Introduction
Hodgkin’s lymphoma (HL) is a frequently occurring
lymphoid cancer, with an annual incidence rate of three to four
novel cases per 100,000 individuals in the Western population
(1). As HL is an aggressive
malignancy and is difficult to diagnose at advanced stages,
identification of reliable diagnostic markers, regulatory factors
and molecular targets is required, and an understanding of the
mechanism of HL must be developed. Previous studies have indicated
that differentially expressed genes and miRNAs determine the
development, metastasis and therapy of HL (2). HL-associated factors (genes and
miRNA) are also important.
Transcription factors (TFs) and microRNAs (miRNAs)
are prominent regulators of gene expression (3). TFs are specific proteins that
activate or repress the transcription of genes by binding to
cis-regulatory elements located in the upstream regions of
genes (4). Alone or combined with
other proteins, TFs regulate gene expression at the transcriptional
level. miRNAs are small (21–24 nt) noncoding RNA molecules, which
affect gene expression at the post-transcriptional level. As miRNAs
repress the translation or degradation of mRNA, they are also
capable of controlling key cellular processes, including
proliferation, differentiation and apoptosis. A previous study
indicated that miRNAs are implicated in a number of human diseases,
including leukemia, lung cancer and HL (5). Gibcus et al(1) demonstrated that miRNAs are
fundamental in HL by investigating the biogenesis and differential
expression of miRNAs, as well as the corresponding potential
involvement of miRNAs in the pathogenesis, progression and
metastasis.
A number of transcription profiling studies of miRNA
transfection have been conducted to investigate the effect of
miRNAs on transcription levels. These identified that miRNAs exert
a widespread impact on the regulation of their target genes
(targets) (6,7). To date, numerous databases, including
computational predicted methods (8) and experimentally validated databases
(9,10) have provided abundant resources to
investigate the association between miRNAs and their targets.
Host genes are the genes to which miRNAs locate.
Rodriguez et al(11)
indicated that miRNAs are transcribed in parallel with their host
transcripts and two transcription classes of miRNAs, exonic and
intronic, have been identified. Baskerville et al(12) demonstrated a close correlation
between intronic miRNA and its host gene. Intronic miRNA and the
host gene are coordinately expressed in biological progression and
act as potential partners to achieve biological function and affect
the alteration of pathways (13).
Molecular biologists and medical researchers have
identified numerous differentially expressed genes and miRNAs
through a large number of studies on HL (14). However, these studies were are
largely based on a single element, gene or miRNA, rendering it
difficult to analyze the general pathogenesis of HL. In the current
study, the underlying networks of miRNAs, targets of miRNAs, TFs
and host genes of miRNAs were investigated to identify the key
pathways of HL and understand their control mechanisms in HL. Three
types of experimentally validated associations were identified,
including miRNA targets, TFs regulating miRNAs and miRNA host
genes. Differentially expressed elements and HL-associated elements
were manually collected from databases and the literature. A number
of predicted TFs were obtained by the P-match method and were
considered to be HL-associated genes. Three regulatory networks,
differentially expressed, associated and global networks, were
constructed based on the associations observed according to the
degree of correlation between the elements and HL. The global
network was constructed with all the experimentally validated
associations at the time of writing. Due to the complexity of
constructing all pathways associated with HL, the regulatory
associations of differently expressed elements were extracted and
TFs were predicted to complete the pathways. Comparing the
similarities and differences is of significance in distinguishing
key pathways and elements. The regulatory pathways of
differentially expressed elements have an important effect on the
progression of HL and abnormal modulation of the pathways results
in the development of HL.
Materials and methods
Material collection and data
processing
An experimentally validated dataset of human miRNAs
and their targets was extracted from Tarbase 5.0 (http://diana.cslab.ece.ntua.gr/tarbase/)
and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/). The official and
unified symbol of National Center for Biotechnology Information
(NCBI) database was used to unify all miRNAs and genes. The NCBI
database may be accessed online at http://www.ncbi.nlm.nih.gov/gene/. These experimental
data support the current study. The complete data may be located in
supplement file 1 and are designated as set
A1.
All data sets may be obtained by request from
zmhmay@sina.com
A human experimentally-validated dataset of TFs and
miRNAs was extracted from TransmiR (15). Data from TransmiR was obtained from
public literature and biological experiments. The complete data are
designated as set A2.
The host gene of human miRNA was manually extracted
from miRBase (16) and NCBI. The
official symbol and official ID was used to sign each host gene.
The complete data are designated as set A3.
In the current study, the differentially-expressed
genes, including genetic mutation, abnormally expressed protein,
SNPs, over expression genes, downregulated, upregulated and
variation genes, were collected from Cancer Genetics Web
(http://www.cancerindex.org/geneweb/index.html), NCBI
SNP database (http://www.ncbi.nlm.nih.gov/snp/) and the relevant
literature. HL-associated genes were collected from GeneCards
database (17) and relevant
literature, including genes that affect tumor growth, migration,
radiation therapy, prevention, diagnosis, development and the
clinical outcome of human HL (18). Differentially expressed elements
were considered to be associated elements. In addition, 35
important TFs were extracted by the P-match method (19). These were considered to be
HL-associated genes and the TFs included in transmiR were focused
on. Promoter region sequences (1,000 nt) of targets that are
targeted by differentially-expressed miRNAs were downloaded from
the UCSC database (20). The
P-match method, which combines pattern matching and weight matrix
approaches to identify transcription factor binding sites (TFBSs)
in 1,000 nt promoter region sequences, was used and TFBSs were
mapped onto the promoter region of targets. The matrix library of
P-match, as well as sets of known TF-binding sites were collected
in TRANSFAC to search for a large variety of different TF binding
sites. The vertebrate matrix was used with restricted high quality
criterion for the matrix. The complete data are designated as set
A4.
