a Gel Documentation
System (Model Gel Doc 2000; Bio-Rad, Hercules, CA, USA).
Statistical analysis
Results are expressed as the mean ± SD. Analysis of
variance was used to determine significant differences between
groups using SPSS Software (SPSS Inc, Chicago, IL, USA). P<0.05
and P<0.01 were considered to indicate a statistically
significant difference.
Results
Effect of STBT on symptoms of CIA
Paw and knee joint swelling was an external
objective indicator for evaluating the severity of inflammation in
the RA model. This was monitored by an independent examiner who did
not have prior knowledge of the experimental groups. The changes of
paw swelling and body weight are presented in Tables I and II. Approximately three days after the
second immunization, the rat knee joints began to swell and over
time, the joint and paw size increased. Paw and knee joint volume
increased and reached a maximum on ~day 18. The groups receiving
votalin ointment and high-dose STBT showed significant inhibition
(P<0.01), compared with the model group. The body weight of the
control group linearly increased, but the model group only
increased 13 days prior to the generation of swelling and then
decreased, particularly between the 13th and 16th day.
 | Table IChanges of paw swelling. |
Table I
Changes of paw swelling.
| Group | Foot volume prior to
inflammation (ml) | Paw swelling (ml)
following inflammation at various times |
|---|
|
|---|
| 7 days | 10 days | 13 days | 16 days | 19 days | 22 days | 25 days | 28 days | 31 days |
|---|
| Control | 1.61±0.07 | 1.61±0.07 | 1.62±0.06 | 1.62±0.08 | 1.62±0.08 | 1.63±0.07 | 1.65±0.08 | 1.66±0.05 | 1.70±0.08 | 1.71±0.07 |
| Model | 1.61±0.05 | 1.65±0.09 |
1.72±0.09b |
1.85±0.09b |
2.40±0.12b |
3.06±0.15b |
3.08±0.15b |
3.06±0.14b |
2.97±0.14b |
2.87±0.13b |
| Votalin ointment | 1.62±0.03 | 1.67±0.06 |
1.69±0.07c |
1.78±0.01c |
2.19±0.13d |
2.61±0.16d |
2.58±.16d |
2.55±0.50d |
2.35±0.23d |
2.25±0.14d |
| STBT low | 1.63±0.08 | 1.62±0.08 |
1.67±0.08c |
1.79±0.09c |
2.58±0.12c | 3.01±0.65 | 3.02±0.55 |
2.87±0.34c |
2.73±0.43c |
2.32±0.24d |
| STBT high | 1.61±0.05 | 1.65±0.06 |
1.65±0.09d |
