Introduction
Oligodendrogliomas, which are predominantly composed
of cells that morphologically resemble oligodendrocytes, are the
third most common type of glioma, comprising 2–5% of primary brain
tumors and 4–15% of all gliomas (1,2). The
actual frequency of oligodendrogliomas may have been largely
underestimated due to a of lack of specific markers for tumoral
oligodendrocytes (1). As these
tumors are capable of affecting regions of the brain that control
speech, vision and motor functions, surgery may be associated with
the risk of disability. Thus, an understanding of their molecular
pathogenesis may provide important alternative therapeutic options
(2). Furthermore, using current
treatment strategies, oligodendrogliomas are considered to be
incurable (3). The standard
chemotherapy regimen for patients with oligodendroglioma involves a
combination treatment with procarbazine, lomustine and vincristine.
Each of these drugs was developed several decades ago (4,5), and
there is an urgent requirement for novel drugs or therapies
(6).
The endothelin (ET) family includes three 21-amino
acid peptides, ET-1, ET-2 and ET-3, that are released from their
large precursor peptides, preproETs, by the action of ET-converting
enzymes. ETs induce their effects via the stimulation of two G
protein-coupled receptors: ET A receptor (ETAR) and ET B receptor
(ETBR) (7). ETAR preferentially
binds to ET-1, while ETBR binds to all three ETs with a similar
affinity (1). ETAR mRNA
appears to have a restricted distribution and is predominantly
expressed in the vascular smooth muscle cells of peripheral
tissues, bronchial smooth muscle cells, myocardium and the
pituitary gland. By contrast, ETBR mRNA is more widely
distributed, with prominent expression in the brain, pedominantly
in glial cells (1). Previous
studies have demonstrated the expression of the ET system in
various types of gliomas (8).
Egidy et al(9) reported
that while ETAR was highly expressed in glioblastoma vessels and in
certain scattered glioblastoma areas, ETBR was predominantly
identified in tumor cells. Paolillo et al(10) revealed that human glioblastoma cell
lines were capable of releasing ET-1 and expressing functional
ETBR, while ETAR appeared to be absent or barely detectable.
Paolillo et al(11)
demonstrated that selective ETBR, but not ETAR, antagonists blocked
proliferation in astrocytoma cells. Anguelova et al(1) reported that ETBR was predominantly
detected in human oligodendrogliomas rather than in glioblastomas.
The findings suggest that ETBR is important in glioma pathogenesis,
particularly, oligodendrogliomas. In this study, the role of ETBR
in oligodendroglioma proliferation and survival was investigated
in vitro and in vivo.
Materials and methods
Cells line, reagents and mice
The Hs683 human glioma cell line (HTB-138) was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Human ETBR cDNA was subcloned into a
pcDNA3.1 expression vector. ETBR (sc-270098-V) short hairpin
RNA (shRNA) lentiviral particles, control shRNA lentiviral
particles-A (sc-108080) and the anti-ETBR (N-21) (sc-21199)
antibody were purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). The anti-Ki67 antibody (ab15580) was purchased from Abcam
(Hong Kong, China). BQ123, BQ788, G418, puromycin, extracellular
signal-regulated kinases-1 and 2 (ERK1/2) inhibitor U0126 and
anti-ERK (M3807) and anti-phosphorylated ERK antibodies were
purchased from Sigma (St. Louis, MO, USA). The Superfect™
transfection reagent was purchased from Qiagen (Valencia, CA, USA).
The methlythiazoletetrazolium (MTT) cell proliferation and
viability assay kit was purchased from R&D Systems
(Minneapolis, MN, USA). Eight-week-old BALB/C female nude mice were
purchased from Central South University (Changsha, China) and were
housed at the Xiangya Hospital BioResources Centre (Changsha,
China).
Transfection and lentiviral
transduction
The ETBR expression construct was transfected into
Hs683 cells using the Superfect transfection reagent according to
the manufacturer’s instructions. Pools of stable transfectants were
generated via selection with G418 (1.25 mg/ml). ETBR shRNA
lentiviral particles contain expression constructs, which encode a
target-specific 19–25 nt (plus hairpin) shRNA, designed to
specifically knockdown ETBR gene expression. Control shRNA
lentiviral particles contained a scrambled shRNA sequence that did
not lead to the degradation of any cellular mRNA, and was used as a
negative control for ETBR shRNA lentiviral particles. Lentiviral
transduction was performed in Hs683 cells. Pools of stable
transductants were generated via selection with puromycin (5 μg/ml)
according to the manufacturer’s instructions (Santa Cruz
Biotechnology).
In vitro cell proliferation assay
In vitro cell proliferation was determined
using the MTT cell proliferation and viability assay kit, according
to the manufacturer’s instructions. Cells were cultured at a
density of 5×103 cells per well in 96-well tissue
culture plates and were incubated at 37ºC for 24 h. At the end of
the culture period, the cells were washed with phosphate-buffered
saline, MTT reagents were added according to the manufacturer’s
instructions and the absorbance was measured at 570 nm using an
enzyme-linked immunosorbent assay plate reader (Hongda Biotech,
Shanghai, China). Each experiment was repeated three times.
