Introduction
Colorectal cancer (CRC) is one of the most common
malignancies, with over one million new cases and more than half a
million deaths each year worldwide (1,2).
Although complete resection of the tumor is the best prognosis for
long-term survival, CRC patients frequently present with metastatic
disease at the time of their diagnosis, and surgery cannot always
extirpate the recurrence of advanced CRC (3,4).
Therefore, chemotherapy remains one of the major therapeutic
approaches for patients with invasive and metastatic CRC, with
5-fluorouracil (5-FU)-based regimens being considered as standard
chemotherapy treatment. However, due to drug resistance and
unacceptable levels of toxicity against normal cells, systemic
chemotherapy using 5-FU-based regimens produces objective response
rates of only 10–20% (5–8). Thus, novel therapeutic strategies
should be developed and antitumor agents should be identified.
Malignant tumors arise from a small fraction of
cancer cells that possess stem cell features and are therefore
termed cancer stem cells (CSCs) (9). The existence of CSCs has been
demonstrated in the majority of leukemias and many solid tumors
including CRC (10–14). Similar to normal stem cells, CSCs
possess properties of continuous self-renewal and multi-directional
differentiation, which confers CSCs the ability of unlimited
proliferation facilitating the long-term maintenance of the cancer,
and differentiation into different cell types to develop new tumors
(15). More importantly, it has
been shown that CSCs are naturally resistant to chemotherapeutic
agents through various mechanisms (16). CSCs specifically overexpress the
ATP-binding cassette (ABC) family of transporter proteins such as
ABCB1 and ABCG2, which pump various xenobiotics out of the cell,
reducing the intracellular accumulation of chemotherapeutic drugs
(17–19). In addition, CSCs usually contain
high levels of DNA repair mechanism and anti-apoptotic proteins
such as Bcl-2 and survivin, further conferring CSCs a survival
advantage (20,21). CSCs also exhibit relative cell
cycle quiescence resulting in a slow rate of cell turnover, thereby
assisting CSCs to evade the cytotoxic effects of conventional
chemotherapies that are designed to target rapidly replicating
cells (22,23). Therefore, CSCs are, not only
responsible for tumor initiation and progression, but also play
important roles in drug resistance leading to cancer relapse and
metastasis and eventually the failure of clinical treatment. Thus,
targeting CSCs is a promising approach for anticancer
treatments.
Due to the drug resistance and cytotoxicity of
currently used chemotherapies, traditional Chinese medicines (TCM)
have received attention due to few side-effects as compared to
modern chemotherapeutics and have been used for thousands of years
as important alternative remedies for various diseases (24,25).
TCM formula is a complex combination of many natural products, each
of which contains numerous chemical compounds. TCM formulas
therefore are considered to be multi-component and multi-target
agents that exert their therapeutic activities in a more holistic
way. Pien Tze Huang (PZH) is a well-known TCM formula that was
first prescribed by a royal physician >450 years ago in the Ming
Dynasty. The main ingredients of PZH include Moschus,
Calculus Bovis, Snake Gall and Radix Notoginseng.
These products together confer PZH properties of heat-clearing,
detoxification, promotion of blood circulation, reduction of blood
stasis, dissipation of hard mass, detumescence and analgesia
(26). In the TCM system,
accumulation of toxic dampness and heat is one of the major
causative factors in the pathogenesis of cancers, and therefore
clearing heat and detoxification is a principle of anticancer
treatment. Thus PZH, which has also been used in China and
Southeast Asia for centuries as a folk remedy for various types of
cancer, is believed to be effective for anti-cancer treatment.
Modern pharmacological studies suggested that PZH exhibits
therapeutic effects in clinical trials of tumors such as
hepatocellular carcinoma and colon cancer (27,28).
In addition, in experimental animals PZH inhibits the growth of
Ehrlich-Ascites tumor, gastric carcinoma, and hepatoma (29). Moreover, it was reported that PZH
inhibits colon cancer growth in vivo and in vitro via
the promotion of cancer cell apoptosis and inhibition of cell
proliferation and tumor angiogenesis, which is probably mediated by
its regulatory effect on multiple intracellular pathways (30–35).
