Introduction
Acute pancreatitis is currently one of the most
intractable diseases in the surgical acute abdomen. Due to the high
incidence rate and rapid progression, particularly for severe acute
pancreatitis (SAP) with systemic inflammatory response syndrome
(SIRS), the mortality rate of acute pancreatitis is high (1,2).
SIRS is an early manifestation of multiple organ dysfunction
syndrome (MODS) and multiple organ failure (MOF) (1,2).
It has been previously demonstrated that the
polymorphonuclear neutrophil (PMN) life cycle is prolonged and
apoptosis is delayed during infection, trauma and other stresses,
which subsequently promotes inflammatory reactions that result in
organ injury (3–8). Inflammatory reactions are worsened
unless apoptotic PMNs are ameliorated. Therefore, delayed apoptosis
of PMNs and the insufficient phagocytosis of apoptotic cells can
increase inflammation. The majority of apoptotic cells in
vivo are removed by macrophages (MΦs). MΦs identify, adhere and
phagocytoze apoptotic PMNs to subsequently inhibit inflammatory
reactions and promote inflammation resolution (9). Membrane-bound CD14 (mCD14) is one of
the main receptors for MΦ recognition, inducing the phagocytosis of
apoptotic neutrophils (10). In
the present study, a model of SAP/SIRS was established in rats to
investigate the in vitro effects of emodin compared with
that of dexamethasone (DEX) on the expression levels of mCD14
protein in peritoneal MΦs (pMΦs).
Materials and methods
Animals
Healthy male Sprague-Dawley (SD) rats (weight,
220–250 g) were provided by the Laboratory Animal Center of Dalian
Medical University (Dalian, China). Forty SD rats were randomly
divided into the sham-operated group (SO) (n=10) and the model
group (n=30). After 24 h, pMΦs were harvested and the model group
was randomly divided into three groups (n=10 in each group); the 5
μg/ml emodin group (EMO), the 0.1 μmol/ml DEX and the SIR/SAP group
(SI). The therapeutic agents were administered following pMΦ
adhesion for 24 h. The study was approved by the Ethics Committee
of the First Affiliated Hospital of Dalian Medical University
(Dalian, Liaoning, China).
Equipment
High-speed refrigerated 5840R centrifuge (Eppendorf,
Hamburg, Germany), flow cytometry (BD Biosciences, Franklin Lakes,
NJ, USA) and an immunofluorescence microscope (CX31-32RFL; Olympus,
Tokyo, Japan) were used in this study.
Reagents and drugs
RPMI-1640 medium, fetal bovine serum, fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit, rabbit anti-CD14
polyclonal antibody and emodin were purchased from Sigma (St.
Louis, MO, USA). Dextran T500 was purchased from Sigma.
SAP/SIRS model establishment
The SAP/SIRS rat model was established as described
previously (11). Briefly, rats
fasted for 12 h prior to surgery, but had access to water ad
libitum. Rats were then anesthetized intraperitoneally with an
injection of 10% chloral hydrate (Sigma) at a dose of 0.3 ml/100 g.
To expose the duodenum, a midline laparotomy was performed. A 1 ml
syringe needle was inserted through the contralateral intestinal
wall of the duodenal papilla into the bile and pancreatic ducts,
and clamped using a noninvasive bulldog clamp, followed by slow
retrograde perfusion of 1.5% sodium deoxycholate (0.1 ml/100 mg)
for 60 sec. The duodenal papilla was pinched to prevent back flow.
The SO group were only subjected to a celiotomy.
Isolation, purification, culture and
administration of pMφs
Trypan blue staining was performed to detect the pMφ
survival rates and cell purity was >98 and >95% in the SO and
model groups, respectively. The majority of cells showed the
morphological features of Mφs. pMφs from each group were seeded in
6-well culture plates and treated with 5 μg/ml emodin or 0.1
μmol/ml DEX. The SI and SO groups were untreated. Cells were then
incubated at 37°C with 5% CO2 for 24 h.
Pathomorphological observation of
pancreatic tissue
Pancreatic tissue sections, cut from the
formalin-fixed, paraffin-embedded tissues, were routinely assessed
with hematoxylin and eosin staining. The pathological scoring was
performed according to the method of Kaiser et al(11).