Differentially expressed miRNAs included
overexpressed, downregulated and upregulated miRNAs. Differentially
expressed miRNAs were collected from mir2Disease (21). Relevant literature was manually
observed to extract HL-associated miRNAs. The complete data are
designated as set A5.
Three networks construction
The following method was used to construct the
differentially expressed network, associated network and global
network. All regulatory associations between TFs, miRNAs, targets
and host genes were extracted from A1,
A2 and A3. Following the
combination of all associations, the global network was
constructed. Differentially expressed elements were separately
extracted from A4 and A5 and
the association between differentially expressed elements from the
global network were selected, resulting in the differentially
expressed network. A similar method was used to construct the
related network. The complete data is located for differentially
expressed network in supplement file 6, for the related network in
supplement file 7 and the global network in supplement file 8.
Results
Differentially-expressed network of
HL
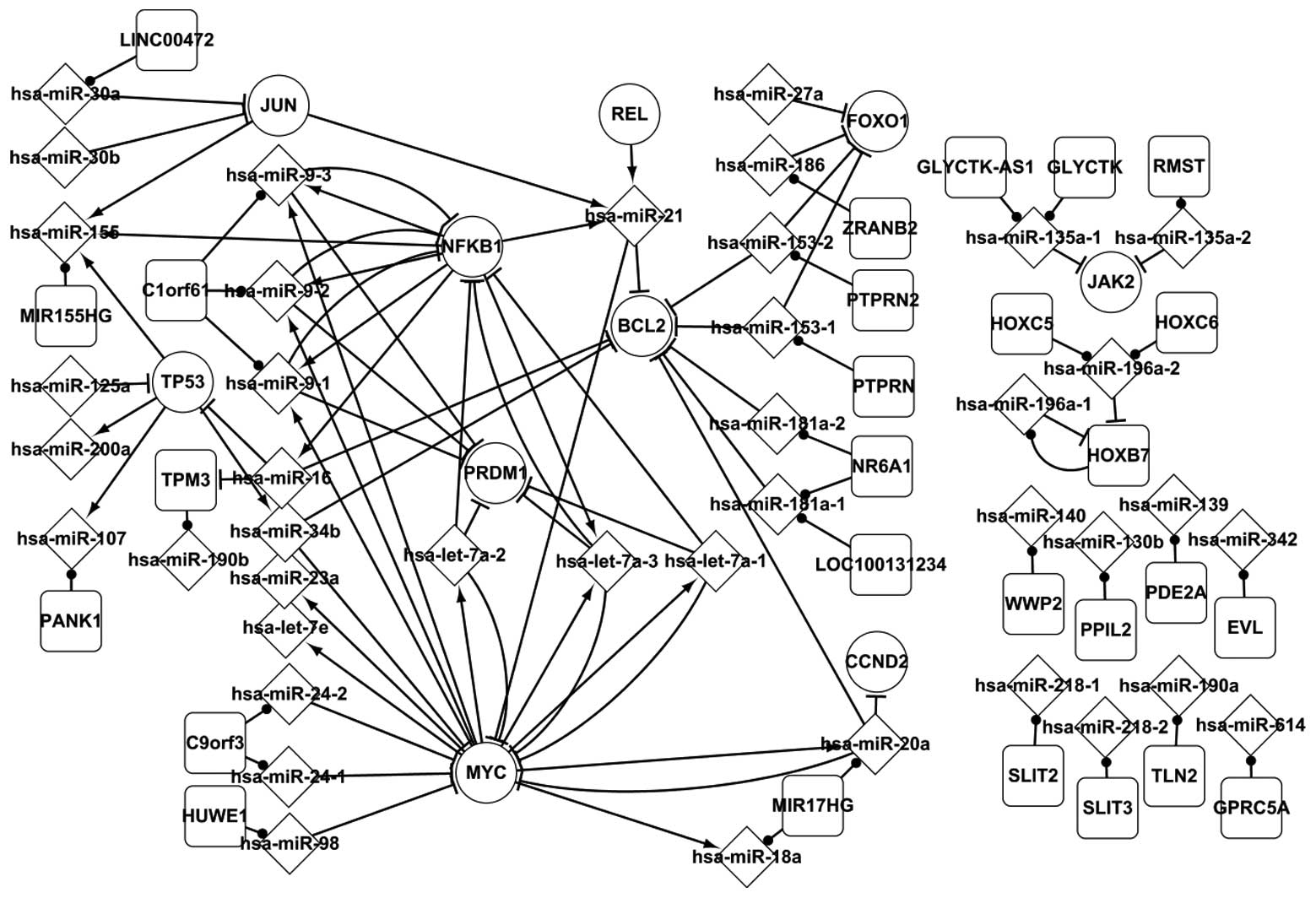
Fig. 1 shows the
significant regulatory associations between differentially
expressed elements in HL. This network is composed of five TFs,
TP53, MYC, NFKB1, JUN and REL, targets of miRNAs, 41 miRNAs and
their host genes. The elements are all differentially-expressed
except from the host genes. Fig. 1
shows three types of associations between each pair in HL, which
are miRNA targets and host genes, including miRNAs and genes
regulating miRNAs. A number of specific features of TFs and miRNAs
are highlighted in Fig. 1. For
example, hsa-miR-98 targets MYC, which regulates has-let-7a (7a-1,
7a-2 and 7a-3) and has-miR-9 (9-1, 9-2 and 9-3). Nie et
al(22) hypothesized that
has-let-7a and has-miR-9 target PRDM1 in HL cell lines, indicating
that MYC indirectly affects PRDM1 by has-let-7a and has-miR-9. A
self-adaptation association was identified between MYC and
has-let-7a, MYC and has-miR-20a and NFκB1 and has-miR-9. A number
of specific features between miRNAs and their host genes are
highlighted in Fig. 1. A host gene
may include one or several miRNAs targeting other genes. For
example, C9orf3 includes hsa-miR-24-1 and hsa-miR-24-2, which
target MYC, CDKN1B, HNF4A and NOTCH1. An miRNA may be located in
one or several genes. For example hsa-miR-181a-1 is located in
NR6A1 and LOC100131234. The differentially expressed network partly
revealed the regulatory mechanism of HL.