To elucidate the mechanism of the tumoricidal activity of PZH, the
effect of PZH on colorectal cancer stem cells was investigated.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), B27 supplement (50X), penicillin-streptomycin,
trypsin-EDTA and TRIzol reagent were purchased from Invitrogen
(Carlsbad, CA, USA). SuperScript II reverse transcriptase was
obtained from Promega (Madison, WI, USA). Any other chemicals used,
unless otherwise stated, were obtained from Sigma Chemicals (St.
Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by Zhangzhou
Pien Tze Huang Pharmaceutical Co., Ltd., China (Chinese FDA
approval no. Z35020242). Stock solution of PZH was prepared
immediately prior to use by dissolving the PZH powder in
phosphate-buffered saline (PBS) to a concentration of 40 mg/ml. The
working concentrations of PZH were obtained by diluting the stock
solution in the culture medium.
Cell culture
Human colon carcinoma HT-29 cells were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The cells were grown in DMEM containing 10% (v/v) FBS, and 100 U/ml
penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2.
Side population analysis
Based on the protocol by Goodell et
al(36), HT-29 cells were
digested with 0.25% trypsin-EDTA, and resuspended in DMEM culture
(supplemented with 2% FBS) at a concentration of 2.5×106
cells/ml. Fresh Hoechst 33342 dye (10 μg/ml; final concentration)
was added for 30 min at 37°C in a rotary shaker. As a control, some
cells were incubated with Hoechst 33342 dye in the presence of 50
μM verapamil. At the end of incubation, the cells were washed and
resuspended in cold PBS, 1 mg/ml propidium iodide was added, and
the cells were kept at 4°C in the dark. Cell analysis and sorting
were performed on Moflo XDP™ cell sorter flow cytometry (Beckman
Coulter, Fullerton, CA, USA). Excitation of Hoechst dye was
performed using a UV laser at 355 nm, and the fluorescence was
measured with a 450±25 nm filter (Hoechst blue) and a 620±15 nm
filter (Hoechst red). Doublets and dead cells were gated out.
Inhibition of the SP phenotype by verapamil was used as guidance
for drawing of sorting gates.
Stem cell culture
Stem cells were cultured in serum-free stem cell
culture medium, which contains DMEM/F12 culture medium, B27 (1X),
20 ng/ml EGF and 20 ng/ml bFGF, to prevent differentiation of the
stem cells.
Sphere formation assay
Sorted cells were seeded at a density of 1,000
cells/well in 6-well plates (Corning, Lowell, MA, USA) and cultured
in the above-mentioned serum-free stem cell culture medium in a
humidified incubator (5% CO2) at 37°C. The medium was
added every 3 days. When the spheroids sufficiently large (>50
cells within a sphere was considered to be a full sphere), they
were collected and transferred onto a 96-well dish, and images were
captured by BD Pathway™ 855 under ×10 objective in the form of
10×10 montage (BD Biosciences Bioimaging, Rockville, MD, USA).
Cell viability evaluation
Cell viability was assessed by the WST-1 assay.
HT-29 stem-like cells (20,000/well in 96-well plates) were
incubated with the above-mentioned serum-free stem cell culture
medium at 37°C for 48 h. The cells were then treated with various
concentrations of PZH for 24 h. At the end of the treatment, 10 μl
WST-1 was added to each well, and the samples were incubated for an
additional 2 h at 37°C. The absorbance was measured at 450 nm using
an ELISA reader (Model ELX800, BioTek, USA).
RT-PCR analysis
Total RNA was isolated with TRIzol reagent.
Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega) according to the
manufacturer's instructions. The obtained cDNA was used to
determine the mRNA amount of CD133, CD44, Oct4, ABCB1, ABCG2 by
PCR. GAPDH was used as an internal control.
Statistical analysis
Data were analyzed using the statistical software
SPSS13.0. Statistical analysis of the data was performed using the
Student's t-test and one-way analysis of variance (ANOVA).