1.72±0.03d |
2.02±0.01d |
2.44±0.17d |
2.50±0.04d |
2.42±0.14d |
2.32±0.14d |
2.12±0.24d |
 | Table IIChanges in body weight at various
times. |
Table II
Changes in body weight at various
times.
|
Body weight
(g) at various times |
|---|
|
|
|---|
| Group | 1 day | 4 days | 7 days | 10 days | 13 days | 16 days | 19 days | 22 days | 25 days | 28 days | 31 days |
|---|
| Control | 198.93±9.94 | 215.35±10.76 | 231.48±11.57 | 243.86±12.19 | 257.95±12.89 | 269.73±3.48 | 282.9±14.14 | 293.28±14.66 | 306.67±15.33 | 325.40±16.27 | 336.91±16.34 |
| Model | 198.30±9.71 | 215.16±10.55 | 221.80±11.09 |
227.93±11.39a |
231.32±11.56b |
216.2±10.81b |
209.96±10.49b |
208.68±10.43b |
210.74±10.53b |
213.73±10.68b |
217.56±10.87b |
| Votalin
ointment | 197.73±9.88 | 218.34±10.41 | 232.92±10.64 | 234.56±10.72 | 238.23±10.91 | 215.8±10.99 | 217.96±11.09 |
220.33±11.21d |
223.72±11.38c |
225.80±11.49d |
226.52±11.52c |
| STBT low | 198.2±10.11 | 216.4±11.02 | 227.58±11.57 | 234.13±11.90 | 238.79±12.13 | 227.80±11.39 |
224.65±11.23c | 219.62±10.98 |
225.17±11.25c |
231.94±11.59c |
237.13±11.85d |
| STBT high | 198.07±9.34 | 218.12±9.76 | 237.00±11.22 | 239.67±11.01 |
243.19±11.56c |
230.21±10.56c |
225.98±11.13c |
227.26±11.11d |
229.08±9.90c |
239.74±12.38d |
239.07±13.12d |
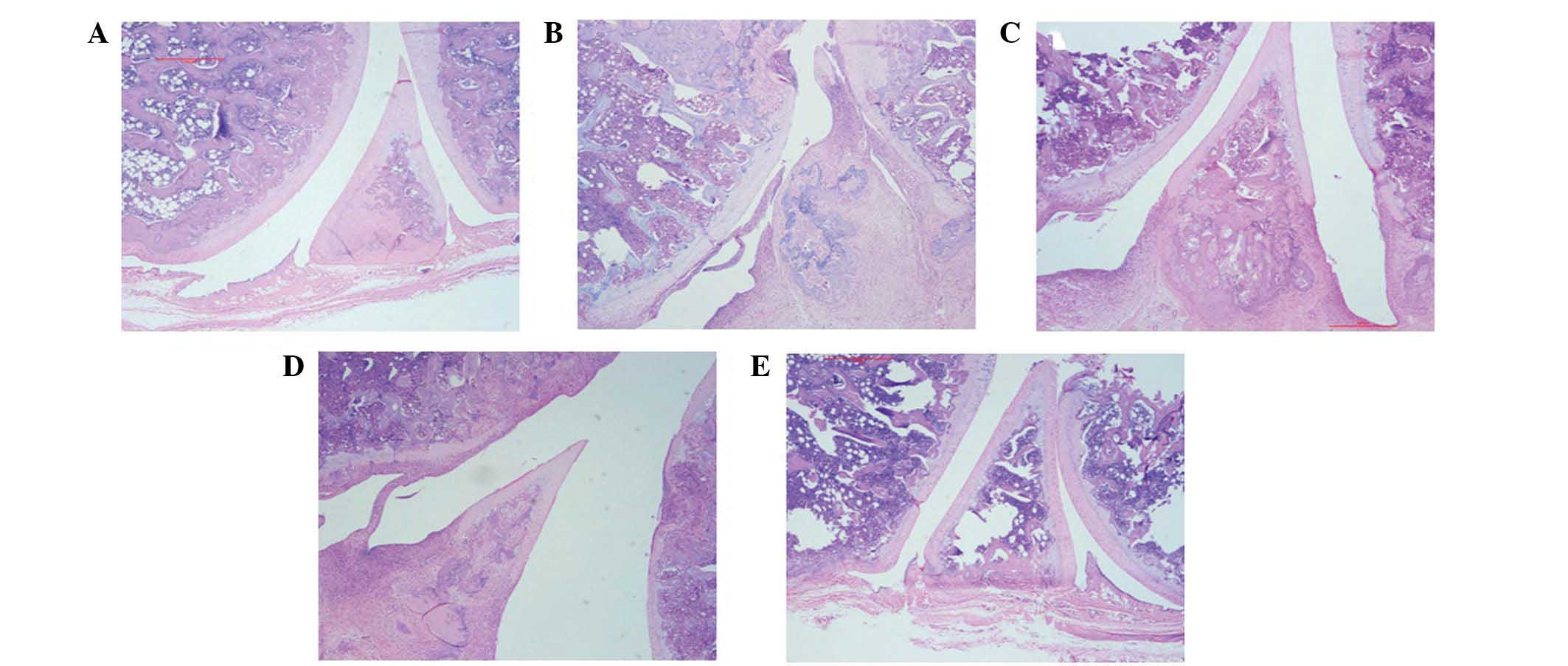
Effects of STBT on histopathological
changes
Representative histopathological lesions in the hind
knee joint of control, model, votalin ointment, STBT low- and
high-dose groups are shown in Fig.
2. Synovium hyperplasia, disorganized arrangement, infiltration
of inflammatory cells, cartilage destruction, bone destruction, a
number of small blood vessels and pannus formation were observed in
the model group. Histopatholgical changes were ameliorated in the
treated groups to a different extent. The STBT high-dose group
showed a significant improvement. This group exhibited only mild
synovium proliferation, regular order of the cell morphology,
infiltration of a small number of inflammatory cells, no typical
pannus formation, the surface of the cartilage was smooth and there
was no marked damage in cartilage and bone erosion.