Establishment of orthotopic xenograft
oligodendroglioma mouse models
In vivo nude mice xenografts were obtained by
grafting 1,000,000 Hs683 cells to the left temporal lobes of the
nude mice, as described previously (12). For experiments without BQ123 or
BQ788 treatment, the mice were divided into four experimental
groups: i) the vector control (VC) group (n=9), where the mice were
grafted with cells stably transfected with the empty pcDNA3.1
vector; ii) the ETBR group (n=9), where the mice were grafted with
cells stably transfected with the pcDNA-ETBR expression vector;
iii) the scramble control (SC) group (n=9), where the mice were
grafted with cells stably transduced with SC shRNA; and iv) the
ETBR-shRNA group (n=18), where the mice were grafted with cells
stably transduced with ETBR shRNA. For experiments with BQ123 or
BQ788 treatment, the mice were grafted with Hs683 cells into the
left temporal lobes on day zero, and subsequently injected
intratumorally with saline, BQ123 or BQ788 (50 μg/mouse/day in 0.1
ml saline) for five consecutive days from days five to nine. The
mice were divided into three experimental groups: i) The normal
control (NC) group (n=9), where the mice were grafted with normal
Hs683 cells and subsequently received an intratumoral injection of
saline; ii) the NC+BQ123 group (n=9), where the mice were grafted
with normal Hs683 cells and subsequently treated with an
intratumoral injection of BQ123; and iii) The NC+BQ788 group
(n=18), where the mice were grafted with normal Hs683 cells and
subsequently treated with an intratumoral injection of BQ788. The
mice were monitored three times per week for signs of distress
until they died or were sacrificed by cervical dislocation when the
mice showed dyspnea, abnormal posture, >20% body weight loss,
difficulty with ambulation or any other clinical sign of
progressive disease resulting in significant pain or distress
according to the institutional guidelines of Xiangya Hospital
(Central South University, Changsha, China). Twenty-seven days
following grafting, all mice in the VC, SC, ETBR, NC and NC+BQ123
groups (n=9 each group) had died or been sacrificed, and half of
the mice in the ETBR-shRNA and the NC+BQ788 groups (n=9 each group)
were sacrificed for the purpose of comparing immunohistochemical
Ki67 staining with that of the other groups. The other half of the
ETBR-shRNA and the NC+BQ788 groups (n=9 each group) were monitored
until they had died or been sacrificed. All animal care, breeding,
and surgical procedures were approved by the Laboratory Animal
Users Committee at Xiangya Hospital, Central South University,
Changsha, China.
Western blot analysis
Cells were lysed in 250 μl 2× sodium dodecyl sulfate
(SDS) loading buffer (62.5 mm Tris-HCl, pH 6.8; 2% SDS, 25%
glycerol, 0.01% bromphenol blue, 5% 2-mercaptoethanol) and
incubated at 95ºC for 10 min. Equal quantities of proteins (100 μg)
for each sample were separated by 8–15% SDS-polyacrylamide gel and
blotted onto a polyvinylidene difluoride microporous membrane