Detection of mCD14 protein expression in
pMφs using immunofluorescence
Glass slides were placed into 24-well culture
plates. PMφ concentration was adjusted to 5×106
cells/ml. After 30 min, cells in 2 ml RPMI-1640 containing serum
were added to the culture plates and incubated at 37°C with 5%
CO2 for 24 h. Emodin (5 μg/ml) and DEX (0.1 μmol/ml)
were respectively added into separate wells, followed by incubation
at 37°C with 5% CO2 overnight. Cells were harvested,
washed with phosphate-buffered saline (PBS) three times, dried,
fixed with 4% paraformaldehyde for 30 min and then washed again
with PBS three times. One drop of non-immune animal serum was added
to each slide and incubated at room temperature for 30 min.
Additionally, 100 μl rabbit anti-CD14 polyclonal antibody (1:100;
Sigma) was added to each slide and incubated at room temperature
for 30 min in the dark. Slides were washed with PBS three times for
5 min and then dried. Fluorescein isothiocyanate (FITC)-conjugated
goat anti-rabbit antibody (50 μl; Sigma) was added to each slide
and incubated at room temperature for 30 min. Slides were washed
with PBS three times for 5 min, dried and observed under a
fluorescence microscope (Olympus). Positive reactions were detected
under a fluorescence microscope as green fluorescent particles,
indicating the presence of pMφ mCD14 receptors.
Detection of mCD14 expression in pMφs
using flow cytometry
After 24 h of culture, cells were washed three times
in pre-warmed Hank’s solution (Beyotime, Shanghai, China) and then
trypsinized with 0.25% trypsin at 37°C for 5–6 min. When 90% of the
adhered pMφs appeared round and transparent as observed under an
inverted microscope (Nikon Eclipse TS100; Nikon Corporation, Tokyo,
Japan), digestion was terminated with 10–20 ml RPMI-1640 medium and
cells were triturated. Cells were centrifuged at 111.8 × g for 10
min at 4°C. The supernatant was discarded and cells were incubated
with 2 μl rabbit anti-CD14 polyclonal antibody (Sigma) at room
temperature for 1 h, washed with PBS three times, centrifuged at
1,000 r/min (R=10 cm) for 10 min at 4°C, and incubated with 2 μl
FITC-conjugated goat anti-rabbit antibody (Sigma) at room
temperature for 15 min. Cells were then washed three times with PBS
and centrifuged at 1,000 r/min for 10 min at 4°C. Cells were
resuspended in 1 ml PBS and mCD14 expression was determined by flow
cytometry.
Statistical analysis
Data were analyzed using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation. Enumeration data were analyzed using the exact
probability of a 4-fold table and measurement data with completely
random analysis of variance. Paired comparison was performed using
a q-test. P<0.05 was considered to indicate a statistically
significant difference at an α level of 0.05.
Results
Clinical manifestations
Following injection of 1.5% sodium deoxycholate,
rats exhibited rapid breathing and symptoms were aggravated with
prolonged time. Rats also presented with cyanosis of the mucosa of
the skin, unconsciousness and occasionally death (mortality rate
was 20% in the model groups).
Gross observation
Immediately following injection of 1.5% sodium
deoxycholate, the pancreatic gland presented with evident regional
or diffuse hyperemia and edema, with increased pancreatic envelope
tension. After 24 h, pancreatic hemorrhaging, necrosis and bloody
ascites were observed in the surviving rats of the model groups. In
addition to yellow saponaceous spots in the greater omentum and
common bile duct, lung hyperemia, edema, bleeding in the lung,
stomach edema, paralytic expansion, liver swelling and kidney
augmentation were observed. However, in the SO group, mild edema of
gastrointestinal mucosa and exudation in the abdominal cavity were
observed.