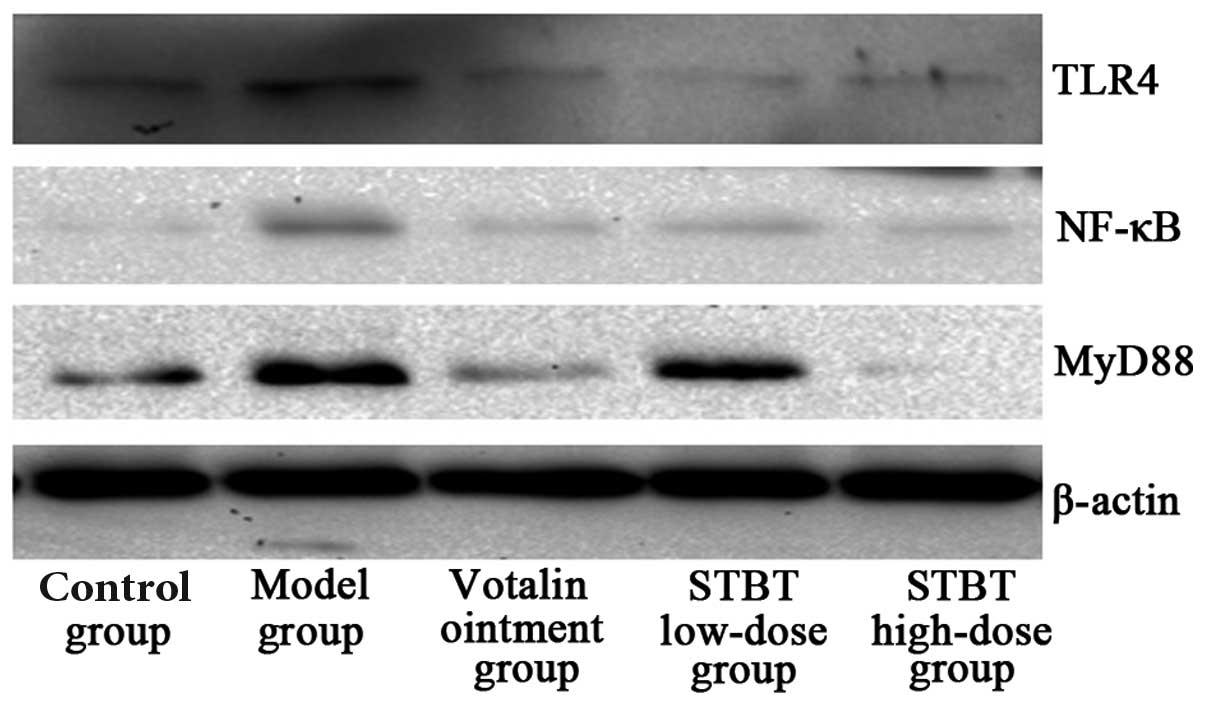
Effect of STBT on the protein expression
of TLR4, MyD88 and NF-κB
As shown in Fig. 3,
the synovial level of TLR4, MyD88 and NF-κB protein expression
significantly increased in the model compared with the control
group. Compared with the model group, treatment with STBT decreased
the synovial levels of TLR4, MyD88 and NF-κB protein expression,
particularly in the high-dose STBT group. The STBT high-dose group
showed significant downregulation of TLR4, MyD88 and NF-κB protein
expression (P<0.01) and the STBT low-dose group exhibited no
marked effect on protein expression.