P<0.05 was considered statistically significant.
Results and Discussion
Human colorectal cancer cell line HT-29
contains stem-like side population cells
One of the commonly used techniques for the
identification and isolation of CSCs is the flow cytometric side
population (SP) analysis (37),
which is based on the ability of CSCs to efflux Hoechst dye due to
the overexpression of ABC transporter proteins (17–19).
SP cells have been identified in various types of cancer including
CRC; and cells in SP exibit stem cell-like characteristics, such as
the ability for self-renewal and tumorigenicity (38–46).
Moreover, it has been shown that the percentage of SP cells in
tumors is correlated with tumor grade and patient prognosis
(46). Therefore, in the present
study, the stem-like cells from the CRC HT-29 cell line were
isolated as SP using fluorescence-activated cell sorting (FACS)
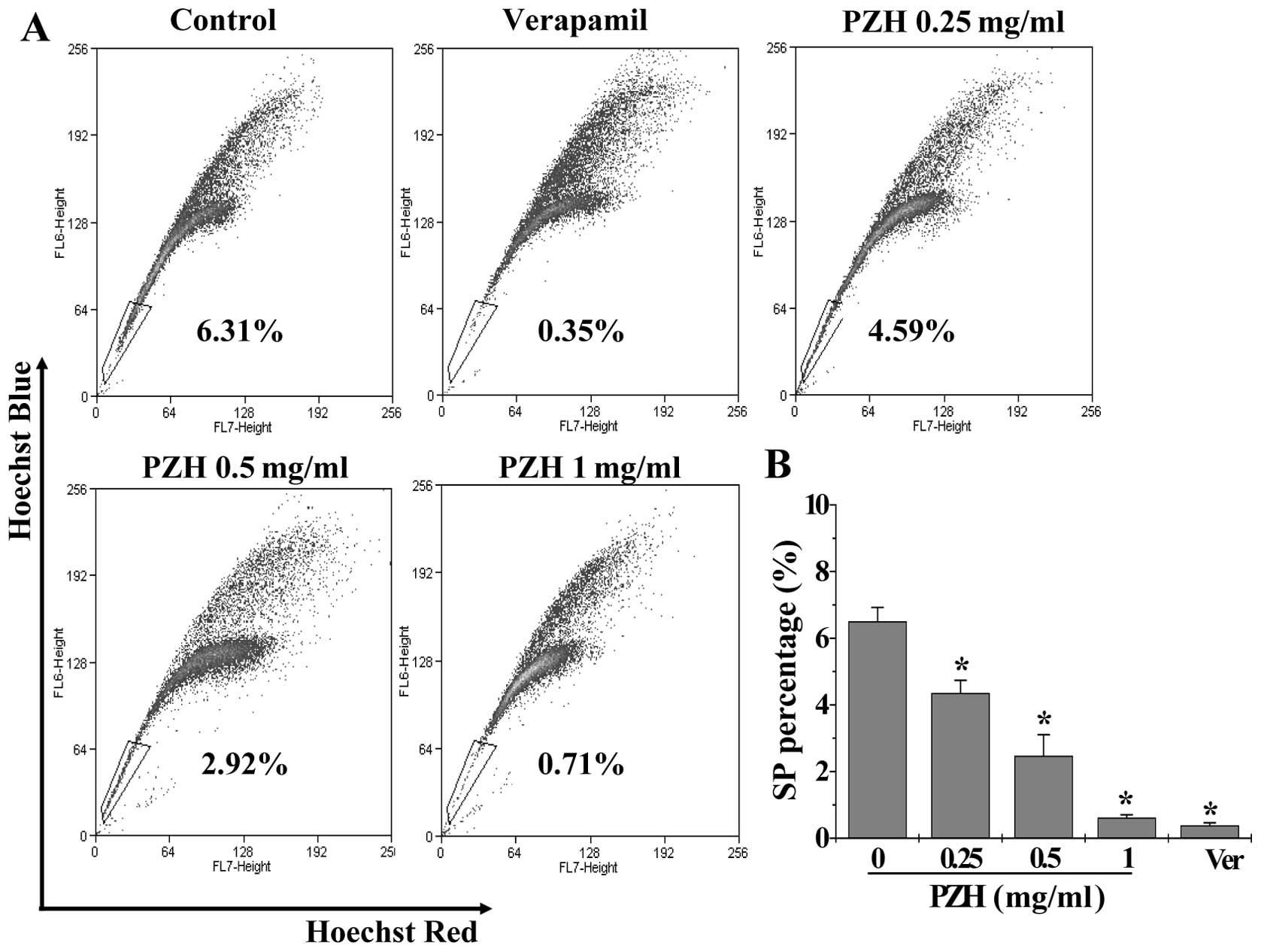
after staining with Hoechst 33342. As shown in Fig. 1, the percentage of SP in HT-29
cells was 6.50±0.42%. Following treatment with verapamil, a
multi-drug transporter inhibitor, the SP was reduced to 0.38±0.08%
(P<0.05).
