Introduction
Vascular tone and blood pressure are regulated by
the opposing actions of vasoconstrictor and vasodilator agonists
that signal via G protein-coupled receptors (GPCRs) (1). Vasoconstrictors such as angiotensin
II, endothelin, vasopressin and norepinephrine bind to their GPCRs
and activate heterotrimeric Gq proteins. The Gq proteins then
trigger several responses in vascular smooth muscle cells (VSMCs),
including Ca2+ release from intracellular stores and
Ca2+ entry through the plasma membrane, which results in
myosin light chain phosphorylation and contraction (1).
The duration and intensity of GPCR signaling is
modulated by the regulator of G protein signaling (RGS) proteins,
which attenuate signaling by stimulating the G protein subunits to
hydrolyze guanosine triphosphate, returning them to the inactive
guanosine diphosphate-bound state (2,3).
RGS2 is one of the predominant RGS proteins in the cardiovascular
system and has been shown to be crucial in the regulation of
vascular tone and blood pressure. RGS2 deficiency in mice is
associated with a hypertensive phenotype and an increased
sensitivity, as well as prolonged responsiveness to
vasoconstrictors (4–6). In addition, genetic polymorphisms of
RGS2 are correlated with hypertension and intima-media thickening
of carotid arteries in humans (7–9).
Adrenomedullin (ADM) is a 52 amino acid peptide,
which was identified and isolated from human pheochromocytoma
extracts (10). Two hallmark
actions of ADM, hypotension and diuresis, suggest that it is
important in vascular tone regulation (11). Intravenous infusion of ADM has been
demonstrated to result in prolonged hypotension in cats, rats,
rabbits, sheep and humans (12–21).
ADM is derived from a larger precursor molecule, proADM (22). ProADM is cleaved by endogenous
peptidases into 4 bioactive peptides: PreproADM22-41,
preproADM45-92, preproADM95-146 (ADM) and preproADM153-185
(23). These peptidases exhibit
different roles in physiological processes; the effects of
preproADM22-41 are similar to that of ADM (24), while PreproADM153-185 leads to
marked contractile activity in cat pulmonary arterial rings and is
termed adrenotensin (ADT) (25).
ADT acts in a modulatory manner to influence vasorelaxation in
response to ADM (26). There
appears to be complex interactions (antagonism or synergism in
vascular activities) between ADM and ADT, and these interactions
may be active in the regulation of circulatory homeostasis
(26).
As GPCR ligands, the vascular activities of ADM and
ADT depend on the modulation of RGS2 expression. However, the
effects and pathways of their modulation remain unknown. This study
aimed to observe the changes of RGS2 expression in response to ADM
and ADT in cultured VSMCs and to clarify the potential signaling
pathways in vitro.
Materials and methods
Animals
Four male Wistar rats (age, 8 weeks) purchased from
the Institute of Laboratory Animal Science, Chinese Academy of
Medical Sciences (Beijing, China), were sacrificed by intravenous
administration of overdosed pentobarbital. The segment of thoracic
aorta between the left subclavian and subcostal arteries was
removed for VSMC isolation using enzymatic digestion, as previously
described (27). This study was
conducted in accordance with the guidelines of Peking University
First Hospital and recommendations for the care and use of
laboratory animals of the Chinese Ministry of Agriculture.
Cell culture
Cells were grown in Dulbecco’s modified Eagle’s
medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10%
fetal bovine serum (HyClone Laboratories Inc., Logan, UT, USA),
penicillin (100 U/ml; Invitrogen Life Technologies, Carlsbad, CA,
USA) and streptomycin (100 μg/ml, Invitrogen Life Technologies) at
37°C in a humidified incubator in a 5% CO2 atmosphere.
Only VSMCs from the third to sixth cell passage were used. The
cells were then cultured in six-well, collagen I-coated BioFlex
plates containing a flexible silicone elastomer substratum
(Flexcell International, Hillsborough, NC, USA).
Drugs
Angiotensin II (002-12), ADM (010-08), and
adrenotensin (010-07) were purchased from Phoenix PharmaLabs, Inc.
(Shanghai, China). Forskolin (66575-29-9), SQ22,536 (17318-31-9)
and chelerythrine chloride (3895-92-9) were purchased from
Sigma-Aldrich.