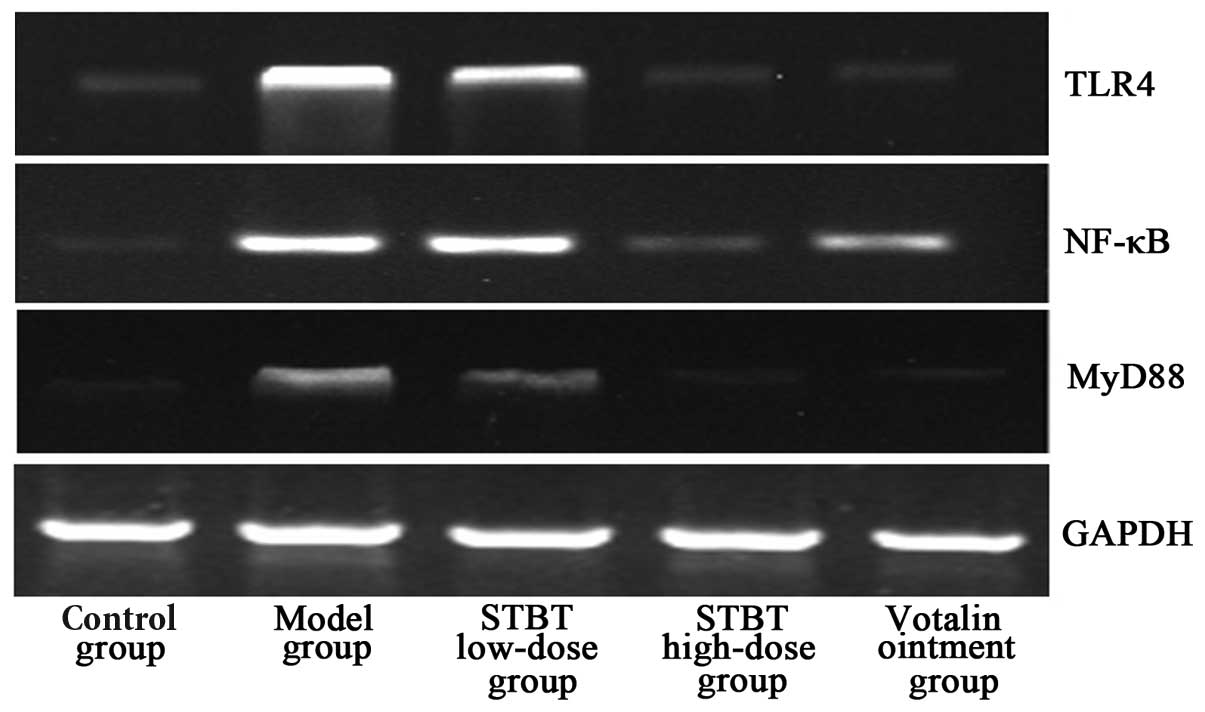
Effect of STBT on the levels of TLR4,
MyD88 and NF-κB mRNA
The results of the RT-PCR assay (Fig. 4) showed that the mRNA levels of
TLR4, MyD88 and NF-κB in the model group was significantly
increased compared with those in the control group (P<0.05).
However, the mRNA levels were decreased following treatment with
votalin ointment or various doses of STBT compared with the model
group.
Discussion
STBT consists of four components, Tripterygium
wilfordii Hook.f., Sinomenium acutum Rehd. et Wils,
Dioscorea nipponica Makino and Glycyrrhiza uralensis
Fisch (Table III). In STBT, the
officinal part of Tripterygium wilfordii Hook.f. is the
root. Its TCM properties are that it is bitter and acrid in taste,
cool in nature and extremely toxic. It is associated with the liver
and kidney channels. Its efficacies are dispeling wind and
eliminating dampness, dredging collaterals, relieving pain and
reducing swelling (25). The root
of Tripterygium wilfordii Hook.f. is one of the most
effective TCMs for the treatment of RA. Long-term clinical practice
has shown that using Tripterygium wilfordii Hook.f. to treat
RA may reduce or replace corticosteroids and/or steroidal
anti-inflammatory drugs and possesses the advantages of high
efficiency and low toxicity (26).
It has been demonstrated that the predominant active ingredient,
triptolide, is effective on the expression and activity of NF-κB in
the synovium of CIA rats (26).
Sinomenium acutum Rehd. et Wils is used in a number of TCM
treatments, including removing wind-dampness and relieving pains,
passing the meridian and increasing urination and it is used to
treat pain and numbness in arthritis, swollen joints, paralysis and
itching (27). Its predominate
active ingredient, sinomenine, was used in the therapy of the RA
and exhibited clear and definite therapeutic effects (28,29).
These include relaxing tendons, removing phlegm, removing
wind-dampness and relieving pains. Dioscorea nipponica
Makino is used to treat pain and numbness in arthritis, paralysis,
Kashin-Beck disease, traumatic injury and bronchitis (30). It was employed in a number of
formulae to clinically treat RA (16,31).