Related network of HL
The HL-associated network contains numerous
regulatory correlations between genes and miRNAs. The associated
network includes the differentially-expressed network. The
associated network includes five differentially expressed TFs, 16
additional TFs, 57 miRNAs and a number of additional targets. The
network shows mass additional pathways of genes and miRNAs. For
instance, ZEB1 and GATA3 regulate hsa-miR-200a that, in turn,
regulates ZEB1 and TFRC. SPI1 regulates hsa-miR-17, which targets
BCL2. Gibcus et al(23)
indicated that in HL, high has-miR-17 expression contributes to a
dysfunctional p53 pathway and thereby to the malignant phenotype.
Smolewski et al(24) showed
that BCL2 may be considered to be a novel prognostic factor in
Hodgkin’s disease. E2F1 regulates hsa-miR-25, which targets TP53
(25). Tanzer et
al(26) demonstrated that
hsa-miR-25 is involved in the pathophysiology of HL. Mutation of
TP53 is involved in the pathogenesis of HL (27). The associated network expands
additional topology associations of differentially expressed
elements and contributes to the understanding of the progression of
HL.
Global network of HL
The global network includes more comprehensive
regulatory correlations that are from the three sets,
A1, A2 and
A3, and is an experimentally validated biological
network in the human body. The global network includes the
differentially expressed and associated networks.
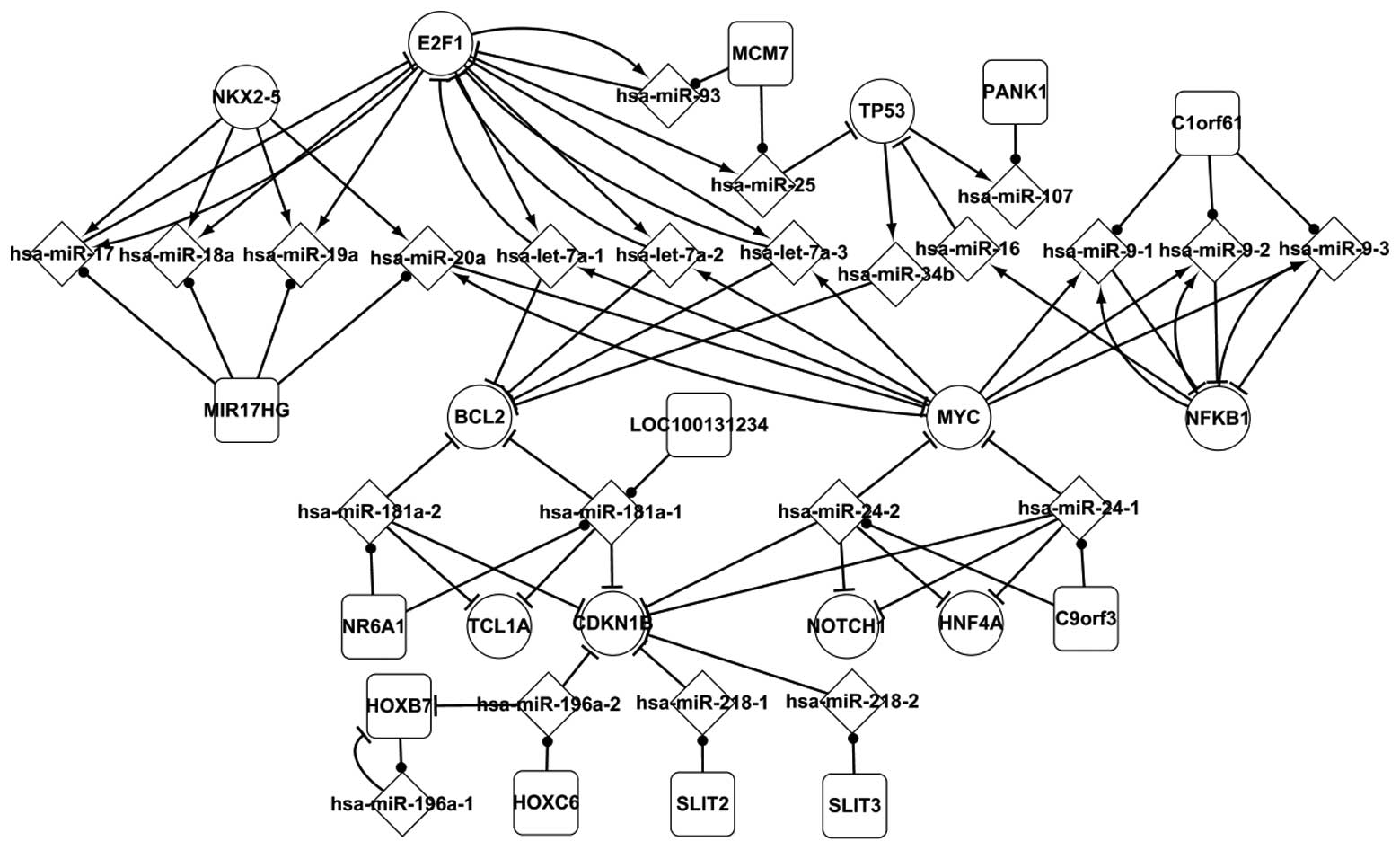
Host genes and the associated miRNAs in
HL
In the current study, the host genes and their
miRNAs showed important characteristics. Although host genes are
not differentially expressed in HL, they were considered as
differentially expressed genes if their miRNAs were differentially
expressed. Numerous associations between host genes and other
elements are shown in Fig. 2.