(Millipore, Billerica, MA, USA). Membranes were incubated for 1 h
with a 1:500 dilution of primary antibody (anti-ETBR; N-21;
Sc-21199; Santa Cruz Biotechnology) and subsequently washed and
revealed using secondary antibodies with horseradish peroxidase
conjugate (1:5,000, 1 h; anti-goat IgG horseradish peroxidase
conjugate; Millipore). The peroxidase was revealed using a GE
Healthcare enhanced chemiluminescence kit (Beijing China). Proteins
were quantified prior to being loaded onto the gel.
Immunohistochemistry
Paraffin-embedded tumor tissues were examined for
Ki67 staining. Briefly, sections (5 μm) of paraffin-embedded
specimens were de-paraffinized in xylene, hydrated in a degraded
series of ethanol and heated in 0.01 M citrate buffer for 10 min in
a microwave oven (Haier, Beijing, China). After cooling for 20 min
and washing in PBS, endogenous peroxidase was blocked with methanol
containing 0.3% H2O2 for 30 min, followed by
incubation with PBS for 30 min. Subsequently, the sections were
incubated with the anti-Ki67 antibody at a dilution of 1:200, and
stained using the avidin-biotin complex method. Coloration was
developed by DAB containing H2O2, and the
sections were counter-stained with hematoxylin. The number of
positive cells was counted in ten high-power view fields and the
percentage was calculated as follows: Ki67 positive cells/total
tumor cells × 100. The slides were independently examined by two
pathologists blinded to the experimental group assignment
information. Cohen’s κ coefficient was calculated in order to
demonstrate interobserver variability. Cohen’s κ coefficient was
0.90 in this study.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 10.0 (IBM, Chicago, IL, USA). Data values were expressed as
the means ± SD. Comparisons of means among multiple groups were
performed using one-way analysis of variance followed by post
hoc pairwise comparisons using Tukey’s tests. A log-rank test
was used to compare the survival times of the mice, defined as the
time to death or sacrifice. The median and mean survival times
within each experimental group were estimated using the
Kaplan-Meier product-limit method. A two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
Western blot analysis
The Hs683 human glioma cell line, whose
oligodendroglial origin has been extensively characterized
(13), was used in this study as a
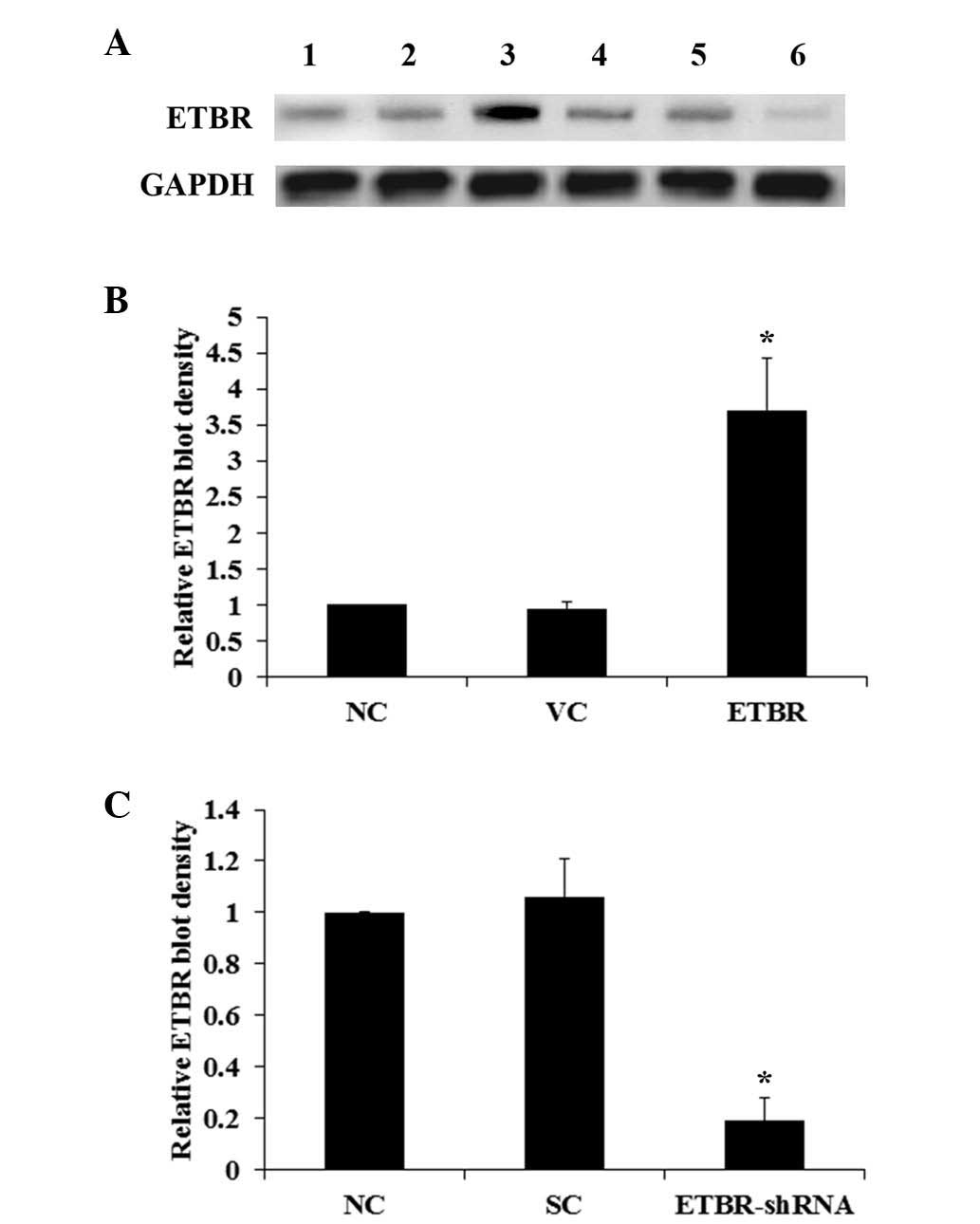
cell model of oligodendroglioma. Western blot analyses revealed
that ETBR was expressed in Hs683 cells (Fig. 1A). Cells were stably transfected
with an ETBR expression vector in order to overexpress ETBR and the
cells stably transduced with ETBR-shRNA in order to knockdown
endogenous ETBR. Compared with the controls, the ETBR level in the
Hs683 cells was overexpressed ~2.5 fold (Fig. 1B) and knocked down by >80%
(Fig. 1C). Endogenous expression
of ETs, ECE-1 and ETAR was also detected in Hs683 cells, the levels
of which were unaltered by the overexpression or knockdown of ETBR
in the cells (data not shown).