Incubation with cultured cells
Following replacement of the collagen-coated BioFlex
plates with serum-free medium for 24 h, cells achieved 70%
confluence and were subjected to incubation with vehicle, ADM, ADT
or angiotensin II for different time periods (10−7 mol/l
for 0, 0.5, 1, 2 or 4 h) or at different concentrations
(10−9, 10−8 and 10−7 mol/l ADM for
0.5 h and ADT for 1 h). The cultured VSMCs were also incubated with
forskolin (10−5 mol/l), a PKA pathway agonist, for
different time periods (0, 0.5, 1, 2 or 4 h). SQ22,536
(10−4 mol/l) and chelerythrine (10−6 mol/l)
were used to block the PKA pathway and PKC pathways by
co-incubation with vehicle or ADM for 0.5 h and vehicle or ADT for
1 h. Subsequent to this, the gene expression of RGS2 was analyzed
by PCR.
Gene expression
Total RNA was extracted from the cultured cells
using TRIzol (Life Technologies Co., Gaithersburg, MD, USA). qPCR
was performed using a Toyobo ReverTra Ace-α-®RT-PCR kit
(Toyobo Life Science, Osaka, Japan). The expression of selected
mRNAs was measured by qPCR using the ABI Prism 7700 Sequence
Detector (Life Technologies). Expression of the mRNAs was
normalized to that of the corresponding β-actin mRNA. PCR reactions
were performed in 25 μl buffer containing 1 μl cDNA, SYBR-Green
Master mix (Life Technologies) and 5 pmol sequence-specific
primers: Forward: 5′-CTG CGT ACC CAT GGA CAA GA-3′ and reverse:
5′-TTG GGC TTC CCA GGA GTA GA-3′ for RGS2; and forward: 5′-AGG CCA
ACC GTG AAA AGA TG-3′ and reverse: 5′-ACC AGA GGC ATA CAG GGA