Pathological changes in pancreatic
tissues observed by light microscopy
The SO group exhibited clear pancreatic lobule
structures. The model groups presented with necrosis in the
pancreatic glandular parenchyma, bleeding, fatty degeneration,
erythrocyte stasis, angiectasis and PMN infiltration into the
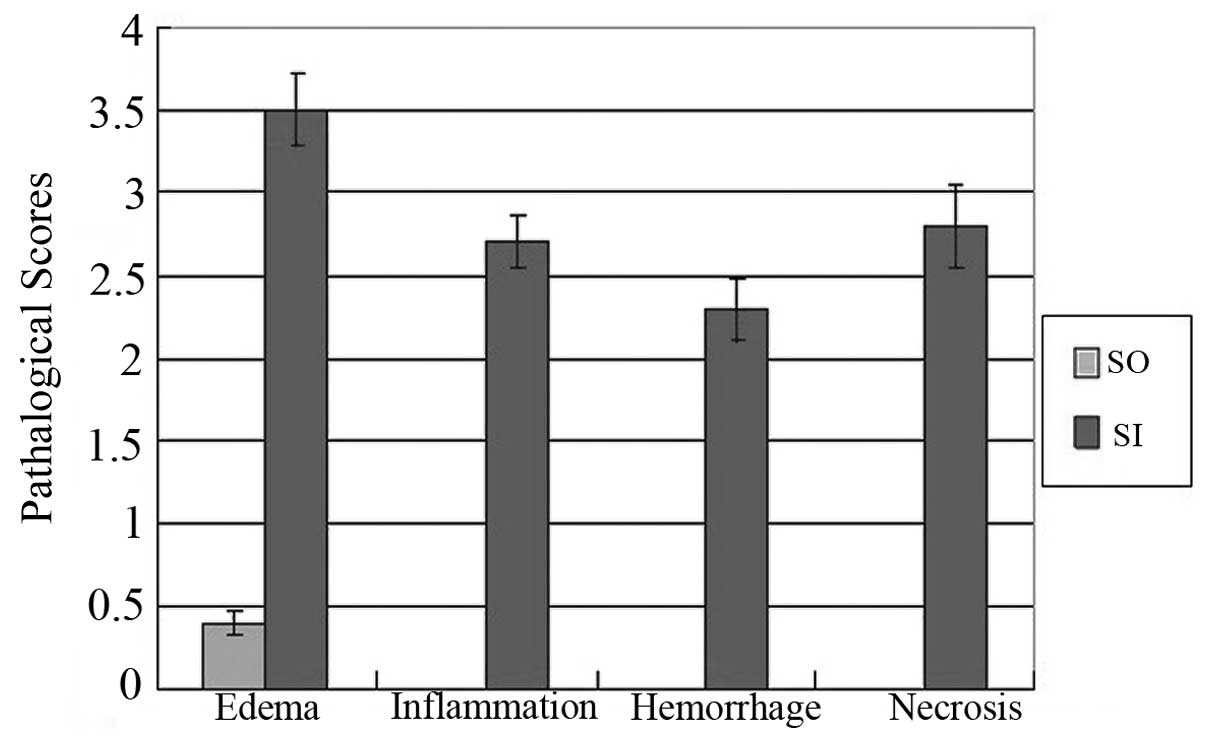
tissue space and parenchyma. Compared with the SO group, the SI
group showed significantly increased pathological changes (edema,
3.50±0.21 vs. 0.40±0.07; inflammation, 2.70±0.16 vs. 0; bleeding,
2.30±0.19 vs. 0 and necrosis, 2.80±0.25 vs. 0; P<0.01; Table I and Fig. 1).
 | Table IComparison of the pathological changes
in the SO and SI groups. |
Table I
Comparison of the pathological changes
in the SO and SI groups.
| Group | No. of rats | Edema | Inflammation | Hemorrhage | Necrosis | P-value |
|---|
| SO | 10 | 0.40±0.07 | 0 | 0 | 0 | <0.01 |
| SI | 8 | 3.50±0.21 | 2.70±0.16 | 2.30±0.19 | 2.80±0.25 | |
mCD14 expression in pMφs in each
group
As detected by immunofluorescence, green fluorescent
particles were observed in the SO, EMO, DEX and SI groups,
indicating the presence of pMφ mCD14 receptors (Fig. 2).