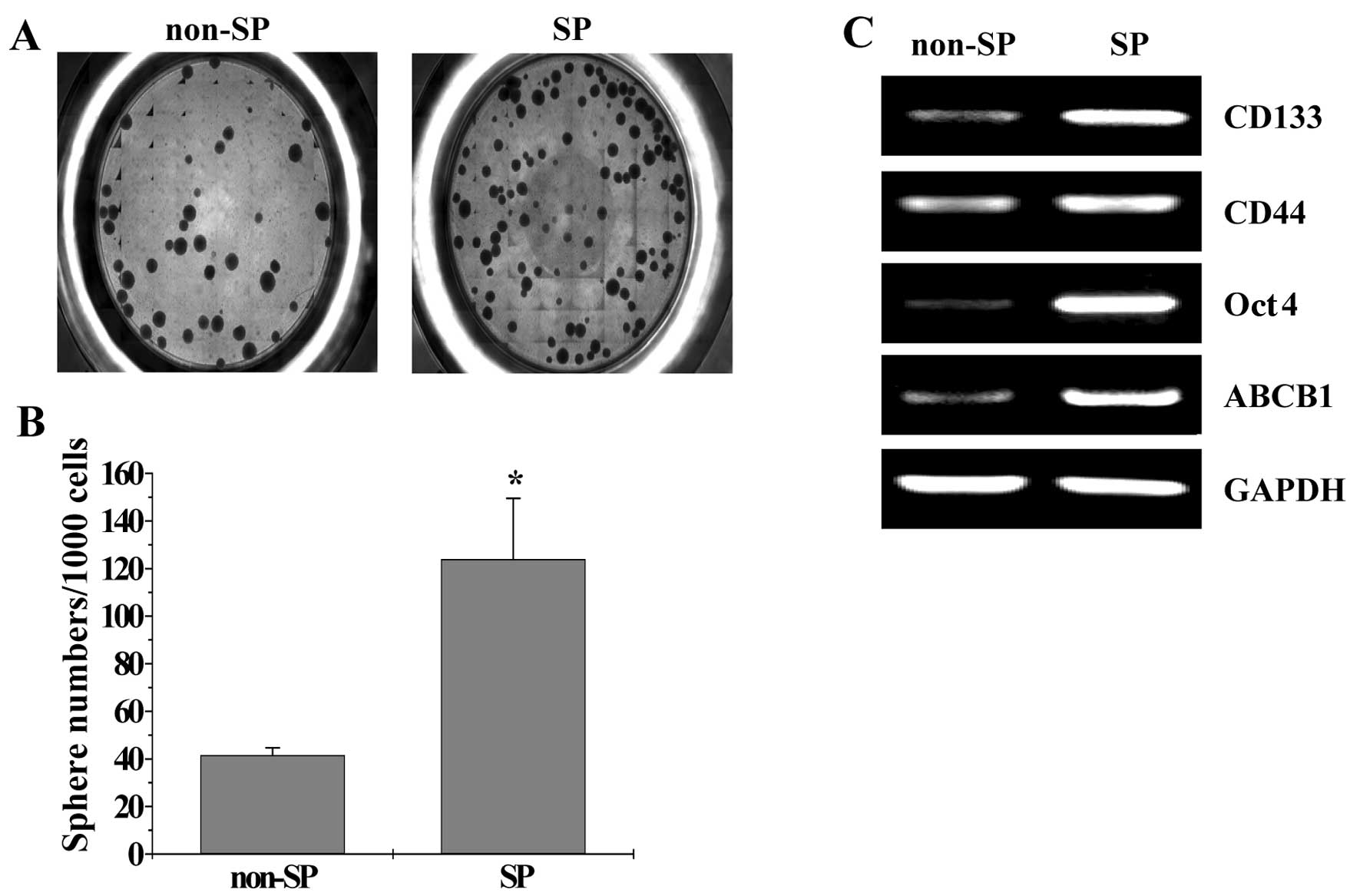
To determine whether the SP cells were enriched for
cancer stem cells (CSCs), the ability of the SP cells to undergo
in vitro sphere formation was examined. The results in
Fig. 2A show that the SP cells had
a higher ability to undergo sphere formation compared with non-SP
cells after 15 days in culture (P<0.05). To verify these
observations, an RT-PCR assay was performed to analyze the
expression of proteins that are either CSC surface markers or
specifically and/or highly expressed in CSCs (CD133, CD44, Oct4 and
ABCB1). As shown in Fig. 2B, the
mRNA expression level of CD133, CD44, Oct4 and ABCB1 in SP cells
was markedly higher than that in non-SP cells. Taken together,
these data suggest that human colorectal cancer HT-29 cells contain
a stem-like population.
PZH reduces the percentage of SP in HT-29
cells
CSCs are naturally resistant to conventional
chemotherapy treatments, leading to cancer relapse and metastasis
and eventually the failure of clinical anticancer treatment.
Therefore, development of novel therapeutic agents targeting CSCs
holds hope for improvement of effectiveness of anticancer
therapies. Natural products, including traditional Chinese medicine
(TCM), have long been used as alternative remedies for cancer.
Recently, natural products received attention in stem cell biology
as some compounds have been reported to attack cancer stem-like
cells as well as to improve the efficacy of conventional
chemotherapies (47,48). PZH is a well-known TCM formula that
has been used to clinically treat various types of cancer. Previous
studies have reported that PZH likely exerts its anticancer
activities via the modulation of multiple intracellular signaling
pathways (30–35). To elucidate the mechanism of the
tumoricidal activity of PZH, its effect was evaluated on colorectal
cancer stem-like cells by examining the size of SP in HT-29 cells
following treatment with various concentrations of PZH. As shown in
Fig. 1, the percentage of SP cells
following treatment with 0, 0.25, 0.5 and 1 mg/ml of PZH was
6.50±0.42, 4.35±0.39, 2.47±0.64 and 0.61±0.09%, respectively
(P<0.05, vs. untreated control cells), suggesting that PZH
possesses anti-CSC activity.