Glycyrrhiza uralensis Fisch. nourishes Qi, alleviates pain,
eliminates phlegm, stops coughing, regulates heat and detoxifies.
Each component of STBT may be important in the treatment of RA.
However, in TCM, a number of herbs are often combined under the
theories of TCM. The combined interactions of these herbs are
hypothesized to contribute more to the anti-RA effect of STBT
compared with single use.
 | Table IIIComposition of STBT. |
Table III
Composition of STBT.
| Pharmaceutical
name | Botanical
source/genus | Part used | Traditional
actions/uses | Quantity (g) |
|---|
| Tripterygium
wilfordii Hook.f. |
Tripterygium | Root | Dispels wind and
eliminate dampness; dredges collaterals and relieves pain; reduces
swelling and eases pain | 30 |
| Sinomenium
acutum Rehd. et Wils |
Sinomenium | Rattan | Removes
wind-dampness and relieves pain, passes the meridian and increases
urination | 25 |
| Dioscorea
nipponica Makino |
Dioscorea | Root and
rhizome | Dispels wind and
eliminates dampness; dredges collaterals and passes the meridian;
promotes blood circulation and relieves pain | 25 |
| Glycyrrhiza
uralensis Fisch. |
Glycyrrhiza | Root and
rhizome | Nourish Qi,
alleviates pain, eliminates phlegm, stops coughing, regulates
temperature and detoxifies | 15 |
CIA is one of the most widely used experimental
arthritis animal models for the identification of genes and
mechanisms, and shares specific immunological and pathological
features with human RA (32,33).
In the current study, it was observed that the swelling dimensions
following treatment with STBT were decreased compared with the
model group. According to the histopathological change of ankle
joint analysis, it was hypothesized that STBT may alleviate the
hyperplasia of the synovial membrane and bone destruction. STBT is
hypothesized as a potential therapeutic agent for treating RA
patients.
TLR signal transduction pathways are important in
the inflammatory responses of RA. There are two primary pathways,
the MyD88-dependent and MyD88-independent pathways. There are a
number of adaptor proteins in the TLR signal transduction pathway.
MyD88 is a protein with a Toll-interleukin receptor structure
domain and is significant in the effect of the TLR signal
transduction pathway. MyD88 identifies pathogen-associated
molecular patterns intra-articularly, magnifies the inflammation
signal and forms a cascade reaction, resulting in the activation of
the NF-κB signaling pathways. The inflammatory response is
initiated and cells synthesize and release a number of inflammatory
factors, including IL-1β and TNF-α. TNF-α provides positive
feedback and activates NF-κB. These activities effect the
inflammatory factors and maintain the inflammation, which destroys
the bone tissue. NF-κB was confirmed to modulate the ectopic
expression of the FLICE inhibitory protein in RA synovial
fibroblasts, inhibiting the apoptosis of synovial membrane
fibroblasts (34). Inhibition of
the NF-κB signal pathway results in the downregulation of the
expression of specific anti-apoptosis and cytokine genes (35).
In the current study TLR4, NF-κB and MyD88 in
synovial tissue were determined by western blot analysis and
RT-PCR. The expression level of NF-κB was consistent with previous
studies (3,27–29)
and TLR4 and MyD88 were downregulated. This may suggest that TLR4
and NF-κB signal transduction pathways are important in the RA
inflammatory response.
In conclusion, the current results suggest that STBT
is effective for the treatment of RA and modulates the inflammatory
response, potentially by downregulating TLR4, NF-κB and MyD88.
However, further studies are required to determine the
pharmacological effects and molecular mechanisms of STBT.
Acknowledgements
This study was supported by grants from the National
Technology Support Project of China (grant no. 2011BAI01B05); the
major projects of Science and Technology Bureau of Fujian Province
(grant no. 2009YZ0001-1-1); the major project of Fujian Provincial
Health Bureau (grant no. WZZY0902); and the Fujian Province
Science-Technology Plan Projects (grant no. 2010Y2004).