MIR17HG includes four miRNAs, hsa-miR-17, hsa-miR-18a, hsa-miR-19a
and hsa-miR-20a, which are regulated by NKX2-5 and E2F1. Hsa-miR-17
and hsa-miR-20a separately form a self-adaptation association with
E2F1 and MYC. MCM7 includes two miRNAs, hsa-miR-93 and hsa-miR-25,
which are regulated by E2F1. C1orf61 includes hsa-miR-9, which
forms a self-adaptation association with NFKB1. NR6A1 includes
hsa-miR-181a-1 and hsa-miR-181a-2, and combined they target BCL2,
TCL1A and CDKN1B. Notably, HOXB7 includes hsa-miR-196a-1 and it is
targeted by hsa-miR-196a-1. Host genes are important for
determination of associations linking specific miRNAs and may aid
in understanding the pathology of HL.
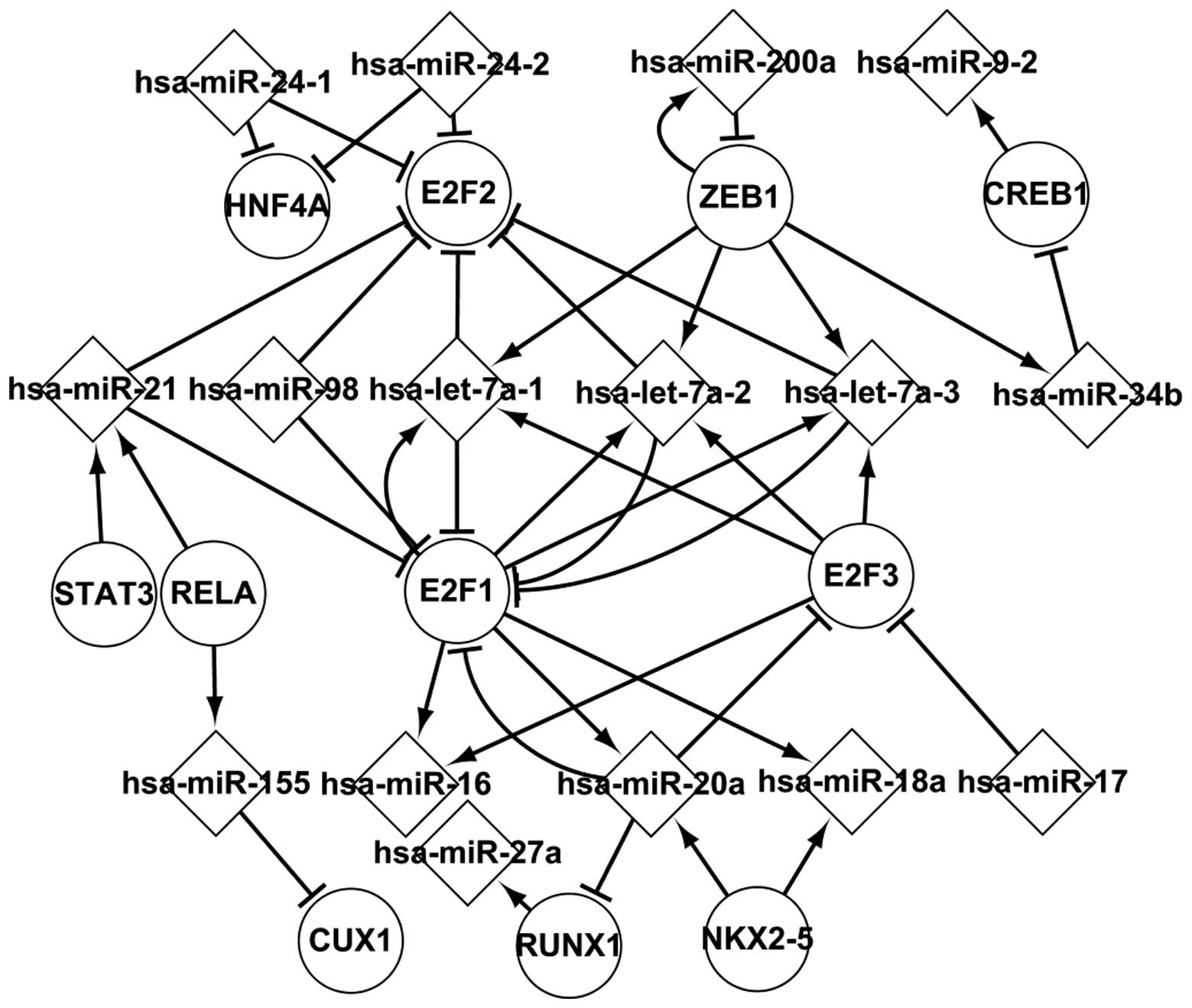
Transcriptional network of predicted
TFs
Sixteen differentially expressed miRNAs, which are
regulated by predicted TFs, were analyzed further. Fig. 3 shows the regulatory associations
of predicted TFs and differentially-expressed miRNAs in HL. These
elements sequentially affect their successors by targeting or
regulating them. Fig. 3 shows that
ZEB1, E2F1 and E2F3 coregulate has-let-7a, which targets E2F2. From
Fig. 3 it is possible to conclude
that a differentially expressed miRNA may target several TFs, a TF
may regulate several differentially expressed miRNAs, a TF
indirectly affects other TFs by several differentially expressed
miRNAs and a differentially expressed miRNA indirectly affects
other miRNAs by TF. For example, hsa-miR-20a targets E2F1, E2F3 and
RUNX1; ZEB1 regulates hsa-miR-200a, hsa-miR-34b and hsa-miR-7a;
NKX2-5 indirectly regulates RUNX1 by hsa-miR-20a; and hsa-miR-17
indirectly affects hsa-miR-16 by E2F3. The transcription network of
predicted TFs and miRNAs is important for the analysis of the
pathogenesis of HL.
Regulatory associations of
differentially-expressed genes
To describe the network of HL more clearly, the
upstream and downstream information of differentially expressed
elements, genes and miRNAs, were extracted and compared, and the
TFs were predicted from the P-match method. The successor nodes and
precursor nodes of the differentially expressed genes from the
three networks were extracted, classed and listed. Specific
regulatory associations were highlighted from the list. Among these
genes, MYC and NFκB1 revealed an important characteristic involving
specific common precursor and on elements in the successor nodes,
indicating that the gene and miRNAs form a self-adaptation
association. The complete data may be identified in supplement file
9.
TFs were selected, the first class of which, had six
types of adjacent nodes (three types of successors and three types