CAA-3′ for β-actin. Thermal cycling conditions consisted of a
preincubation step for 2 min at 50°C, denaturation for 10 min at
95°C followed by 40 cycles of denaturation for 15 sec at 95°C and
annealing/extension for 1 min at 60°C. qPCR reactions were
performed in triplicate.
Statistical analysis
Values were expressed as the mean ± standard error
of the mean. Statistical differences between groups were determined
by either one-way or two-way analysis of variance. When a
statistical difference was detected, Tukey’s method of adjustment
was used for multiple pairwise comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
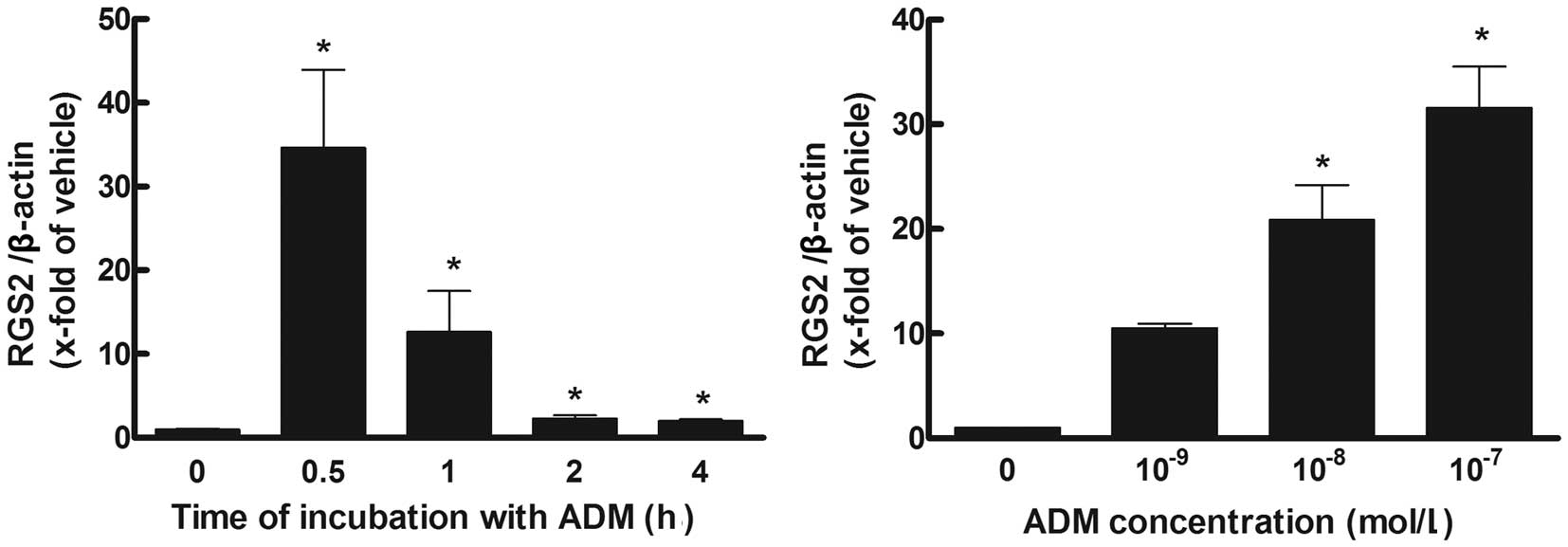
ADM increases RGS2 in a time- and
concentration-dependent manner
Following incubation with ADM (10−7
mol/l), the gene expression of RGS2 was significantly increased at
0.5 h, reaching a peak ~35 fold of that at baseline and gradually
decreasing to~2-fold that of baseline at 4 h (P<0.05). When
incubated for 0.5 h with different concentrations of ADM, ADM at
concentrations of 10−8 and 10−7 mol/l
significantly increased the gene expression of RGS2 (P<0.05;
Fig. 1).
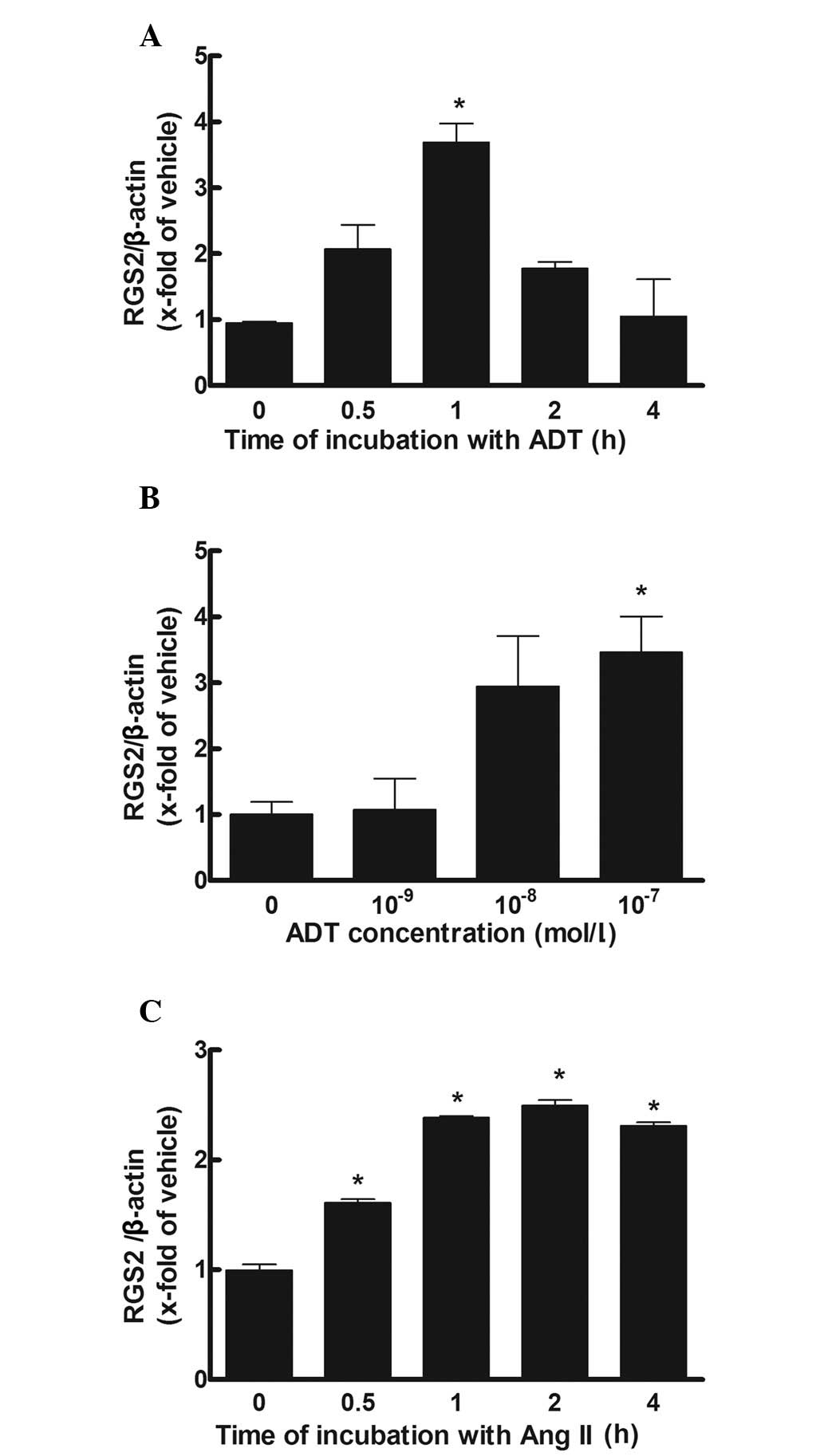
ADT increases RGS2 in a time and
concentration-dependent manner
Following incubation with ADT (10−7
mol/l) the gene expression of RGS2 was significantly increased to
~3.7-fold of that at baseline after 1 h (P<0.05). Subsequent to
incubation for 1 h with different concentrations of ADT, a
concentration of 10−7 mol/l significantly increased the
gene expression of RGS2 (P<0.05; Fig. 2). The expression of RGS2 was also