MTT assays
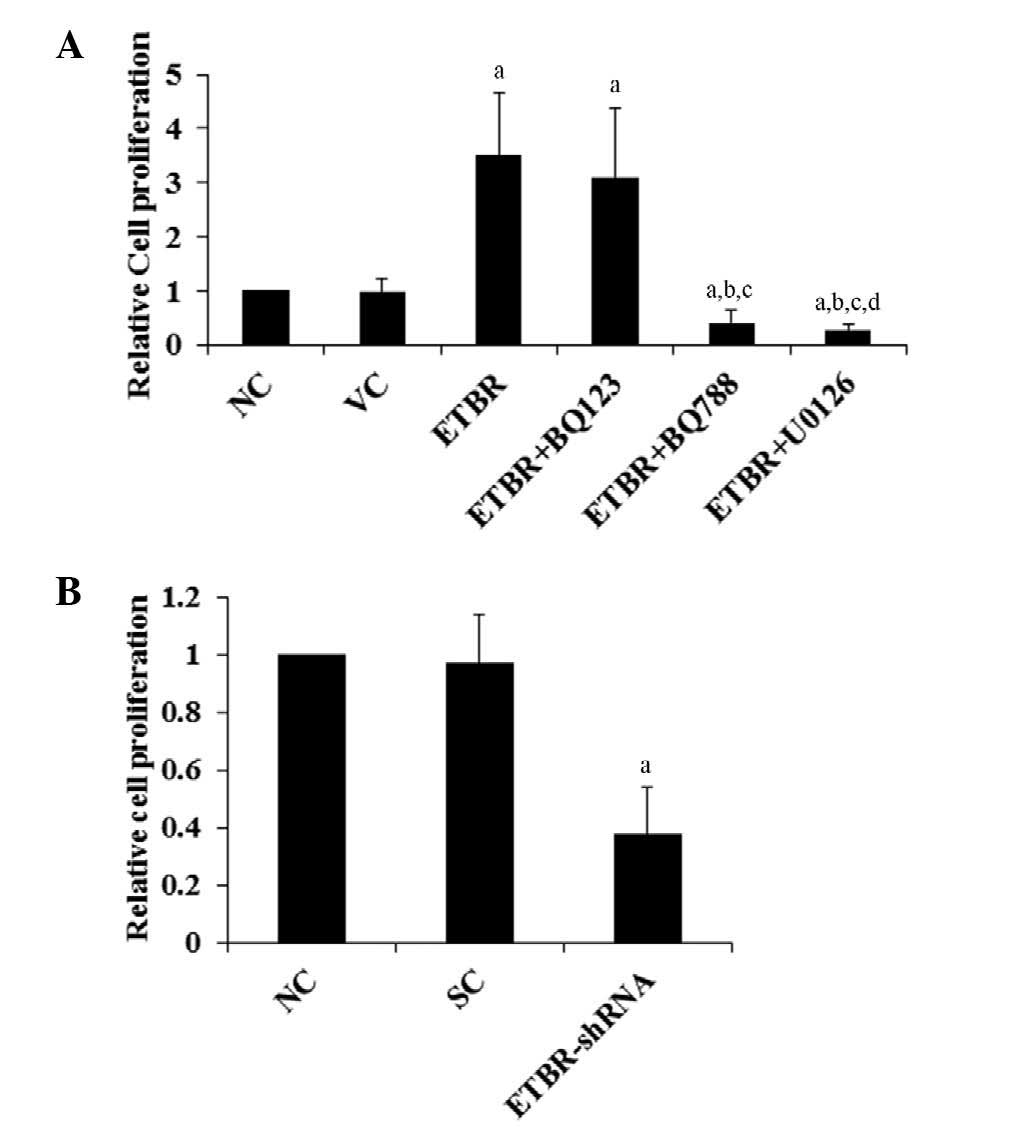
It has been reported that in oligodendroglioma
cells, ETBR is functionally coupled to the activation of ERK, which
is involved in cell proliferation (1). In order to investigate the role of
ETBR in oligodendroglioma cell proliferation and the underlying
mechanisms, Hs683 cell proliferation was examined in vitro
using MTT assays. As shown in Fig.
2A, overexpression of ETBR increased cell proliferation
>2-fold compared with the controls, which was eliminated by
selective ETBR inhibitor BQ788 and ERK-specific inhibitor U0126,
but not selective ETAR inhibitor BQ123. By contrast, the knockdown
of endogenous ETBR decreased cell proliferation to >0.6-fold of
the control level (Fig. 2B). A
similar data trend was observed with the activation
(phosphorylation) status of ERK1/2 in the cells (Fig. 3). The results indicate that ETBR
mediates oligodendroglioma cell proliferation by an ERK-dependent
mechanism.
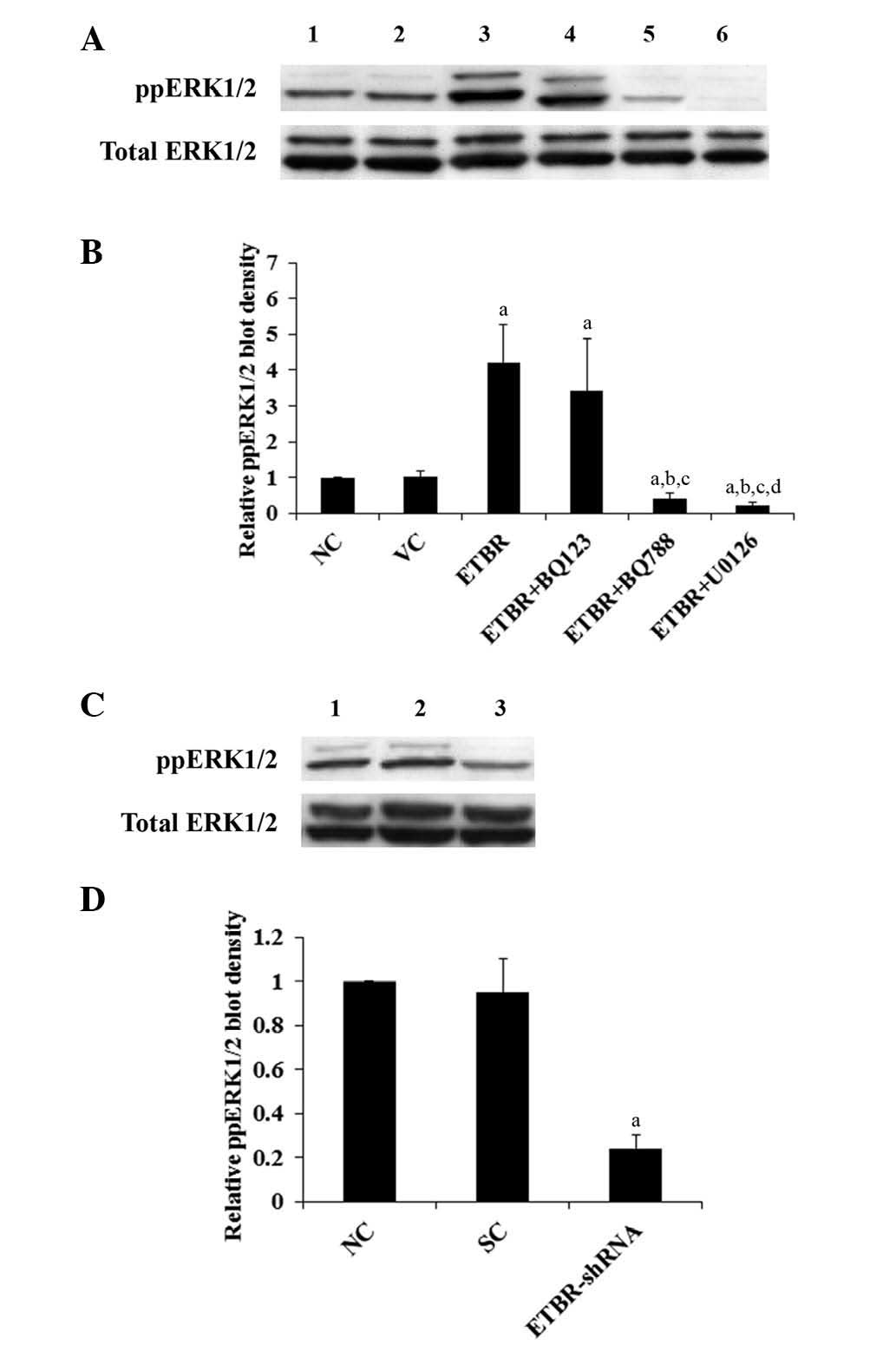 | Figure 3Western blot analysis of
phosphorylated ERK (ppERK1/2) level in Hs683 cells. (A) Lysates
from Hs683 cells were subject to western blot analyses in order to
determine the levels of ppERK1/2 and total ERK1/2: lane 1, normal
Hs683 cells (NC); lane 2, Hs683 cells stably transfected with empty
pcDNA3.1 expression vector (VC); lane 3, Hs683 cells stably
transfected with pcDNA-endothelin B receptor expression vector
(ETBR); lane 4, ETBR+BQ123 (1 μM); lane 5, ETBR+BQ788 (1 μM); lane
6, ETBR+U0126 (10 μM). (B) ppERK1/2 and total ERK1/2 blots were
measured using densitometry. The density of the ppERK1/2 blot was
normalized against that of total ERK1/2 in order to obtain a
relative ppERK1/2 blot density, which was expressed as a fold
change to that of NC (designated as 1). (C) Lane 1, NC; lane 2,
cells stably transduced with scramble control shRNA (SC); lane 3,
cells stably transduced with ETBR-shRNA (ETBR-shRNA). (D) Relative