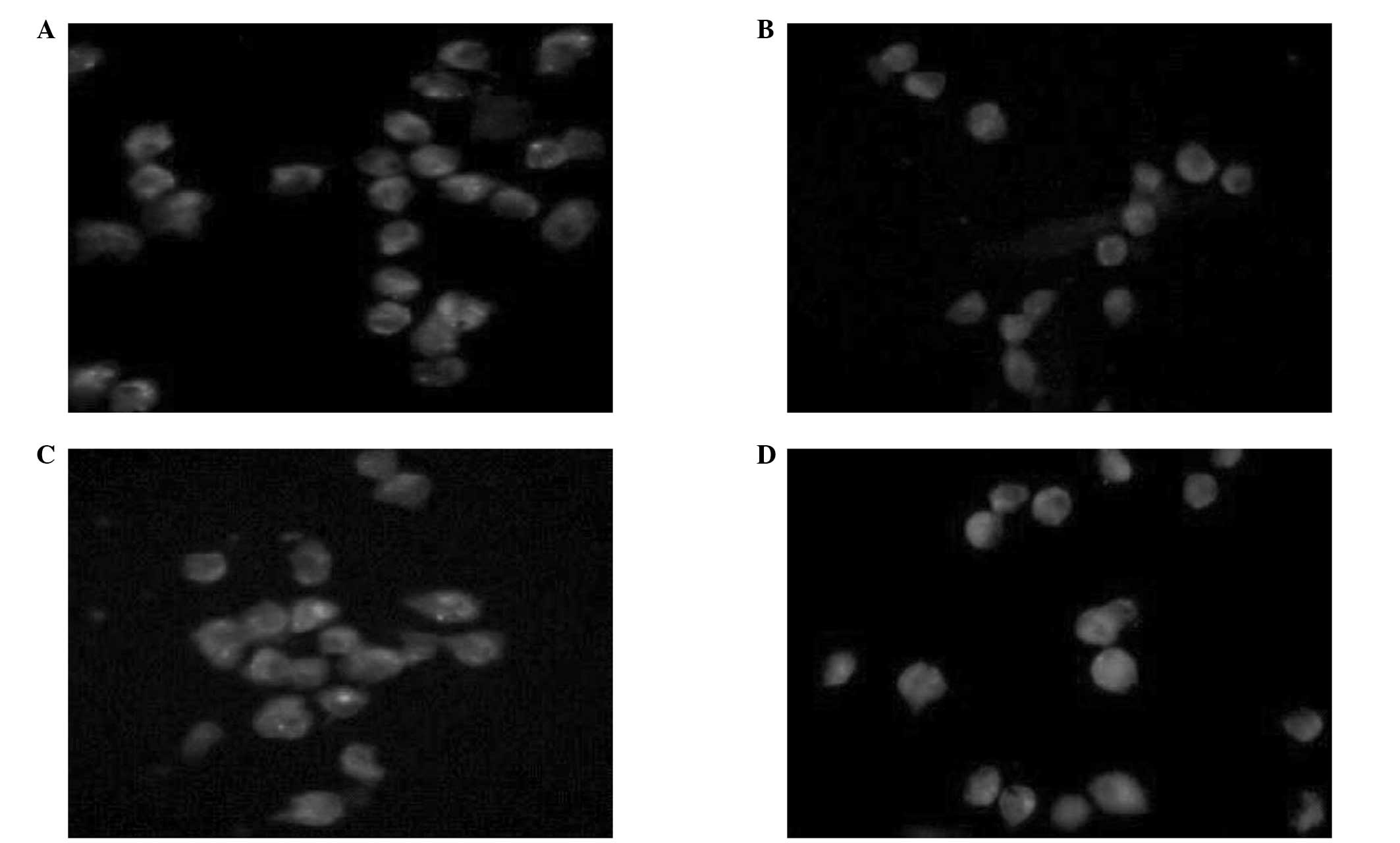 | Figure 2As detected by immunofluorescence,
green fluorescent particles were found in the (A) SO, (B) SI, (C)
EMO and (D) DEX groups, indicating the presence of pMφ mCD14
receptors in each group (magnification, ×100). SO, sham-operated
group; SI, SIRS/SAP group; EMO, emodin group; DEX, dexamethasone
group; pMφ, peritoneal macrophage; mCD14, membrane-bound cluster of
differentiation 14 receptor. |
The mCD14 expression levels in pMφs in each group
detected by flow cytometry (mean ± standard deviation) are shown in
Table II and Fig. 3. Compared with the SO group, the
expression levels of mCD14 in pMφs were significantly decreased in
the SI group (28.55±2.53 vs. 14.76±2.84; P<0.01). Compared with
the SI group, the expression levels of mCD14 in pMφs were
significantly increased in the EMO (25.60±2.79 vs. 14.76±2.84;
P<0.01) and DEX (20.87±1.99 vs. 14.76±2.84; P<0.01) groups.