significantly increased by angiotensin II incubation
(10−7 mol/l) to ~2.5-fold of that at baseline at 2 h
(P<0.05; Fig. 2).
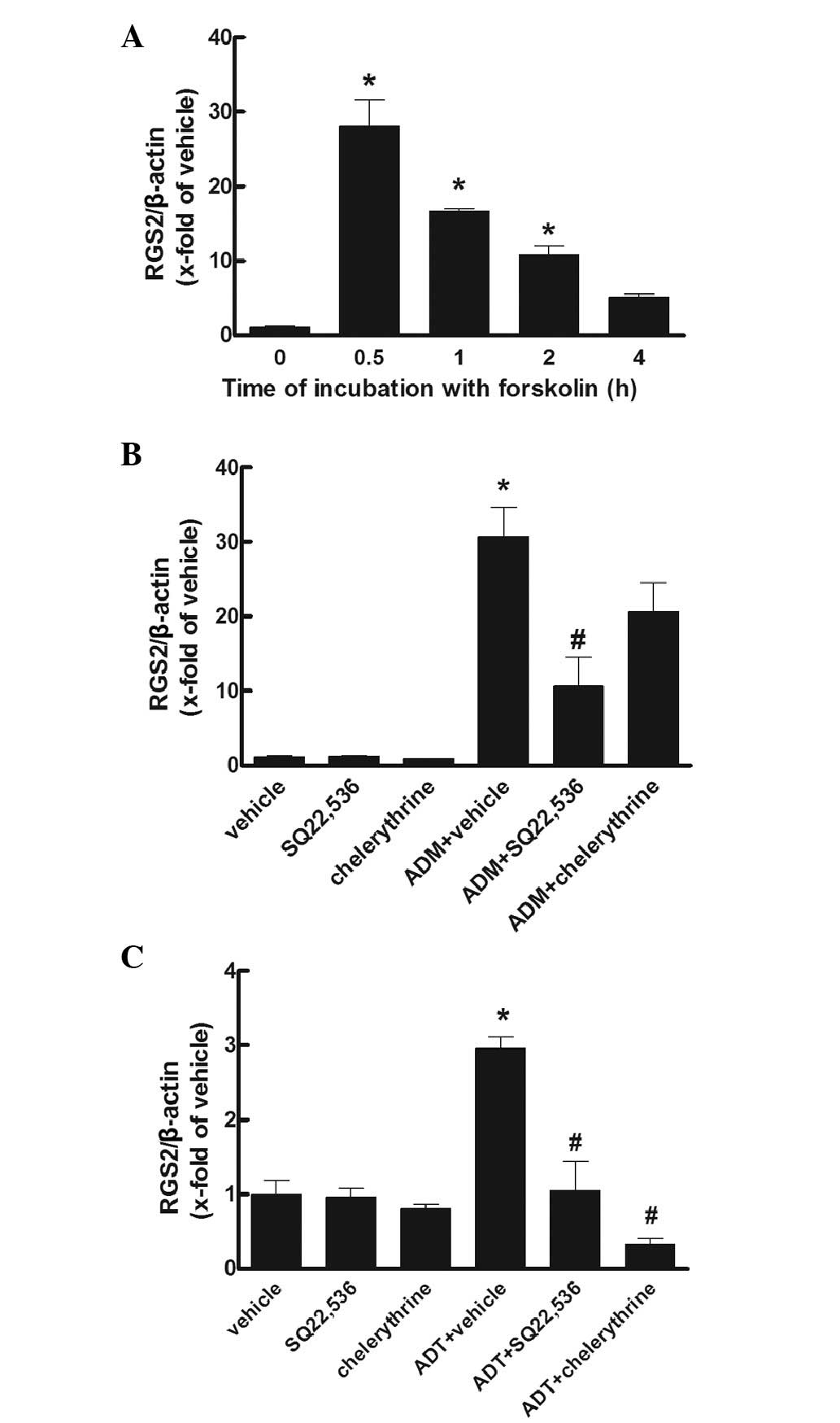
Effects of signaling reagents
Forskolin (10−5 mol/l), a cyclic
adenosine monophosphate (cAMP)-dependent pathway agonist,
significantly increased the gene expression of RGS2 at 0.5 h to a
peak of ~30-fold of that at baseline (P<0.05), which then
gradually decreased to ~5-fold of that at baseline at 4 h. When the
PKA pathway antagonist SQ22,536 (10−4 mol/l) and PKC
inhibitor chelerythrine (10−6 mol/l) was incubated with
the cells treated with ADM (10−7 mol/l), the gene
expression of RGS2 was markedly reduced by SQ22,536 only. Moreover,
when SQ22,536 and chelerythrine was incubated with ADT
(10−7 mol/l), the gene expression of RGS2 was markedly
reduced by SQ22,536 and chelerythrine (P<0.05; Fig. 3).
Discussion
In the present study, it was demonstrated that the
modulation of RGS2 gene expression in cultured VSMCs by ADM and ADT
occurs via different pathways. As ADM and ADT are potential
vascular therapeutic agents and RGS2 may be used as a future
theraputic target for vascular diseases, this study provides
additional information for their potential clinical
application.
RGS2 has been shown to be crucial in the regulation
of vascular tone and hypertension. Haplo-insufficiency or
elimination of the RGS2 gene leads to hypertension in mice
(6). Certain RGS2 haplotypes also
cosegregate with human hypertension (8). Furthermore, RGS2 was shown to