ppERK1/2 blot density was expressed as a fold change to that of NC
(designated as 1). aP<0.05 compared with NC;
bP<0.05 compared with ETBR; cP<0.05
compared with ETBR+BQ123 and dP<0.05 compared with
ETBR+BQ788. |
Orthotopic xenograph mouse model
In order to assess the role of ETBR in
oligodendroglioma proliferation in vivo, an orthotopic
xenograft oligodendroglioma mouse model was established. Hs683
cells stably transfected with the empty expression vector (the VC
group) or the ETBR expression vector (the ETBR group), and Hs683
cells stably transduced with the SC shRNA (the SC group) or the
ETBR-shRNA (the ETBR-shRNA group) were grafted orthotopically into
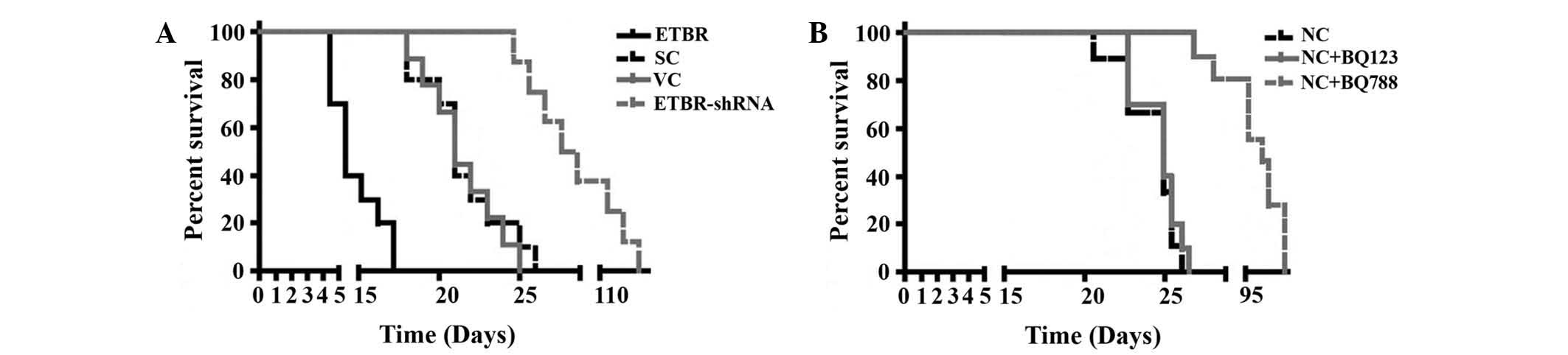
the brains of nude mice. As shown in Fig. 4A, all mice in the ETBR group (n=9)
had died or been sacrificed due to the fact that they were showing
signs of significant distress by day 17 following grafting. All
mice in the VC and the SC groups (n=9 each group) had died or been
sacrificed for showing signs of significant distress by day 27. By
contrast, no mice in the ETBR-shRNA group (n=9) died or displayed
any signs of distress by day 27, and the last mouse in this group
died on day 112. Log-rank tests showed that the ETBR group had a
significantly shorter survival time than the controls, while the
ETBR-shRNA group had a significantly longer survival time compared
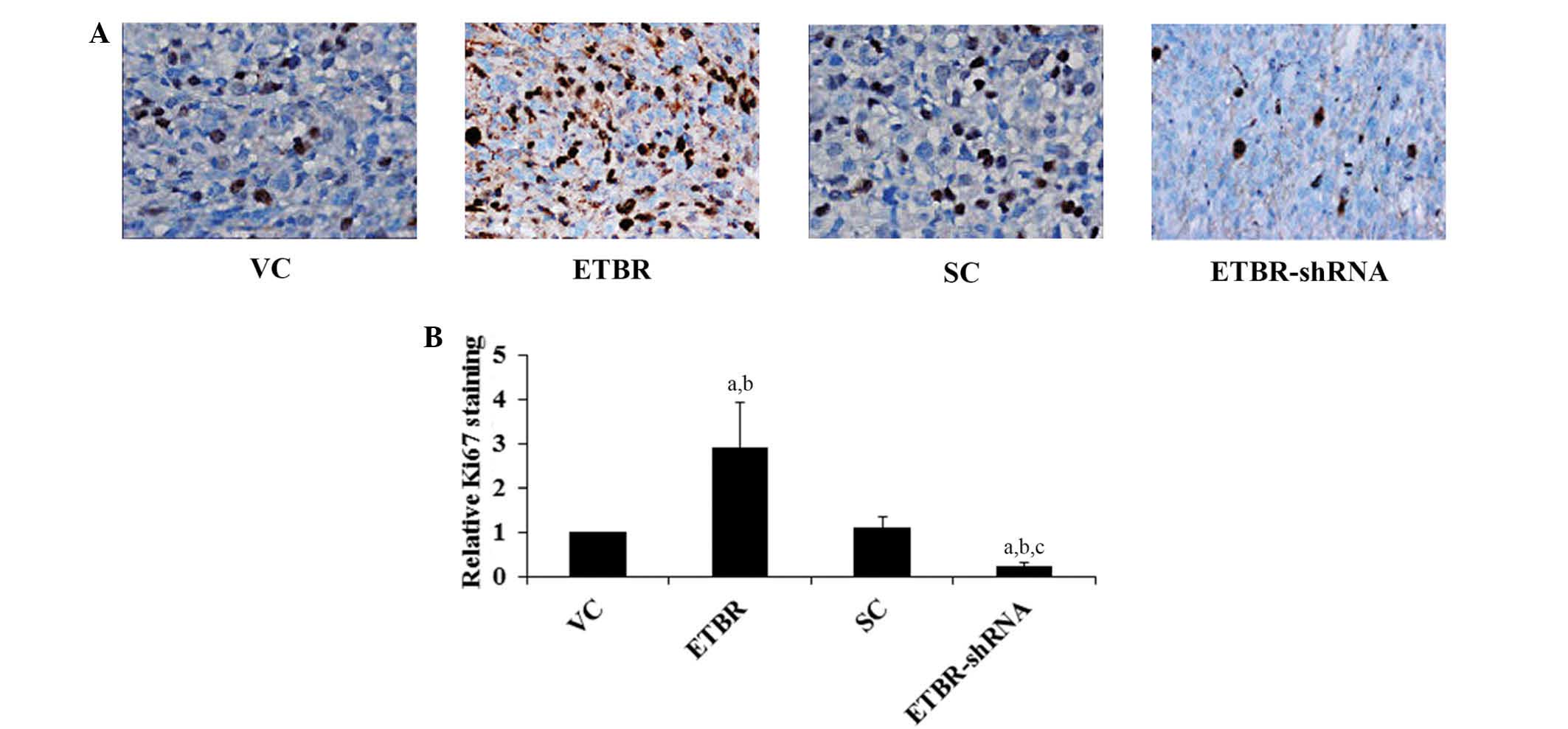
with the controls. Immunohistochemical staining for Ki67, which has
been widely used to assess tumor cell proliferative potential
(14), demonstrated that the ETBR
group had significantly increased levels of Ki67 staining in the
oligodendroglioma xenograft compared with the controls, while the
ETBR-shRNA group had significantly reduced Ki67 staining compared
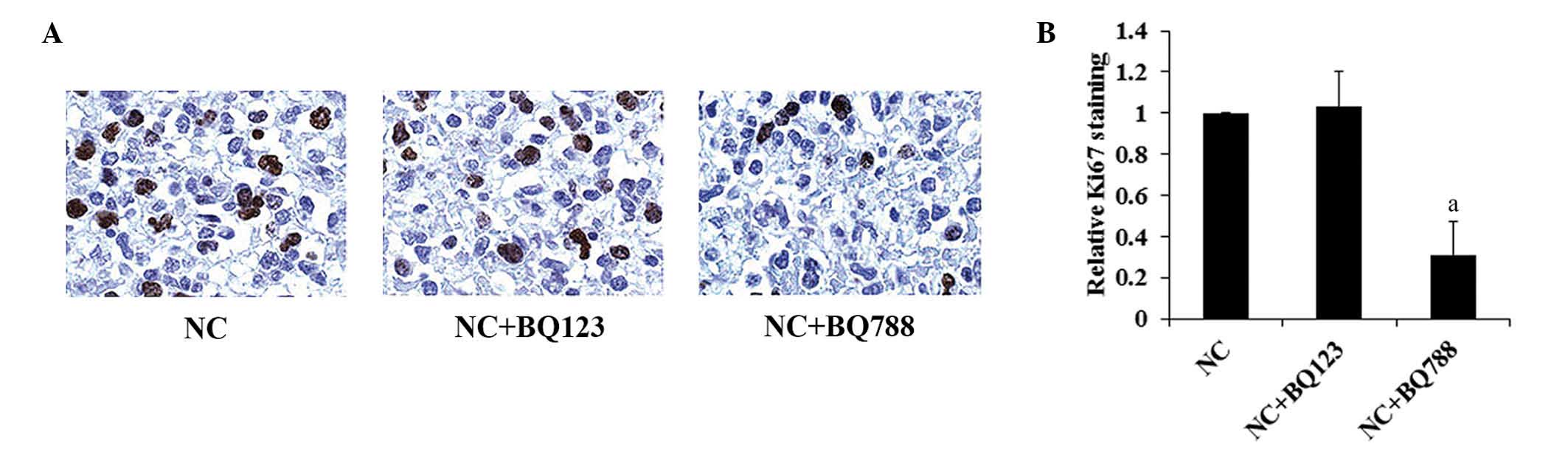
with the controls (Fig. 5).
Intratumoral injection of saline, BQ123
or BQ788