of predecessors). This type of TF includes JUN, MYC, NFκB1 and
TP53. The subsequent experiments focused on MYC.
In Table I, the
precursors and successors of MYC in the differentially-expressed,
associated and global networks are listed. MYC is a notable tumor
suppressor and has significant features in the three networks.
Table I shows that nine miRNAs
target MYC and MYC regulates nine miRNAs in the differentially
expressed network. In the present study it has been hypothesized
that the precursors of MYC indirectly affect the successors
expression via MYC. Hsa-miR-20a and hsa-let-7a target MYC and MYC
regulates them in return, forming a self-adaptation correlation.
Supplement file 9 shows that MYC indirectly affects other genes via
a number of miRNAs. For example, MYC regulates hsa-miR-17, which
targets BCL2. Numerous TFs also indirectly affect MYC via specific
miRNAs. For example, TP53 and NFκB1 regulate hsa-miR-34, which
targets MYC.
 | Table IRegulatory associations between miRNAs
and MYC in Hodgkin’s lymphoma. |
Table I
Regulatory associations between miRNAs
and MYC in Hodgkin’s lymphoma.
| A. miRNAs that target
MYC in the three networks. |
|---|
|
|---|
| Differentially
expressed network | Associated
network | Global network |
|---|
| let-7a-1 | let-7a-1 | let-7a-1 |
| let-7a-2 | let-7a-2 | let-7a-2 |
| let-7a-3 | let-7a-3 | let-7a-3 |
| miR-20a | miR-20a | miR-20a |
| miR-21 | miR-21 | miR-21 |
| miR-24-1 | miR-24-1 | miR-24-1 |
| miR-24-2 | miR-24-2 | miR-24-2 |
| miR-34b | miR-34b | miR-34b |
| miR-98 | miR-98 | miR-98 |
| miR-17 | miR-17 |
| | let-7g |
| | miR-145 |
| | miR-26a |
| | miR-34a |
| | miR-30c-5p |
| | miR-378 |
|
| B. miRNAs regulated
by MYC in the three networks. |
|
| Differentially
expressed network | Associated
network | Global network |
|
| let-7a-1 | let-7a-1 | let-7a-1 |
| let-7a-2 | let-7a-2 | let-7a-2 |
| let-7a-3 | let-7a-3 | let-7a-3 |
| miR-20a | miR-20a | miR-20a |
| miR-18a | miR-18a | miR-18a |
| miR-23a | miR-23a | miR-23a |
| miR-9-1 | miR-9-1 | miR-9-1 |
| miR-9-2 | miR-9-2 | miR-9-2 |
| miR-9-3 | miR-9-3 | miR-9-3 |
| miR-17 | miR-17 |
| miR-19a | miR-19a |
| miR-19b | miR-19b |
| miR-106a | miR-106a |
| miR-106a | miR-106a |
| miR-106a | miR-106a |
| miR-93 | miR-93 |
| | miR-16-1 |
| | miR-34a |
| | miR-23b |
| | miR-141 |
| | miR-221 |
| | miR-195 |
| | miR-22 |
| | miR-29a |
| | miR-29c |
| | miR-26b |
| | miR-26a |
| | let-7b, let-7c |
| | let-7d, let-7e |
| | let-7f, let-7g |
| | let-7i |
| | miR-15a |
| | miR-20b |
| | miR-106b |
| | miR-29b |
The second class of TF had three types of adjacency
nodes (three types of successors), including REL. There is no miRNA
targeting REL and it regulates hsa-miR-21 in three networks.