selectively inhibit Gq signaling and thereby attenuates the action
of vasoconstrictors (28). Aortic
rings from RGS2 knockout mice exhibited increased contraction and
impaired cyclic guanosine monophosphate (cGMP) mediated relaxation
in vitro(4). Previously, it
was suggested that RGS2 functions as a novel target or effector of
the nitric oxide-cGMP pathway to regulate vasoconstrictor signaling
(4). RGS2 mediated the action of
the nitric oxide-cGMP pathway in several tissues as it is widely
expressed, including in vascular smooth muscle and kidney (29). RGS2 regulates blood pressure to a
significant extent by mediating the ability of the nitric
oxide-cGMP pathway to relax the resistance vasculature and
attenuate vasoconstrictor signaling in VSMCs (5). The short-term action of nitric oxide
donor on blood pressure is impaired in RGS2 knockout mice.
Furthermore, the loss of RGS2 in primary smooth muscle cells from
mesenteric resistance arteries increases the magnitude and duration
of agonist-induced Ca2+ responses and blocks the ability
of cGMP analogs to attenuate agonist-induced Ca2+
signaling (4).
In the present study, ADM significantly increased
the expression of RGS2 mRNA at 0.5 h to 35-fold of that at
baseline, whereas ADT and angiotensin II marginally increased the
expression of RGS2 mRNA after 1–2 h. Therefore, the increased RGS2
expression may be crucial in the vascular activities of ADM and the
latent and marginal increase of RGS2 expression following the
stimulation of vasoconstrictors such as ADT and Angiotensin II may
be a compensated change.