PZH inhibits the viability and sphere
formation capacity of SP in HT-29 cells
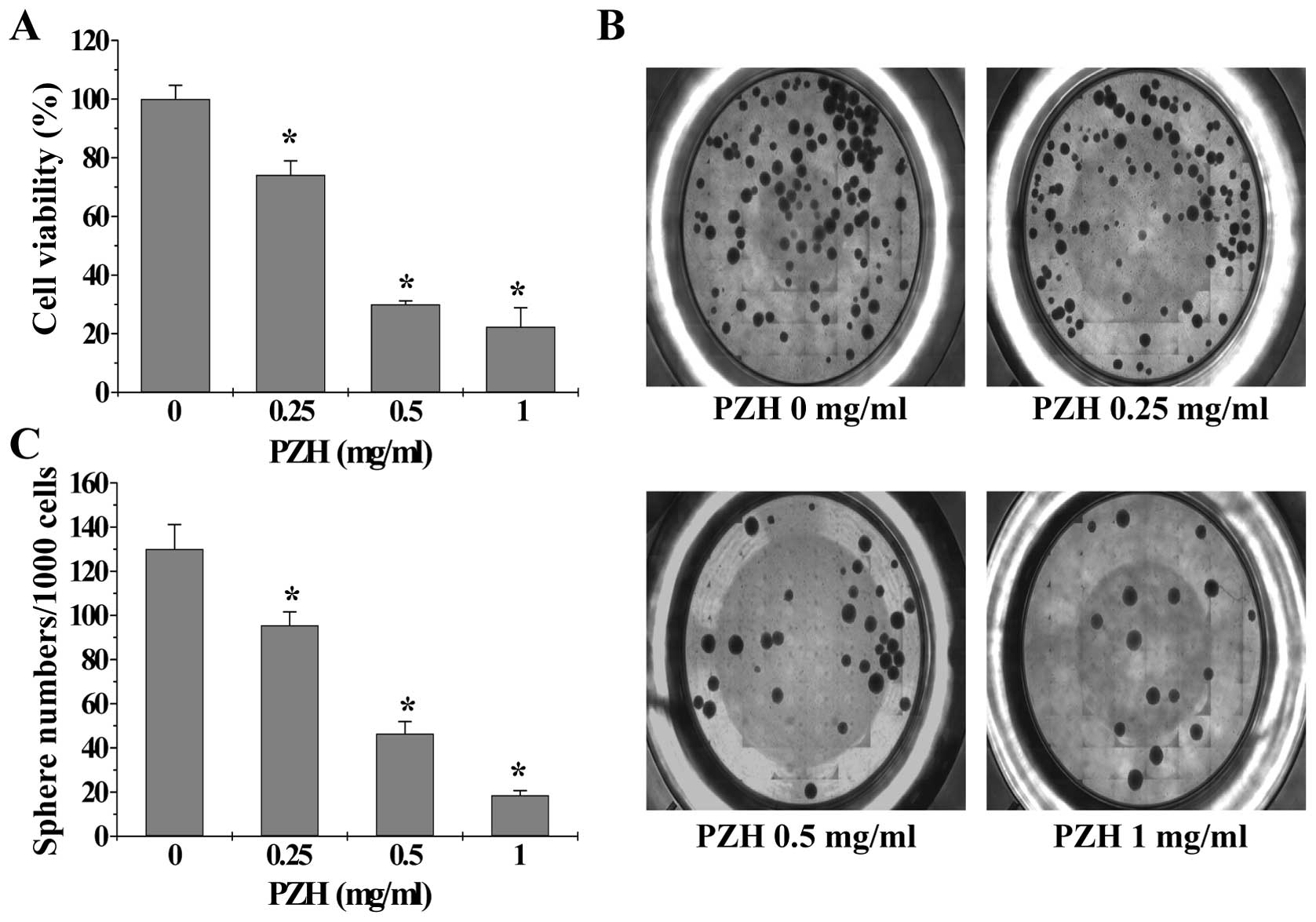
To determine the effect of PZH on the growth of
colorectal cancer stem-like cells, we treated isolated HT-29 SP
cells with PZH and then examined cell viability via WST-1 assay. As
shown in Fig. 3A, treatment with
0.25–1 mg/ml of PZH for 24 h dose-dependently reduced the viability
of HT-29 SP cells by 26.83–77.67% compared with the untreated
control cells (P<0.05). To confirm the growth suppressive
activity of PZH in cancer stem-like cells, we investigated its
effect on the sphere formation ability of SP cells. Data in
Fig. 3B and C show that PZH
dose-dependently suppressed sphere formation in isolated HT-29 SP
cells (P<0.05). These results demonstrate that PZH is potent in
suppressing the growth of colorectal cancer stem cells.