In order to investigate the therapeutic effects of
the ET receptor antagonist on oligodendroglioma in vivo,
normal Hs683 cells were grafted orthotopically into the brains of
nude mice followed by the intratumoral injection of saline, BQ123
or BQ788. As shown in Fig. 4B,
treatment with BQ788, but not BQ123, increased the survival time of
the mice compared with the controls. Immunohistochemical Ki67
staining revealed that BQ788 significantly reduced Ki67 staining in
the oligodendroglioma xenograft (Fig.
6).
Discussion
In the present study, it was demonstrated that ETBR
mediates oligodendroglioma cell proliferation according to an
ERK-dependent mechanism in vitro, and that ETBR is critical
for oligodendroglioma proliferation and survival in
vivo.
The Hs683 human glioma cell line has frequently been
used as an oligodendroglioma cell model for its extensively
characterized oligodendroglial origin (13). Furthermore, an orthotopic xenograft
oligodendroglioma mouse model using Hs683 cells has been well
established (6,13). Thus, Hs683 cells were employed in
the present study. A moderate expression of ETBR was detected in
Hs683 cells, which allowed the effective overexpression and
knockdown of ETBR to be achieved in the cells in the context of the
study goals. ETs secreted from tumor cells reportedly exert
autocrine/paracrine effects through the ET receptors (15). In this study, the expression levels
of endogenous ETs, ECE-1 and ETAR in Hs683 cells were unaltered by
the overexpression or knockdown of ETBR, which enabled the
investigation of the role of ETBR in the relatively stable setting
of the cellular ET system.
ERK, a kinase involved in cell proliferation, is an
established downstream activation target of ETBR in
oligodendroglioma cells (1). In
agreement with previous reports, it was observed that the
overexpression of ETBR significantly increased the activation