ADM is a potent cardiovascular-active peptide that
has hypotensive, natriuretic and diuretic actions. It is considered
to be important in the pathophysiology of numerous disorders, such
as hypertension, acute coronary syndromes and renal failure
(30). It inhibits the angiotensin
II-induced proliferation and migration effects on renal mesangial
cells and exhibits an anti-oxidant action on these cells (30). ADM was observed to stimulate
adenylyl cyclase activity in a platelet bioassay. ADM receptors are
coupled to adenylate cyclase via G-proteins and the cyclic ring
structure and C-terminal amidation are essential for full binding
and activity (10). Moreover, ADM
has been demonstrated to activate the adenylate cyclase-cAMP system
in isolated cardiac myocytes, which is one of the predominant
pathways for the regulation of myocardial contractility (31). In the present study, the effects of
ADM on RGS2 expression was simulated by adenylate cyclase activator
forskolin and blocked by the adenylate cyclase antagonist SQ22,536.
Therefore, the effect of ADM on RGS2 expression occurs via
adenylate cyclase and a PKA pathway.
Consistently, the ADM-mediated effects in calcified
VSMCs and vitamin D3 plus nicotine-treated aortas were also similar
to that of forskolin, while the PKA inhibitor H89 blocked the
effect of ADM (32). Furthermore,
ADM induced the relaxation of rat brain pericytes, which was
associated with the reduced phosphorylation of myosin light chain
through the cAMP/PKA signaling pathway. H89 inhibited the
ADM-induced increase in the number of relaxed pericytes and
returned the phosphorylation of the myosin light chains to that of
the control levels (33).
ADT, which is derived from the common precursor of
ADM, is also an active peptide. ADT has been demonstrated to
produce contractile responses in cat pulmonary arterial rings in a
concentration-dependent manner (34). In anesthetized rats, an intravenous
bolus injection of ADT increased the mean arterial pressure. In
cultured rat vascular smooth muscle cells, 10−7 mol/l
ADT increased 3H-TdR incorporation (26). ADT induced endothelium-dependent
vasoconstriction and elevated blood pressure (25,34).
Moreover, ADT acts in a modulatory manner to influence
vasorelaxation in response to ADM. ADT has been shown to antagonize
the stimulatory effect of ADM on endothelial nitric oxide
generation (26). ADT also induced
an increase in the concentration of medium immunoreactive ADM
(35). In a rat model of pulmonary
hypertension, ADM and ADT exhibited opposite effects on
vasoactivity and showed reciprocal inhibition in their release
(36). However, the signaling
pathway of ADT remains unclear. The results of the present study
showed that SQ22,536 and chelerythrine chloride blocked the effects
of ADT on RGS2 expression significantly, which suggest that the
effect of ADT on RGS2 expression occurred via a PKA and PKC
pathway.
In conclusion, our results showed that ADM
immediately exhibited a significantly increased the gene expression
of RGS2 in VSMCs via cAMP-dependent pathway and ADT gradually
showed a marginal increase in the gene expression of RGS2 via a
cAMP-dependent and a PKC pathway. This suggests the different
responses of RGS2 to vasodilators and vasoconstrictors.
Acknowledgements
The authors are grateful to Ms. Lin Xue and Ms.
Chunyu Zhao for their technical assistance. This study was
supported by grants from the National Nature Science Foundation of
China (grant no. NSFC30600230) and the Chinese Doctoral Foundation
(No. 331).
References
|
1
|
Münzel T, Feil R, Mülsch A, Lohmann SM,
Hofmann F and Walter U: Physiology and pathophysiology of vascular
signaling controlled by guanosine 3′,5′-cyclic
monophosphate-dependent protein kinase [Corrected]. Circulation.
108:2172–2183. 2003.
|
|
2
|
Ross EM and Wilkie TM: GTPase-activating
proteins for heterotrimeric G proteins: regulators of G protein
signaling (RGS) and RGS-like proteins. Annu Rev Biochem.