PZH inhibits the expression of ABCB1 and
ABCG2 of SP in HT-29 cells
ATP-binding cassette (ABC) transporter proteins
belong to the superfamily of membrane pumps that expel various
xenobiotics out of cells, such as chemotherapeutic drugs and
lipophilic fluorescent dyes, contributing to the SP phenotype and
chemotherapy resistance. ABC transporters are commonly
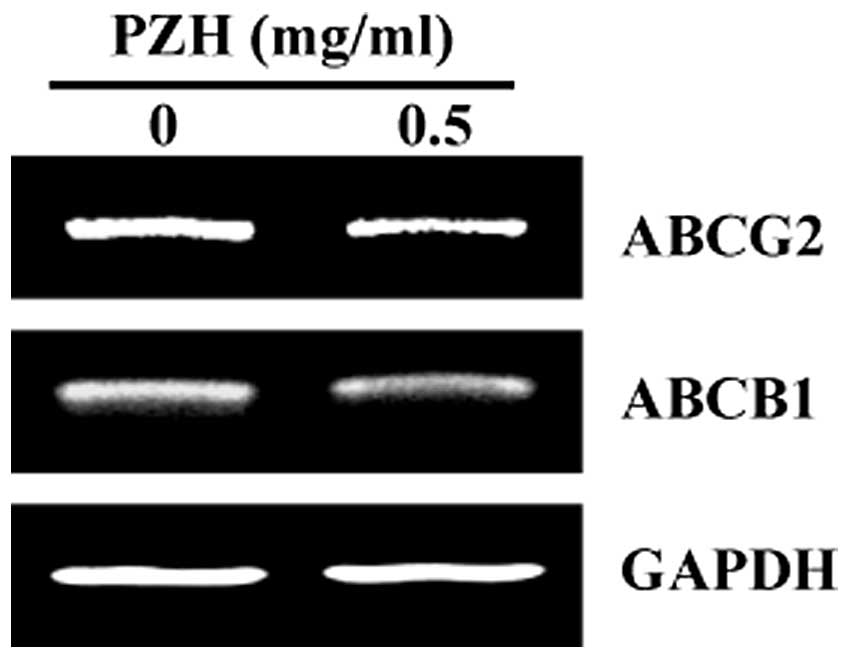
overexpressed in multi-drug-resistant tumors and CSCs. To explore
the mechanism whereby PZH inhibited the growth of colorectal cancer
stem-like cells, we examined the mRNA expression of ABCB1 and
ABCG2, which are considered the best-characterized ABC members.
Results from RT-PCR showed that PZH treatment markedly reduced the
mRNA levels of ABCB1 and ABCG2 in HT-29 SP cells (Fig. 4).
In conclusion, our findings in this study suggest
that attacking CSC is a potential mechanism by which PZH exerts its
anticancer activities.
Acknowledgements
This study was sponsored by the National Natural
Science Foundations of China (nos. 81073097 and 81202790) and the
China Postdoctoral Science Foundation (no. 2013T60636).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PZH
|
Pien Tze Huang
|
|
CSCs
|
cancer stem cells
|
|
SP
|
side population
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar
|
|
4
|
Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH,
Jiang HP and Teng LS: Molecular biomarkers of colorectal cancer:
prognostic and predictive tools for clinical practice. J Zhejiang
Univ Sci B. 13:663–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E and Costa F: Progress in the
adjuvant treatment of colon cancer: has it influenced clinical
practice? JAMA. 294:2758–2760. 2005.PubMed/NCBI
|
|
6
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
7
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 3:5232006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin L, Liu Y, Li H, Li PK, Fuchs J,
Shibata H, Iwabuchi Y and Lin J: Targeting colon cancer stem cells
using a new curcumin analogue, GO-Y030. Br J Cancer. 105:212–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
17
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H and Sorrentino BP: The ABC transporter Bcrp1/ABCG2 is
expressed in a wide variety of stem cells and is a molecular
determinant of the side-population phenotype. Nat Med. 7:1028–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: an
overview. Adv Drug Deliv Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: a perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar
|
|
20
|
Madjd Z, Mehrjerdi AZ, Sharifi AM,
Molanaei S, Shahzadi SZ and Asadi-Lari M: CD44+ cancer
cells express higher levels of the anti-apoptotic protein Bcl-2 in
breast tumours. Cancer Immun. 9:42009.