(phosphorylation) of ERK and Hs683 cell proliferation, whereas the
knockdown of ETBR resulted in the opposite effects. Selective ETBR,
but not an ETAR, antagonist eliminated the effects of ETBR
overexpression, suggesting that ETBR, but not ETAR, mediates the
proliferation of oligodendroglioma cells.
Based on the in vitro results, the role of
ETBR in oligodendroglioma proliferation and survival was
investigated in vivo. The majority of the experimental
models used currently include either murine gliomas, which have
biologic characteristics markedly different from those of human
gliomas, or human glioma cell lines grafted subcutaneously onto the
flanks of immunodeficient mice. In these models, diffuse
invasiveness into the brain parenchyma, the hallmark of a malignant
human glioma phenotype, is no longer valid (6). Thus, an Hs683 cell orthotopic
xenograft mouse model was employed in the present study.
The in vivo results of the present study were
in line with the in vitro findings. Immunohistochemical
staining for Ki67, which has been widely used to assess tumor cell
proliferative potential (14),
indicated that the overexpression and knockdown of ETBR,
respectively, increased and decreased Hs683 cell proliferation
in vivo. This was confirmed by the survival results. To the
best of our knowledge, this study was the first to evaluate the
in vivo therapeutic effects of the ETBR antagonist on
oligodendroglioma proliferation and survival. Intratumoral
injection of BQ788 was used to increase local drug concentrations
in the tumor xenografts for an efficient initial assessment. BQ788,
but not BQ123, effectively inhibited oligodendroglioma
proliferation and prolonged survival, which suggests a great
potential of selective ETBR antagonists as a therapeutic
alternative for oligodendrogliomas. However, the therapeutic
effects of BQ788 on oligodendroglioma require further confirmation
in other orthotopic xenograft mouse models.