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
STBT
|
shuangtengbitong tincture
|
|
CIA
|
type II collagen-induced arthritis
|
|
TCM
|
traditional Chinese tedicines
|
|
HE
|
hematoxylin and eosin
|
|
TLR4
|
toll-like receptor-4
|
|
MyD88
|
myeloid differentiation factor 88
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Brenner M, Meng HC, Yarlett NC, Joe B,
Griffiths MM, Remmers EF, Wilder RL and Gulko PS: The non-MHC
quantitative trait locus Cia5 contains three major arthritis genes
that differentially regulate disease severity, pannus formation,
and joint damage in collagen- and pristane-induced arthritis. J
Immunol. 174:7894–7903. 2005. View Article : Google Scholar
|
|
2
|
Helliwell PS, Woodburn J, Redmond AC,
Turner DE and Davys HJ: Current concepts in rheumatoid arthritis.
The Foot and Ankle in Rheumatoid Arthritis: A Compehensive Guide.
Churchill Livingstone; Edinburgh: pp. 1–16. 2007
|
|
3
|
Yang M, Xiao C, Wu Q, Niu M, Yao Q, Li K,
Chen Y, Shi C, Chen D, Feng G and Xia C: Anti-inflammatory effect
of Sanshuibaihu decoction may be associated with nuclear
factor-kappa B and p38 MAPK alpha in collagen-induced arthritis in
rat. J Ethnopharmacol. 127:264–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolfe F, Michaud K, Gefeller O and Choi
HK: Predicting mortality in patients with rheumatold arthritis.
Arthritis Rheum. 48:1530–1542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tobón GJ, Youinou P and Saraux A: The
environment, geo-epidemiology, and autoimmune disease: Rheumatoid
arthritis. Autoimmun Rev. 9:A288–A292. 2010.
|
|
6
|
Rat AC and Boissier MC: Rheumatoid
arthritis: direct and indirecteosts. Joint Bone Spine. 71:518–524.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cook DN, Hollingsworth JW Jr and Schwartz
DA: Toll-like receptors and the genetics of innate immunity. Curr
Opin Allergy Clin Immunol. 3:523–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Migita K, Miyashita T, Maeda Y, Nakamura
M, Yatsuhashi H, Kimura H, Ishibashi H and Eguchi K: Toll-like
receptor expression in lupus peripheral blood mononuclear cells. J
Rheumatol. 34:493–500. 2007.PubMed/NCBI
|
|
9
|
Zhang X, Li H, Hu S, Zhang L, Liu C, Zhu
C, Liu R and Li C: Brain edema after intracerebral hemorrhage in
rats: the role of inflammation. Neurol India. 54:402–407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao
RL, Browder W and Williams DL: Modulating Toll-like receptor
mediated signaling by (1→3)-beta-D-glucan rapidly induces
cardioprotection. Cardiovasc Res. 61:538–547. 2004.
|
|
11
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Deng L, Hong M, Akkaraju GR, Inoue
J and Chen ZJ: TAK1 is a ubiquitin-dependent kinase of MKK and IKK.
Nature. 412:346–351. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Deng L, Ea CK, Xia ZP and Chen ZJ:
The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation
by BCL10 and MALT1 in T lymphocytes. Mol Cell. 14:289–301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Y, Commane M, Ninomiya-Tsuji J,
Matsumoto K and Li X: IRAK-mediated translocation of TRAF6 and TAB2
in the interleukin-1-induced activation of NFkappa B. J Biol Chem.
276:41661–41667. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang L, Ninomiya-Tsuji J, Qian Y,
Mastsumoto K and Li X: Interleukin-1 (IL-1) receptor-associated
kinase-dependent IL-1-induced signaling complexes phosphorlate TAK1
and TAB2 at the plasma membrane and activate TAK1 in the cytosol.
Mol Cell Biol. 22:7158–7167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam FF, Ko IW, Ng ES, Tam LS, Leung PC and
Li EK: Analgesic and anti-arthritic effects of Lingzhi and San Miao
San supplementation in a rat model of arthritis induced by Freund’s
complete adjuvant. J Ethnopharmacol. 120:44–50. 2008.PubMed/NCBI
|
|
17
|
Chen L: Bizheng of TCM and sub clinical. J
Chin Med. 3:1162011.(In Chinese).
|
|
18
|
Deng ZZ, He YT and Wang ZQ: Research on
the anti-inflammatory and analgesia effects of fufang leigongteng
film on rheumatoid arthritis. Chinese Journal of Hospital.