|
|
21
|
Carter BZ, Qiu Y, Huang X, Diao L, Zhang
N, Coombes KR, Mak DH, Konopleva M, Cortes J, Kantarjian HM, Mills
GB, Andreeff M and Kornblau SM: Survivin is highly expressed in
CD34(+)38(−) leukemic stem/progenitor cells and predicts poor
clinical outcomes in AML. Blood. 120:173–180. 2012.
|
|
22
|
Terpstra W, Ploemacher RE, Prins A, van
Lom K, Pouwels K, Wognum AW, Wagemaker G, Löwenberg B and Wielenga
JJ: Fluorouracil selectively spares acute myeloid leukemia cells
with long-term growth abilities in immunodeficient mice and in
culture. Blood. 88:1944–1950. 1996.PubMed/NCBI
|
|
23
|
Paterson SC, Smith KD, Holyoake TL and
Jørgensen JHG: Is there a cloud in the silver lining for imatinib?
Br J Cancer. 88:983–987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
26
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010, (In
Chinese).
|
|
27
|
Xu YY and Yu EX: Clinical analysis of the
effect of Pien Tze Huang in treatment of 42 patients with moderate
or advanced liver cancer. Shanghai J Tradit Chin Med (Chin).
12:4–5. 1994.(In Chinese).
|
|
28
|
Gu ZX: Therapeutical observation of
advanced colon cancer. Chin Tradit Patent Med (Chin). 15:231993.(In
Chinese).
|
|
29
|
Liu CS: Review of pharmacology and
clinical application of Pien Tze Huang. Med Pharm World (Chin).
7:64–66. 2006.(In Chinese).
|
|
30
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang-induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang QC, Hong F, Shen AL, Zheng LP, Zeng
JW, Lin W, Chen YQ, Sferra TJ, Hong ZF and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in colorectal cancer mouse. Int J
Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
32
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen AL, Hong F, Liu LY, Lin JM, Wei LH,
Cai QY, Hong ZF and Peng J: Pien Tze Huang inhibits the
proliferation of human colon carcinoma cells by arresting G1/S cell
cycle progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
34
|
Shen AL, Chen YQ, Hong F, Lin JM, Wei LH,
Hong ZF, Sferra TJ and Peng J: Pien Tze Huang suppresses
IL-6-inducible STAT3 activation in human colon carcinoma cells
through induction of SOCS3. Oncol Rep. 28:2125–2130.
2012.PubMed/NCBI
|
|
35
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013.PubMed/NCBI
|
|
36
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Golebiewska A, Brons NH, Bjerkvig R and
Niclou SP: Critical appraisal of the side population assay in stem
cell and cancer stem cell research. Cell Stem Cell. 8:136–147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chua C, Zaiden N, Chong KH, See SJ, Wong
MC, Ang BT and Tang C: Characterization of a side population of
astrocytoma cells in response to temozolomide. J Neurosurg.
109:856–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mitsutake N, Iwao A, Nagai K, Namba H,
Ohtsuru A, Saenko V and Yamashita S: Characterization of side
population in thyroid cancer cell lines: cancer stem-like cells are
enriched partly but not exclusively. Endocrinology. 148:1797–1803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2− cancer cells are
similarly tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar
|
|
46
|
Wu C, Wei Q, Utomo V, Nadesan P, Whetstone
H, Kandel R, Wunder JS and Alman BA: Side population cells isolated
from mesenchymal neoplasms have tumor initiating potential. Cancer
Res. 67:8216–8222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Efferth T: Stem cells, cancer stem-like
cells, and natural products. Planta Med. 78:935–942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weber DA, Wheat JM and Currie GM: Cancer
stem cells and the impact of Chinese herbs, isolates and other
complementary medical botanicals: a review. Zhong Xi Yi Jie He Xue
Bao. 10:493–503. 2012. View Article : Google Scholar : PubMed/NCBI
|