Compared with the DEX group, mCD14 expression levels in pMφs were
significantly increased in the EMO group (25.60±2.79 vs.
20.87±1.99; P<0.01).
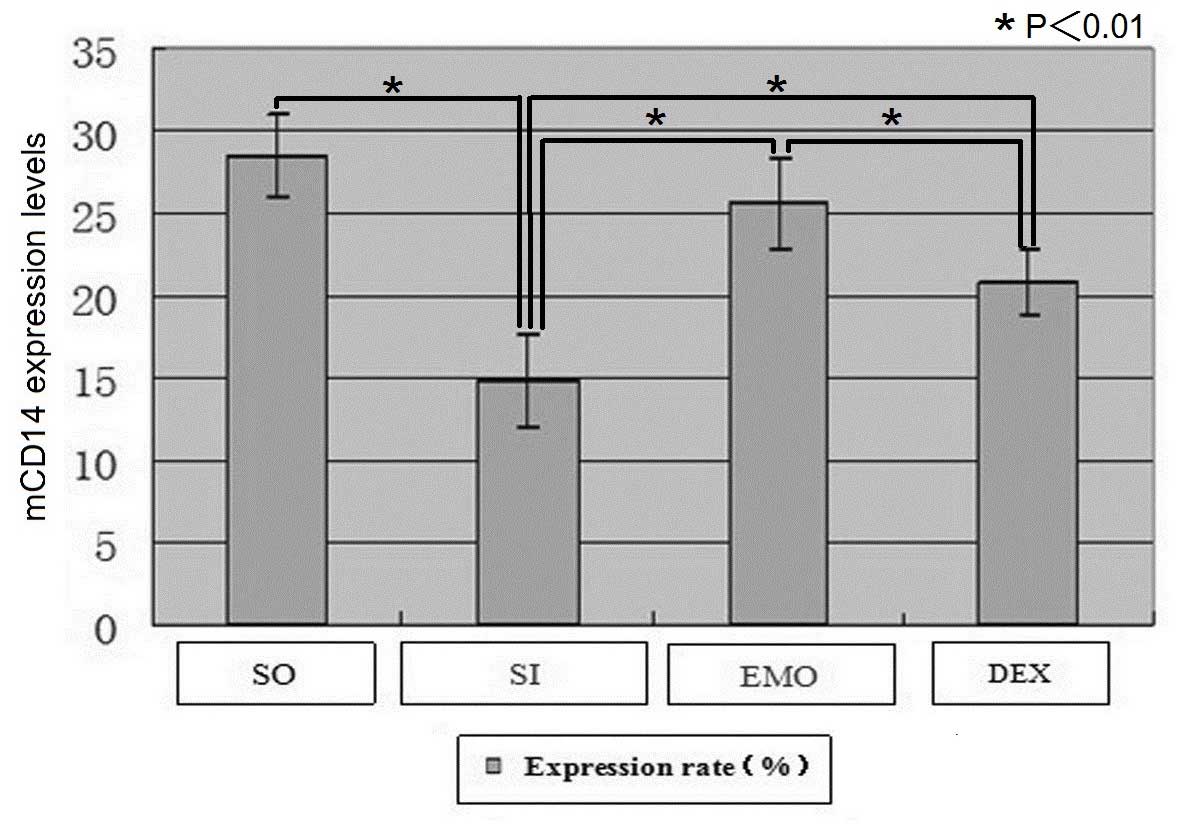 | Figure 3mCD14 expression levels in pMφs in
each group detected by flow cytometry. Compared with the SO group,
mCD14 expression levels in pMφs were significantly decreased in the
model groups. Compared with the SO group, mCD14 expression levels
in pMφs were significantly decreased in the SI group (P<0.01).
Compared with the SI group, mCD14 expression levels were
significantly increased in the EMO (P<0.01) and DEX (P<0.01)
groups. Compared with the DEX group, mCD14 expression levels in
pMφs were significantly increased in the EMO group (P<0.01).