69:795–827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hollinger S and Hepler JR: Cellular
regulation of RGS proteins: modulators and integrators of G protein
signaling. Pharmacol Rev. 54:527–559. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang KM, Wang GR, Lu P, Karas RH,
Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP,
Zhu Y and Mendelsohn ME: Regulator of G-protein signaling-2
mediates vascular smooth muscle relaxation and blood pressure. Nat
Med. 9:1506–1512. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X, Kaltenbronn KM, Steinberg TH and
Blumer KJ: RGS2 is a mediator of nitric oxide action on blood
pressure and vasoconstrictor signaling. Mol Pharmacol. 67:631–639.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heximer SP, Knutsen RH, Sun X, Kaltenbronn
KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin
AJ, Steinberg TH, et al: Hypertension and prolonged vasoconstrictor
signaling in RGS2-deficient mice. J Clin Invest. 111:12592003.
View Article : Google Scholar
|
|
7
|
Yang J, Kamide K, Kokubo Y, Takiuchi S,
Tanaka C, Banno M, Miwa Y, Yoshii M, Horio T, Okayama A, et al:
Genetic variations of regulator of G-protein signaling 2 in
hypertensive patients and in the general population. J Hypertens.
23:1497–1505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riddle EL, Rana BK, Murthy KK, Rao F,
Eskin E, O’Connor DT and Insel PA: Polymorphisms and haplotypes of
the regulator of G protein signaling-2 gene in normotensives and
hypertensives. Hypertension. 47:415–420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamide K, Kokubo Y, Yang J, Takiuchi S,
Horio T, Matsumoto S, Banno M, Matayoshi T, Yasuda H, Miwa Y, et
al: Association of intima-media thickening of carotid artery with
genetic polymorphisms of the regulator of G-protein signaling 2
gene in patients with hypertension and in the general population.
Hypertens Res. 34:740–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: a novel
hypotensive peptide isolated from human pheochromocytoma. Biochem
Biophys Res Commun. 192:553–560. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samson WK, Resch ZT, Murphy TC, Vargas TT
and Schell DA: Adrenomedullin: is there physiological relevance in
the pathology and pharmacology? News Physiol Sci. 14:255–259.
1999.PubMed/NCBI
|
|
12
|
Ishiyama Y, Kitamura K, Ichiki Y, Sakata
J, Kida O, Kangawa K and Eto T: Haemodynamic responses to rat
adrenomedullin in anaesthetized spontaneously hypertensive rats.
Clin Exp Pharmacol Physiol. 22:614–618. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parkes DG and May CN: Direct cardiac and
vascular actions of adrenomedullin in conscious sheep. Br J
Pharmacol. 120:1179–1185. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hjelmqvist H, Keil R, Mathai M, Hübschle T
and Gerstberger R: Vasodilation and glomerular binding of
adrenomedullin in rabbit kidney are not CGRP receptor mediated. Am
J Physiol. 273:R716–R724. 1997.PubMed/NCBI
|
|
15
|
Lainchbury JG, Cooper GJ, Coy DH, Jiang
NY, Lewis LK, Yandle TG, Richards AM and Nicholls MG:
Adrenomedullin: a hypotensive hormone in man. Clin Sci (Lond).
92:467–472. 1997.PubMed/NCBI
|
|
16
|
Parkes DG: Cardiovascular actions of
adrenomedullin in conscious sheep. Am J Physiol. 268:H2574–H2578.
1995.PubMed/NCBI
|
|
17
|
Feng CJ, Kang B, Kaye AD, Kadowitz PJ and
Nossaman BD: L-NAME modulates responses to adrenomedullin in the
hindquarters vascular bed of the rat. Life Sci. 55:PL433–PL438.
1994.PubMed/NCBI
|
|
18
|
Fukuhara M, Tsuchihashi T, Abe I and
Fujishima M: Cardiovascular and neurohormonal effects of
intravenous adrenomedullin in conscious rabbits. Am J Physiol.
269:R1289–R1293. 1995.PubMed/NCBI
|
|
19
|
Nakamura M, Yoshida H, Makita S, Arakawa
N, Niinuma H and Hiramori K: Potent and long-lasting vasodilatory
effects of adrenomedullin in humans. Comparisons between normal
subjects and patients with chronic heart failure. Circulation.