In addition, the remaining genes that were not TFs
were investigated. The first class of genes had three types of
adjacency nodes, (three types of predecessors), including BCL2,
CCND2, FGFR3, FOXO1, JAK2 and PRDM1. The adjacency nodes were
identified to be targeted by specific miRNAs and did not regulate
any miRNA.
The second class of gene, CD44, had a type of
adjacency node in three networks, which is a type of predecessor.
It is hypothesized that CD44 has the least effect compared with
other differentially expressed genes.
Regulatory associations of differentially
expressed miRNAs
The regulatory associations of each differentially
expressed miRNA were extracted, compared and analyzed by the same
method as differentially expressed genes. The complete data may be
located in supplement file 10. Among these miRNAs, seven
differentially expressed miRNAs, hsa-let-7a-1, hsa-let-7a-2,
hsa-let-7a-3, hsa-miR-20a, hsa-miR-9-1, hsa-miR-9-2 and hsa-miR-9-3
and corresponding genes, form self-adaptation.
The first class of miRNA had six types of adjacent
nodes, three types of predecessors and three types of successors,
including hsa-miR-20a and hsa-miR-34b. The subsequent experiments
focused on hsa-miR-20a.
In Table II, the
precursors and successors of hsa-miR-20a in the differentially
expressed, associated and global networks are listed. Table II shows that MYC regulates
hsa-miR-20a, as well as targeting three genes in the differentially
expressed network. MYC has been concluded to indirectly affect MYC,
BCL2 and CCND2 expression through hsa-miR-20a. Table II shows that MYC and hsa-miR-20a,
CCND1 and hsa-miR-20a and E2F1 and hsa-miR-20a separately form
self-adaptation. Supplement file 10 shows that hsa-miR-20a also
indirectly affects other miRNAs by specific TFs, for example
hsa-miR-20a targets E2F1 that regulates hsa-let-7a, hsa-miR-16 and
hsa-miR-18a. A number of miRNAs also indirectly affect hsa-miR-20a
by targeting certain TFs, for example hsa-miR-155 targets SPI1,
which regulates hsa-miR-20a.
 | Table IIRegulatory relations between
hsa-miR-20a and genes in Hodgkin’s lymphoma. |
Table II
Regulatory relations between
hsa-miR-20a and genes in Hodgkin’s lymphoma.
| A. Genes that
regulate hsa-miR-20a in the three networks. |
|---|
|
|---|
| Differentially
expressed network | Related
network | Global network |
|---|
| MYC | MYC | MYC |
| NKX2-5 | NKX2-5 |
| CCND1 | CCND1 |
| E2F1 | E2F1 |
| SPI1 | SPI1 |
| | TLX1 |
| | TLX3 |
| | ERS1 |
| | STAT5 |
|
| B. Genes targeted
by the hsa-miR-20a in the three networks. |
|
| Differentially
expressed network | Related
network | Global network |
|
| MYC | MYC | MYC, MAP3K12 |
| BCL2 | BCL2 | BCL2, WEE1 |
| CCND2 | CCND2 | CCND2, RB1 |
| E2F1 | E2F1, CDKN1A |
| E2F3 | E2F3, HIF1A |
| RBL1 | RBL1, RBL2 |
| CCND1 | CCND1, APP |
| RUNX1 | RUNX1, NRAS |
| | SMAD4, PTEN |
| | MUC17, BMPR2 |
| | VEGFA, BNIP2 |
| | MAPK9, TGFBR2 |
| | MEF2D, THBS1 |
The second class of miRNA had five types of
adjacency nodes (two types of successors and three types of
predecessors, three types of successors and two types of
predecessors), including hsa-miR-155 and hsa-miR-27a.
The third class of miRNA had four types of adjacency
nodes, (three types of successors and a type of predecessor, two
types of successors and two types of predecessors and a type of
successor and three types of predecessors), including hsa-miR-125a
and hsa-let-7e.
The fourth class of miRNA had three types of
adjacency nodes (three types of successors, two types of successors
and a kind of predecessor and a type of successor and two types of
predecessors), including hsa-miR-98, hsa-miR-130b and
hsa-miR-206.
The fifth class of miRNA had two types of adjacency
nodes (two types of successors or a type of successor and a type of
predecessor), including hsa-miR-150 and hsa-miR-140.
The sixth class of miRNA had a type of adjacency
node (a type of successor), including hsa-miR-196a-1.