PMφ, peritoneal macrophage; mCD14, membrane-bound cluster of
differentiation 14 receptor; SO, sham-operated group; SI, SIRS/SAP
group; EMO, emodin group; DEX, dexamethasone group. |
 | Table IImCD14 expression levels in pMφs in
each group as detected by flow cytometry. |
Table II
mCD14 expression levels in pMφs in
each group as detected by flow cytometry.
| Groups | No. of rats | Expression rate
(%) |
|---|
| SO | 10 | 28.55±2.53 |
| SI | 8 | 14.76±2.84a |
| EMO | 8 | 25.60±2.79b |
| DEX | 8 | 20.87±1.99b,c |
Discussion
There are numerous receptors, which are involved in
the recognition and phagocytosis of apoptotic cells, on the surface
of phagocytes. The protein, CD14, is one of the five macrophage
receptors located on the surface of phagocytes, which is involved
in the recognition and phagocytosis of apoptotic cells. The
expression of CD14 is tissue and cell specific. CD14 is mainly
expressed on the surface of mononuclear Mφs and is weakly expressed
on the surface of neutrophils and lymphocytes (12–15).
The expression levels of CD14 are different from that of various
types of mononuclear Mφs; the expression of CD14 is stronger on the
surface of pMφs and weaker on the surface of alveolar macrophages,
Kupffer and microglia cells (16).
Moreover, numerous factors can affect the expression levels of CD14
in the development process of monocytes to Mφs. There are two forms
of CD14, termed mCD14 and soluble CD14 receptor (sCD14). sCD14
appears in the serum due to proteolytic cleavage and phospholipase
D-induced mCD14 shedding from monocytes (10). mCD14 is one of the predominant
receptors that Mφs recognize inducing the phagocytosis of apoptotic
cells. CD14 is a receptor for the serum lipopolysaccharide (LPS)
complex or LPS binding protein (LBP) and may be shed from the
monocyte surface to inhibit the release of tumor necrosis factor-α
(TNF-α) (17). In addition,
Marchant et al(18) found
that a low concentration of LPS increased the expression levels of
monocyte mCD14. In the present study, mCD14 protein expression
levels in pMφs were significantly decreased following the induction
of SAP/SIRS compared with that of the SO group. Therefore, it was
speculated that SIRS induced intestinal mucosal barrier damage and
bacterial translocation causes intestinal endotoxemia. After
binding to LBP receptors on Mφs thereby forming LPS-LBP complexes,
endotoxins could induce Mφs to synthesize and release a large
quantity of TNF-α and other inflammatory mediators that trigger
inflammatory reactions (16,19–22).
Additionally, mCD14 may be shed from the monocyte surface
accompanied by a decrease in the levels of mCD14.
Dahuang (Rhubarb), a Chinese herb, has been found to
be clinically effective for the treatment of acute pancreatitis
(23–26). Emodin is the main active component
of Dahuang. Previous studies have shown that emodin affects
bacteriostasis, catharsis, releases Oddi sphincter spasms, inhibits
abnormal metabolism of vasoactive substances (eicosenoic acid),
improves microcirculation and antagonizes coagulation and thrombus
formation (23,27,28).
The present study showed that emodin significantly increased mCD14
protein expression levels in pMφs in rats with SAP/SIRS compared
with that of the SI (P<0.01), which subsequently enhanced the
identification and phagocytosis capacity of pMφs and relieved
inflammatory reactions.
In 1952, Stephensen et al(29) first reported the application of
glucocorticoids to treat acute pancreatitis. However, the
mechanisms underlying their effect have not been fully elucidated.
The effects of glucocorticoids on inflammation via receptor
mediation may trigger anti-inflammation. Glucocorticoids inhibit
inflammatory exudation, leukocytic infiltration, inflammatory
mediator production and release, as well as improve
microcirculation, alleviate endotoxemia and induce apoptosis of
pancreatic acinar cells, thereby reducing the degree of pancreatic
necrosis in SAP (30,31). A previous study demonstrated that
pancreatic cell apoptosis occurs during acute pancreatitis
(32). Animal studies have also
indicated that DEX induces pancreatic cell apoptosis, stabilizes
the internal environment and attenuates inflammation in pancreatic
tissues (31,33). In the present study, it was
demonstrated that DEX increased mCD14 expression levels in
pMφs.
In this study, it was demonstrated that compared
with the SO group, mCD14 expression levels in pMφs were
significantly decreased in the model groups. In vitro emodin
or DEX administration increased the expression levels of mCD14 in
pMφs. Notably, emodin exhibited more significant effects than DEX,
suggesting that emodin and DEX may be used together due to their
respective advantages.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81072918/H2902).