95:1214–1221. 1997. View Article : Google Scholar
|
|
20
|
Shirai M, Shimouchi A, Ikeda S, Ninomiya
I, Sunagawa K, Kangawa K and Matsuo H: Vasodilator effects of
adrenomedullin on small pulmonary arteries and veins in
anaesthetized cats. Br J Pharmacol. 121:679–686. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Champion HC, Lambert DG, McWilliams SM,
Shah MK, Murphy WA, Coy DH and Kadowitz PJ: Comparison of responses
to rat and human adrenomedullin in the hindlimb vascular bed of the
cat. Regul Pept. 70:161–165. 1997. View Article : Google Scholar
|
|
22
|
Dieplinger B, Mueller T, Kollerits B,
Struck J, Ritz E, von Eckardstein A, Haltmayer M and Kronenberg F;
MMKD Study Group. Pro-A-type natriuretic peptide and
pro-adrenomedullin predict progression of chronic kidney disease:
the MMKD Study. Kidney Int. 75:408–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hinson JP, Kapas S and Smith DM:
Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev.
21:138–167. 2000.PubMed/NCBI
|
|
24
|
Samson WK: Proadrenomedullin-derived
peptides. Front Neuroendocrinol. 19:100–127. 1998. View Article : Google Scholar
|
|
25
|
Gumusel B, Chang JK, Hyman A and Lippton
H: Adrenotensin: an ADM gene product with the opposite effects of
ADM. Life Sci. 57:PL87–PL90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Qiu Z, Ye C, Di L, Liu X, Tang C
and Zhao Y: Vasoactive effects of adrenotensin and its interactions
with adrenomedullin. Chin Med J (Engl). 113:269–271.
2000.PubMed/NCBI
|
|
27
|
Hitomi H, Fukui T, Moriwaki K, Matsubara
K, Sun GP, Rahman M, Nishiyama A, Kiyomoto H, Kimura S, Ohmori K,
Abe Y and Kohno M: Synergistic effect of mechanical stretch and
angiotensin II on superoxide production via NADPH oxidase in
vascular smooth muscle cells. J Hypertens. 24:1089–1095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muslin AJ: Tuning cardiomyocyte Gq
signaling with RGS2. J Mol Cell Cardiol. 41:14–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kehrl JH and Sinnarajah S: RGS2: a
multifunctional regulator of G-protein signaling. Int J Biochem
Cell Biol. 34:432–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beltowski J and Jamroz A: Adrenomedullin -
what do we know 10 years since its discovery? Pol J Pharmacol.
56:5–27. 2004.PubMed/NCBI
|
|
31
|
Sato A, Canny BJ and Autelitano DJ:
Adrenomedullin stimulates cAMP accumulation and inhibits atrial
natriuretic peptide gene expression in cardiomyocytes. Biochem
Biophys Res Commun. 230:311–314. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai Y, Teng X, Pan CS, Duan XH, Tang CS
and Qi YF: Adrenomedullin up-regulates osteopontin and attenuates
vascular calcification via the cAMP/PKA signaling pathway. Acta
Pharmacol Sin. 31:1359–1366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takata F, Dohgu S, Nishioku T, Takahashi
H, Harada E, Makino I, Nakashima M, Yamauchi A and Kataoka Y:
Adrenomedullin-induced relaxation of rat brain pericytes is related
to the reduced phosphorylation of myosin light chain through the
cAMP/PKA signaling pathway. Neurosci Lett. 449:71–75. 2009.
View Article : Google Scholar
|
|
34
|
Gumusel B, Chang JK, Hao Q, Hyman A and
Lippton H: Adrenotensin: an adrenomedullin gene product contracts
pulmonary blood vessels. Peptides. 17:461–465. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi YF, Bu DF, Niu DD, Shi YR, Wang SH,
Pang YZ, Tang CS and Du JB: Effects of different peptide fragments
derived from proadrenomedullin on gene expression of adrenomedullin
gene. Peptides. 23:1141–1147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cuifen Z, Lijuan W, Li G, Wei X, Zhiyu W
and Fuhai L: Changes and distributions of peptides derived from
proadrenomedullin in left-to-right shunt pulmonary hypertension of
rats. Circ J. 72:476–481. 2008. View Article : Google Scholar : PubMed/NCBI
|