Regulatory associations of predicted
transcription factors
Using the aforementioned method, predicted TF data
from the P-match method was processed. The complete data may be
located in supplement file 10. Two TFs, E2F1 and ZEB1, and
corresponding miRNAs were identified to form self-adaptation
associations.
The first class of TF had six types of adjacent
nodes (three types of successors and three types of predecessors),
including CREB1, E2F1, E2F3, RUNX1 and ZEB1. The subsequent
experiments focused on E2F1.
In Table III, the
precursors and successors of E2F1 in the differentially expressed,
associated and global networks are listed. There were six
differentially expressed miRNAs that target E2F1 and E2F1 regulates
six differentially expressed miRNAs. Four of which, hsa-let-7a-1,
hsa-let-7a-2, hsa-let-7a-3 and hsa-miR-20a, separately form
self-adaptation with E2F1 in differentially expressed networks.
 | Table IIIRegulatory relations between miRNAs
and E2F1 in Hodgkin’s lymphoma. |
Table III
Regulatory relations between miRNAs
and E2F1 in Hodgkin’s lymphoma.
| A. miRNAs that
target E2F1 in the three networks. |
|---|
|
|---|
| Differentially
expressed network | Related
network | Global network |
|---|
| let-7a-1 | let-7a-1 | let-7a-1 |
| let-7a-2 | let-7a-2 | let-7a-2 |
| let-7a-3 | let-7a-3 | let-7a-3 |
| miR-20a | miR-20a | miR-20a |
| miR-21 | miR-21 | miR-21 |
| miR-98 | miR-98 | miR-98 |
| miR-106a | miR-106a |
| miR-17 | miR-17 |
| miR-93 | miR-93 |
| | miR-34a |
| | miR-106b |
| | miR-126 |
| | miR-223 |
| | miR-330 |
| | miR-23b |
| | miR-149 |
|
| B. miRNAs regulated
by E2F1 in the three networks. |
|
| Differentially
expressed network | Related
network | Global network |
|
| let-7a-1 | let-7a-1 | let-7a-1 |
| let-7a-2 | let-7a-2 | let-7a-2 |
| let-7a-3 | let-7a-3 | let-7a-3 |
| miR-16 | miR-16 | miR-16 |
| miR-18a | miR-18a | miR-18a |
| miR-20a | miR-20a | miR-20a |
| miR-106a | miR-106a |
| miR-17 | miR-17 |
| miR-19a | miR-19a |
| miR-19b | miR-19b |
| miR-25 | miR-25 |
| miR-92a | miR-92a |
| miR-93 | miR-93 |
| | miR-15a |
| | miR-15b |
| | miR-195 |
| | miR-106b |
| | let-7i |
| | miR-18b |
| | miR-20b |
| | miR-223 |
| | miR-363 |
| | miR-449a |
| | miR-449b |
| | miR-449c |
The second class of TF had four types of adjacent
nodes (three types of successors and a type of predecessors),
including STAT3 and RELA.
The third class of TF had three types of adjacent
nodes (three types of successors or three types of predecessors),
including NKX2-5 and HNF4A.
The fourth class of TF had two types of adjacent
nodes (two types of successors or a type of successors and a type
of predecessor), including HOXA5 and YY1.
The fifth class of TF has one adjacent node (a type
of successor or a type of predecessor), including TCF3 and
PLAU.
Discussion
The present study highlights a number of important
pathways of differentially-expressed genes, differentially
expressed miRNAs and predicted TFs in HL.
For the differentially-expressed elements, the
current results show numerous important pathways, including three
or more elements. For example, hsa-miR-30a targets JUN and JUN
regulates hsa-miR-21. These pathways exhibit a key biological
function in HL. Certain pathways have not only been observed in HL,
but also in other carcinomas. For example, hsa-miR-16 induces
apoptosis by targeting BCL2 in chronic lymphocytic leukemia
(28). Hsa-miR-16 was hypothesized
to target the BCL2 pathway, thus, the role of hsa-miR-16 requires
further investigation in studies of carcinoma. Pathways in HL have
been observed to be associated with the progression of various
other carcinomas. For example, hsa-miR-19a targets BCL2L11 in
T-cell acute lymphoblastic leukemia (29).
The TFs predicted from the P-match method indicate
the potential associations between the differentially expressed
miRNAs and TFs. Further studies are required to validate the close
associations between HL and miRNAs and TFs. For the pathways of
predicted TFs, a number have been identified in other carcinomas,
for example, NKX2-5 targets miR-17-92 and concomitantly reduces
E2F1, thereby enhancing the survival rate of leukemic T-cells
(30). This is likely to enable
further knowledge of the linkage between genes in various
carcinomas and highlight current gaps in research in the pathology
of HL.
In the present study, three regulatory topological
networks of elements involved in HL were constructed. Three figures
were extracted from the networks to highlight certain important
pathways and elements. To further investigate the network, the
successors and precursors of the elements in the three networks
were investigated. The entire experimentally validated data of HL
are presented in the current study. A number of were formed with
the P-match method, which provides specific starting points for
further studies of HL. Future studies are likely to focus on the
interaction between proteins and the regulatory patterns
(upregulation and downregulation) enabling the construction of a
comprehensive and extensive network of HL.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 60973091 and
60905022).