Abbreviations:
|
mCD14
|
membrane-bound CD14 receptor
|
|
sCD14
|
soluble CD14 receptor
|
|
SO
|
sham-operated group
|
|
EMO
|
emodin group
|
|
DEX
|
dexamethasone group
|
|
SI
|
SIRS/SAP group
|
|
SAP
|
severe acute pancreatitis
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
MODS
|
multiple organ dysfunction
syndrome
|
|
MΦs
|
macrophages
|
|
pMΦs
|
peritoneal macrophages
|
|
PMN
|
polymorphonuclear neutrophil
|
|
LPS
|
lipopolysaccharide
|
|
LPB
|
lipopolysaccharide binding protein
|
References
|
1
|
Frossard JL, Steer ML and Pastor CM: Acute
pancreatitis. Lancet. 371:143–152. 2008. View Article : Google Scholar
|
|
2
|
Doctor N, Agarwal P and Gandhi V:
Management of severe acute pancreatitis. Indian J Surg. 74:40–46.
2012. View Article : Google Scholar
|
|
3
|
Blomgran R, Patcha Brodin V, Verma D, et
al: Common genetic variations in the NALP3 inflammasome are
associated with delayed apoptosis of human neutrophils. PLoS One.
7:e313262012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paunel-Görgülü A, Kirichevska T, Lögters
T, Windolf J and Flohé S: Molecular mechanisms underlying delayed
apoptosis in neutrophils from multiple trauma patients with and
without sepsis. Mol Med. 18:325–335. 2012.PubMed/NCBI
|
|
5
|
Schwartz JT, Bandyopadhyay S, Kobayashi
SD, et al: Francisella tularensis alters human neutrophil
gene expression: insights into the molecular basis of delayed
neutrophil apoptosis. J Innate Immun. 5:124–136. 2013. View Article : Google Scholar
|
|
6
|
Terashima M, Aoyama-Ishikawa M, Ueda T, et
al: The effects of n-3 polyunsaturated fatty acid-rich total
parenteral nutrition on neutrophil apoptosis in a rat endotoxemia.
J Clin Biochem Nutr. 52:154–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang D, Wang BZ, Bi W, Chen HL, Qi QH and
Guan FL: Abnormal polymorphonuclear neutrophils apoptosis in
systemic inflammatory response syndrome. Dalian Yike Daxue Xuebao.
28:161–163. 2006.
|
|
8
|
Zhang J, He J, Xia J, Chen Z and Chen X:
Delayed apoptosis by neutrophils from COPD patients is associated
with altered Bak, Bcl-xl, and Mcl-1 mRNA expression. Diagn Pathol.
7:652012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willems CH, Urlichs F, Seidenspinner S,
Kunzmann S, Speer CP and Kramer BW: Poractant alfa
(Curosurf®) increases phagocytosis of apoptotic
neutrophils by alveolar macrophages in vivo. Respir Res.
13:172012.
|
|
10
|
Schütt C: CD14. Int J Biochem Cell Biol.
31:545–549. 1999.
|
|
11
|
Kaiser AM, Saluja AK, Sengupta A, Saluja M
and Steer ML: Relationship between severity, necrosis, and
apoptosis in five models of experimental acute pancreatitis. Am J
Physiol. 269:C1295–C1304. 1995.PubMed/NCBI
|
|
12
|
Pietruska M, Zak J, Pietruski J and
Wysocka J: Evaluation of mCD14 expression on monocytes and the
blood level of sCD14 in patients with generalized aggressive
periodontitis. Adv Med Sci. 51(Suppl 1): S166–S169. 2006.PubMed/NCBI
|
|
13
|
Wright SD, Ramos RA, Tobias PS, Ulevitch
RJ and Mathison JC: CD14, a receptor for complexes of
lipopolysaccharide (LPS) and LPS binding protein. Science.
249:1431–1433. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klaassen EM, Thönissen BE, van Eys G,
Dompeling E and Jöbsis Q: A systematic review of CD14 and toll-like
receptors in relation to asthma in Caucasian children. Allergy
Asthma Clin Immunol. 9:102013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fadok VA, Warner ML, Bratton DL and Henson
PM: CD36 is required for phagocytosis of apoptotic cells by human
macrophages that use either a phosphatidylserine receptor or the
vitronectin receptor (alpha v beta 3). J Immunol. 161:6250–6257.