13:14–16. 1998.(In Chinese).
|
|
19
|
Liang GX and Duan JM: Clinical effect
observation of Silongsanmiao Powder on 68 rheumatoid arthritis
cases. CJGMTCM. 22:86–87. 2007.(In Chinese).
|
|
20
|
Wang JF, Cai CZ, Kong CY, Cao ZW and Chen
YZ: A computer method for validating traditional Chinese medicine
herbal prescriptions. Am J Chin Med. 33:281–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang SH, Sung HC, Choi Y, Ko SY, Lee BE,
Baek DH, Kim SW and Kim JK: Suppressive effect of AIF, a water
extract from three herbs, on collagen-induced arthritis in mice.
Int Immunopharmacol. 5:1365–1372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Setty AR and Sigal LH: Herbal medications
commonly used in the practice of rheumatology: mechanisms of
action, efficacy, and side effects. Semin Arthritis Rheum.
34:773–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Xu W, Chu K, Chen L and Zhang X:
Determination of triptolide in Bitongling tincture by HPLC. Chin J
Inform Trad Chin Med. 19:51–52. 2012.(In Chinese).
|
|
24
|
Ganesan K, Tiwari M, Balachandran C,
Manohar B and Puvanakrishnan R: Estrogen and testosterone attenuate
extracellular matrix loss in collagen-induced arthritis in rats.
Calcif Tissue Int. 83:354–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu P and Hu Y: Re-understanding of
Tripterygium wilfordii Hook.f. J Chin Integr Med Rheumatol.
5:1691996.(In Chinese).
|
|
26
|
Tu S, Hu Y, Zeng K, Zhang M, Lai X and
Weishen Z: Effects of triptolide on the expression and activity of
NF-kappaB in synovium of collagen-induced arthritis rats. J
Huazhong Univ Sci Technolog Med Sci. 25:543–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Pharmacopoeia Committee. Chinese
Pharmacopoeia. 1st ed. TCM Science and Technology Publishing House;
Beijing: pp. 181–182. 2010, (In Chinese).
|
|
28
|
Fang Y, Wang Y, Zhou X, Zhong B, Bai G and
Zhang C: Effect and mechanism of sinomenine on the signal
transduction of the synovial cell nuclear factor-κB in rats with
adjuvant arthritis. Chin J Clin Rehabil. 9:204–205. 2005.(In
Chinese).
|
|
29
|
Fang Y, Zhang Y and Wang Y: Effects of
sinomenine on svnovioctve apoptosis, cell cycle and change of bcl-2
protein in patients with rheumatoid arthritis. Chinese Remedies
& Clinics. 4:261–264. 2008.
|
|
30
|
National Pharmacopoeia Committee. Chinese
Pharmacopoeia. 1st ed. TCM Science and Technology Publishing House;
Beijing: pp. 250–251. 2010, (In Chinese).
|
|
31
|
Liu Y: Clinical observation of rheumatoid
arthritis treated with Sanxiao ymzi decoction combined with
lefiunomide. J TCM Univ Hunan. 31:17–20. 2011.(In Chinese).
|
|
32
|
Brand DD, Kang AH and Rosloniec EF:
Immunopathogenesis of collagen arthritis. Springer Semin
Immunopathol. 25:3–18. 2003. View Article : Google Scholar
|
|
33
|
Einhorn TA, Buckwalter JA and Sheldon RS:
Orthopaedic Basic Science. People’s Health Publishing House;
Beijing: pp. 987–1204. 2001, (In Chinese).
|
|
34
|
Bai S, Liu H, Chen KH, Eksarko P, Perlman
H, Moore TL and Pope RM: NF-kappaB-regulated expression of cellular
FLIP protects rheumatoid arthritis synovial fibroblasts from tumor
necrosis factor alpha-mediated apoptosis. Arthritis Rheum.
50:3844–3855. 2004. View Article : Google Scholar
|
|
35
|
Osorio Y, Fortéa J, Bukulmez H,
Petit-Teixeira E, Michou L, Pierlot C, Cailleau-Moindrault S,
Lemaire I, Lasbleiz S, Alibert O, Quillet P, Bardin T, Prum B,
Olson JM and Cornélis F: Dense genome-wide linkage analysis of
rheumatoid arthritis, including covariates. Arthritis Rheum.
50:2757–2765. 2004. View Article : Google Scholar : PubMed/NCBI
|