Abbreviations:
|
miRNA
|
microRNA
|
|
TFs
|
transcription factors
|
|
targets
|
target genes
|
|
HL
|
Hodgkin’s lymphoma
|
|
NCBI
|
National Center for Biotechnology
Information
|
|
TFBSs
|
transcription factor binding sites
|
References
|
1
|
Gibcus JH, Tan LP, Harms G, et al: Hodgkin
lymphoma cell lines are characterized by a specific miRNA
expression profile. Neoplasia. 11:167–176. 2009.PubMed/NCBI
|
|
2
|
Xie L, Ushmorov A, Leithäuser F, et al:
FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood.
119:3503–3511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran DH, Satou K, Ho TB and Pham TH:
Computational discovery of miR-TF regulatory modules in human
genome. Bioinformation. 4:371–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M, Li J, Ding X, He M and Cheng SY:
microRNA and cancer. AAPS J. 12:309–317. 2010. View Article : Google Scholar
|
|
6
|
Naeem H, Küffner R and Zimmer R: MIRTFnet:
analysis of miRNA regulated transcription factors. PLoS One.
6:e225192011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao F, Zuo Z, Cai G, et al: miRecords: an
integrated resource for microRNA-target interactions. Nucleic Acids
Res. 37(Database issue): D105–D110. 2009.PubMed/NCBI
|
|
8
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA. org resource: targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008.PubMed/NCBI
|
|
9
|
Papadopoulos GL, Reczko M, Simossis VA,
Sethupathy P and Hatzigeorgiou AG: The database of experimentally
supported targets: a functional update of TarBase. Nucleic Acids
Res. 37:D155–D158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu SD, Lin FM, Wu WY, et al: miRTarBase:
a database curates experimentally validated microRNA-target
interactions. Nucleic Acids Res. 39:D163–D169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baskerville S and Bartel DP: Microarray
profiling of microRNAs reveals frequent coexpression with
neighboring miRNAs and host genes. RNA. 11:241–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao G, Huang B, Liu Z, et al: Intronic
miR-301 feedback regulates its host gene, ska2, in A549 cells by
targeting MEOX2 to affect ERK/CREB pathways. Biochem Biophys Res
Commun. 396:978–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gibcus JH, Tan LP, Harms G, et al: Hodgkin
lymphoma cell lines are characterized by a specific miRNA
expression profile. Neoplasia. 11:167–176. 2009.PubMed/NCBI
|
|
15
|
Wang J, Lu M, Qiu C and Cui Q: TransmiR: a
transcription factor-microRNA regulation database. Nucleic Acids
Res. 38:D119–D122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozomara A and Griffiths-Jones S: miRBase:
integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39:D152–D157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Safran M, Dalah I, Alexander J, et al:
GeneCards Version 3: the human gene integrator. Database (Oxford).
2010:baq0202010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nature Protoc. 4:44–57. 2009.PubMed/NCBI
|
|
19
|
Chekmenev DS, Haid C and Kel AE: P-Match:
transcription factor binding site search by combining patterns and
weight matrices. Nucleic Acids Res. 33:W432–W437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita PA, Rhead B, Zweig AS, et al: The
UCSC Genome Browser database: update 2011. Nucleic Acids Res.
39:D876–D882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Q, Wang Y, Hao Y, et al:
miR2Disease: a manually curated database for microRNA deregulation
in human disease. Nucleic Acids Res. 37:D98–D104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nie K, Gomez M, Landgraf P, et al:
MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in
Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in
Hodgkin lymphomas. Am J Pathol. 173:242–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibcus JH, Kroesen BJ, Koster R, et al:
MiR-17/106b seed family regulates p21 in Hodgkin’s lymphoma. J
Pathol. 225:609–617. 2011.PubMed/NCBI
|
|
24
|
Smolewski P, Niewiadomska H, Błonski JZ,
Robak T and Krykowski E: Expression of proliferating cell nuclear
antigen (PCNA) and p53, bcl-2 or C-erb B-2 proteins on
Reed-Sternberg cells: prognostic significance in Hodgkin’s disease.
Neoplasma. 45:140–147. 1998.
|
|
25
|
Massip A, Arcondéguy T, Touriol C, et al:
E2F1 activates p53 transcription through its distal site and
participates in apoptosis induction in HPV-positive cells. FEBS
Lett. 587:3188–3194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanzer A and Stadler PF: Molecular
evolution of a microRNA cluster. J Mol Biol. 339:327–335. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feuerborn A, Möritz C, Von Bonin F,
Dobbelstein M, Trümper L, Stürzenhofecker B and Kube D:
Dysfunctional p53 deletion mutants in cell lines derived from
Hodgkin’s lymphoma. Leuk Lymphoma. 47:1932–1940. 2006.PubMed/NCBI
|
|
28
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye H, Liu X, Lv M, et al: MicroRNA and
transcription factor co-regulatory network analysis reveals miR-19
inhibits CYLD in T-cell acute lymphoblastic leukemia. Nucleic Acids
Res. 40:5201–5214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagel S, Venturini L, Przybylski GK, et
al: Activation of miR-17-92 by NK-like homeodomain proteins
suppresses apoptosis via reduction of E2F1 in T-cell acute
lymphoblastic leukemia. Leuk Lymphoma. 50:101–108. 2009. View Article : Google Scholar : PubMed/NCBI
|