1998.PubMed/NCBI
|
|
16
|
Ziegler-Heitbrock HW and Ulevitch RJ:
CD14: cell surface receptor and differentiation marker. Immunol
Today. 14:121–125. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bazil V and Strominger JL: Shedding as a
mechanism of down-modulation of CD14 on stimulated human monocytes.
J Immunol. 147:1567–1574. 1991.PubMed/NCBI
|
|
18
|
Marchant A, Duchow J, Delville JP and
Goldman M: Lipopolysaccharide induces up-regulation of CD14
molecule on monocytes in human whole blood. Eur J Immunol.
22:1663–1665. 1992. View Article : Google Scholar
|
|
19
|
Nathens AB, Bitar R, Marshall JC, et al:
Antioxidants increase lipopolysaccharide-stimulated TNF alpha
release in murine macrophages: role for altered TNF alpha mRNA
stability. Shock. 16:361–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsiao HB, Wu JB, Lin H and Lin WC:
Kinsenoside isolated from Anoectochilus formosanus
suppresses LPS-stimulated inflammatory reactions in macrophages and
endotoxin shock in mice. Shock. 35:184–190. 2011.
|
|
21
|
Li S, Ni Z, Cong B, et al: CCK-8 inhibits
LPS-induced IL-1beta production in pulmonary interstitial
macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock.
27:678–686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boonstra A, Rajsbaum R, Holman M, et al:
Macrophages and myeloid dendritic cells, but not plasmacytoid
dendritic cells, produce IL-10 in response to MyD88- and
TRIF-dependent TLR signals, and TLR-independent signals. J Immunol.
177:7551–7558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Xia X, Zhang S, Zhang A, Bo W and
Zhou R: Up-regulation of Toll-like receptor 4 was suppressed by
emodin and baicalin in the setting of acute pancreatitis. Biomed
Pharmacother. 63:120–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Sun B, Gao Y, Meng QH and Jiang
HC: The effect of emodin-assisted early enteral nutrition on severe
acute pancreatitis and secondary hepatic injury. Mediators Inflamm.
2007:296382007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang CY, Shen L, Xie ZG, Jiang X, Liang N
and Chen ZH: Experimental studies of therapeutic effect of Rheum
officinale on acute pancreatitis. Zhong Yao Cai. 34:84–88. 2011.(In
Chinese).
|
|
26
|
Zheng SH, Tong QY, Zhu ZY, Li ZY and You
H: Effect of Rhubarb administered via different routes on blood
inflammatory cytokines levels of patients with severe acute
pancreatitis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:437–438.
2011.(In Chinese).
|
|
27
|
Kuo YC, Meng HC and Tsai WJ: Regulation of
cell proliferation, inflammatory cytokine production and calcium
mobilization in primary human T lymphocytes by emodin from
Polygonum hypoleucum Ohwi. Inflamm Res. 50:73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li A, Dong L, Duan ML, Sun K, Liu YY, Wang
MX, et al: Emodin improves lipopolysaccharide-induced
microcirculatory disturbance in rat mesentery. Microcirculation.
20:617–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stephenson HE Jr, Pfeffer RB and Saypol
GM: Acute hemorrhagic pancreatitis; report of a case with cortisone
treatment. AMA Arch Surg. 65:307–308. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jingmin O, Xiping Z, Chun W, Ping Y and
Qian Y: Study of dexamethasone, baicalin and octreotide on brain
injury of rats with severe acute pancreatitis. Inflamm Res.
61:265–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XP, Chen L, Hu QF, et al: Effects of
large dose of dexamethasone on inflammatory mediators and
pancreatic cell apoptosis of rats with severe acute pancreatitis.
World J Gastroenterol. 13:5506–5511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jha RK, Ma Q, Sha H and Palikhe M: Acute
pancreatitis: a literature review. Med Sci Monit. 15:RA147–RA156.
2009.PubMed/NCBI
|
|
33
|
Ou JM, Zhang XP, Wu CJ, Wu DJ and Yan P:
Effects of dexamethasone and Salvia miltiorrhiza on multiple
organs in rats with severe acute pancreatitis. J Zhejiang Univ Sci
B. 13:919–931. 2012.
|