The endothelin axis consists of three 21-amino acid
peptides, ET-1, ET-2 and ET-3, two distinct receptor subtypes ETAR
and ETBR, and ECEs, which catalyze the generation of biologically
active ET (15). ET-1 is an
established promoting factor for a wide variety of cancers
(15). There is a growing body of
evidence implicating ET-2 in the progression of breast cancer
(16). ET-2 mRNA has been reported
to be overexpressed in basal cell carcinoma compared with normal
skin, an effect controlled by the Hedgehog signaling pathway
(17). A study has suggested that
unlike ET-1 and ET-2, ET-3 may act as a natural tumor suppressor in
breast cancer (18).
Pharmacological studies have demonstrated that ETAR preferentially
binds to ET-1, while ETBR binds to all three ETs with a similar
affinity (1). In this study, the
role of ETBR in oligodendroglioma was analyzed without assessing
its interaction with ETs, this requires further investigation in
future studies.
In conclusion, it was demonstrated in vitro
that ETBR mediates oligodendroglioma cell proliferation according
to an ERK-dependent mechanism. Using an orthotopic xenograft
oligodendroglioma mouse model, it was demonstrated in vivo
that ETBR markedly promotes the proliferation of oligodendroglioma
and that a selective ETBR antagonist may effectively inhibit the
proliferation of oligodendroglioma cells and prolong survival
times. This study provides a novel insight into the role of ETBR in
the proliferation of oligodendroglioma and survival, and provides
the first in vivo evidence that ETBR-specific antagonists
may be potential therapeutic alternatives for
oligodendrogliomas.
Acknowledgements
This study was supported by the Hunan Provincial
Natural Science Foundation (grant no. 12F5587), Hunan, China.
References
|
1
|
Anguelova E, Beuvon F, Leonard N, et al:
Functional endothelin ET B receptors are selectively expressed in
human oligodendrogliomas. Brain Res Mol Brain Res. 137:77–88. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HL, Chew LJ, Packer RJ and Gallo V:
Modulation of the Wnt/beta-catenin pathway in human
oligodendroglioma cells by Sox17 regulates proliferation and
differentiation. Cancer Lett. 35:361–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005.PubMed/NCBI
|
|
4
|
Burton E and Prados M: New chemotherapy
options for the treatment of malignant gliomas. Curr Opin Oncol.
11:157–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nutt CL, Noble M, Chambers AF and
Cairncross JG: Differential expression of drug resistance genes and
chemosensitivity in glial cell lineages correlate with differential
response of oligodendrogliomas and astrocytomas to chemotherapy.
Cancer Res. 60:4812–4818. 2000.PubMed/NCBI
|
|
6
|
Branle F, Lefranc F, Camby I, et al:
Evaluation of the efficiency of chemotherapy in in vivo orthotopic
models of human glioma cells with and without 1p19q deletions and
in C6 rat orthotopic allografts serving for the evaluation of
surgery combined with chemotherapy. Cancer. 95:641–655. 2002.
View Article : Google Scholar
|
|
7
|
Levin ER: Endothelins. New Engl J Med.
333:356–363. 1995. View Article : Google Scholar
|
|
8
|
Schinelli S: Pharmacology and
physiopathology of the brain endothelin system: an overview. Curr
Med Chem. 13:627–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egidy G, Eberl LP, Valdenaire O, et al:
The endothelin system in human glioblastoma. Lab Invest.
80:1681–1689. 2000. View Article : Google Scholar
|
|
10
|
Paolillo M, Barbieri A, Zanassi P and
Schinelli S: Expression of endothelins and their receptors in
glioblastoma cell lines. J Neurooncol. 79:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paolillo M, Russo MA, Curti D, Lanni C and
Schinelli S: Endothelin B receptor antagonists block proliferation
and induce apoptosis in glioma cells. Pharmacol Res. 61:306–315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathieu V, De Nève N, Le Mercier M, et al:
Combining bevacizumab with temozolomide increases the antitumor
efficacy of temozolomide in a human glioblastoma orthotopic
xenograft model. Neoplasia. 10:1383–1392. 2008.
|
|
13
|
Le Mercier M, Fortin S, Mathieu V, et al:
Galectin 1 proangiogenic and promigratory effects in the Hs683
oligodendroglioma model are partly mediated through the control of
BEX2 expression. Neoplasia. 11:485–496. 2009.PubMed/NCBI
|
|
14
|
Schilling H, Sehu KW and Lee WR: A
histologic study (including DNA quantification and Ki-67 labeling
index) in uveal melanomas after brachytherapy with ruthenium
plaques. Invest Ophthalmol Vis Sci. 38:2081–2092. 1997.PubMed/NCBI
|
|
15
|
Bagnato A, Loizidou M, Pflug BR, Curwen J
and Growcott J: Role of the endothelin axis and its antagonists in
the treatment of cancer. Br J Pharmacol. 163:220–233. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grimshaw MJ, Hagemann T, Ayhan A, Gillett
CE, Binder C and Balkwill FR: A role for endothelin-2 and its
receptors in breast tumor cell invasion. Cancer Res. 64:2461–2468.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanese K, Fukuma M, Ishiko A and Sakamoto
M: Endothelin-2 is upregulated in basal cell carcinoma under
control of Hedgehog signaling pathway. Biochem Biophys Res Commun.
391:486–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiesmann F, Veeck J, Galm O, et al:
Frequent loss of endothelin-3 (EDN3) expression due to epigenetic
inactivation in human breast cancer. Breast Cancer Res. 11:R342009.
View Article : Google Scholar : PubMed/NCBI
|