Introduction
Since the 1980’s, when transgenic mice were first
produced by Gordon et al (1), transgenic biotechnology has been
widely used to produce genetically modified animals for scientific
and pharmaceutical research, animal husbandry and agricultural
purposes (2–4). Over the past 30 years, scientists
have continued to search for more efficient and easier transgenic
methods. To date, various transgenic approaches have been
developed, including pronuclear microinjection (PM), in
vitro transfection of embryonic cells, particularly stem cells
and embryonic stem cells, and the use of retroviruses and germ
cells as vectors. Among the current methods, the use of
retroviruses and germ cells as vectors for gene transfer are
considered to be the most successful (5).
There are several germ cell-mediated gene transfer
(GCMGT) methods, including male and female GCMGT (MGCMGT and
FGCMGT, respectively). MGCMGT can be divided into sperm-mediated
gene transfer (SMGT) (6) and
testis-mediated gene transfer (TMGT) (7,8),
while FGCMGT is divided into oocyte-mediated gene transfer
(9) and ovary-mediated gene
transfer (10). Compared with the
conventional PM approach, TMGT and ovary-mediated gene transfer
(OMGT) are markedly cheaper and faster, and provide quick and
effective delivery of genes to the target tissue (5). However, the transgenic offspring and
mechanism of TMGT and OMGT require further investigation.
The replication-competent avian sarcoma-leukosis
virus (ASLV) long terminal repeat with splice acceptor-tumor virus
A (RCAS-TVA) system is one type of gene transfer method that is
used extensively. Using this method, TVA, a receptor for the ASLV-A
in avian cells, is transferred to mammalian cells. Subsequently,
using RCAS, an avian retrovirus, the foreign DNA is transferred
into the mammalian cells. RCAS-TVA has been widely used to study
gene function, as well as to investigate gene therapy,
developmental processes, tumorigenesis and diseases (11–19).
To apply the RCAS-TVA system in gene research, the
production of TVA transgenic animals is required. The key elements
involved are the tissue- or organ-specific promoters that determine
the specific expression of TVA in target organs, tissues or cells.
To examine the effect of certain oncogenes on ovarian
tumorigenesis, the present study produced transgenic mice that
expressed TVA in their ovaries. Of the promoters that are able to
control gene expression in the ovary, including β-actin (20), keratin (21) and ovarian-specific promoter 1
(OSP1) (22,23), OSP1 is accepted as the most
specific (20–23); therefore, OSP1 was selected to
control TVA expression in the mice ovaries in the present
study.
In this study, transgenic mice expressing TVA under
the control of OSP1 in their ovaries were produced. The results
confirmed that a TVA gene with an OSP1 promoter could be
transferred to the eggs via the sperm during fertilization, whereby
transgenic F1 and F2 individuals were produced. The transgenic
nature of the offspring was verified using polymerase chain
reaction (PCR), southern blot hybridization and semi-quantitative
reverse transcription (RT)-PCR. The results confirmed that a
foreign gene is capable of being transferred to a transgenic F1
animal genome and subsequently to F2 descendants.
Materials and methods
Animals
Six-week-old adult Imprinting Control Region (ICR)
mice were purchased from the Laboratory Animal Center of the
Academy of Military Medical Science (Beijing, China) and housed in
our laboratory’s specific-pathogen free animal unit (Peking
University People’s Hospital, Beijing, China), under controlled
temperature (20–26°C) and humidity (40–70%), with a 12-h light/dark
cycle. The experimental protocol for the use of mice in this study
was approved by the Animal Care and Use Committee at Peking
University People’s Hospital.
Production of vectors
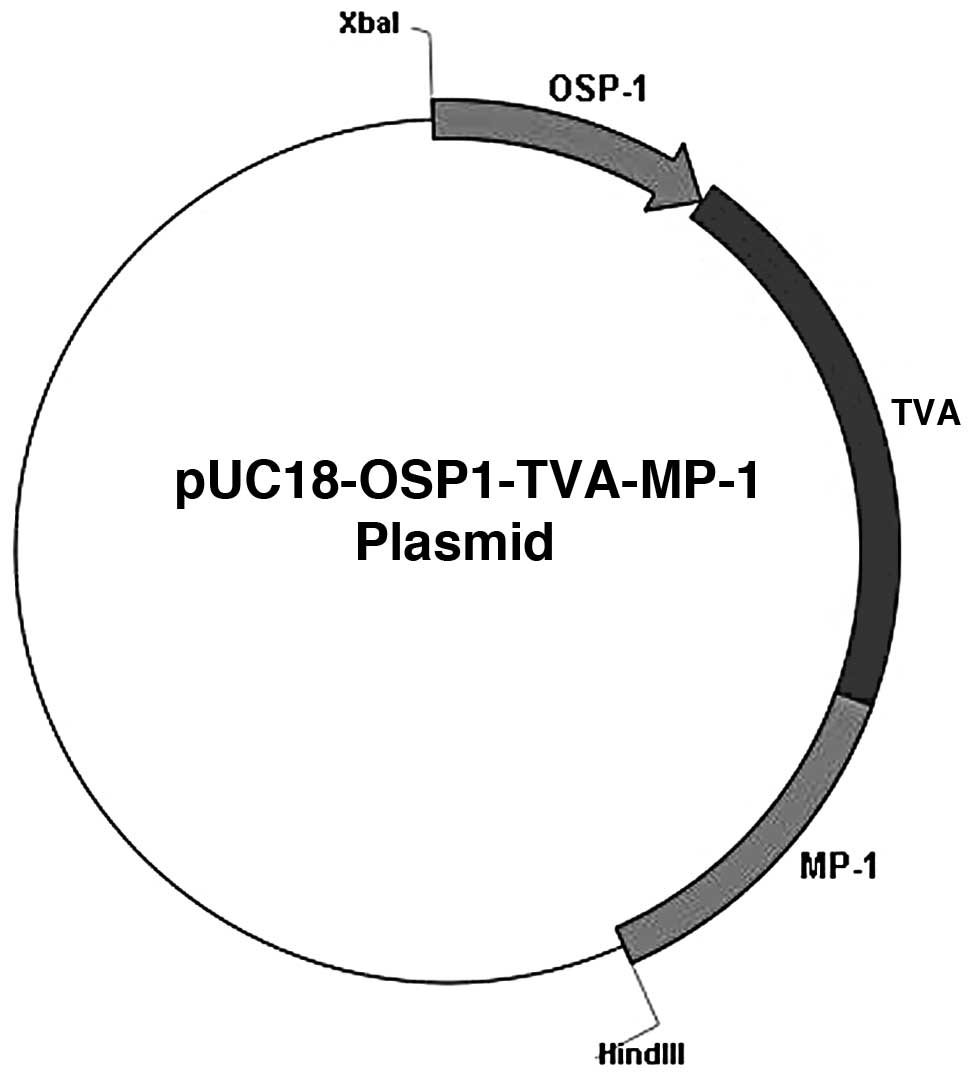
The plasmid, pUC18-MMTV-TVA-MP-1 was provided by
Professor Li Yi (Baylor College of Medicine, Houston, TX, USA). The
plasmid contained the MMTV promoter, TVA protein cDNA and a mouse
MP-1 signal. A novel plasmid, pUC18-OSP1-TVA-MP-1, was constructed
by replacing the MMTV promoter with the OSP1 promoter. The
restriction map of the vector pUC18-OSP1-TVA-MP-1 is shown in
Fig. 1. The novel plasmid DNA was
prepared by using a plasmid extraction kit (Tiangen Biotech Co.,
Ltd., Beijing, China), accoring to the manufacturer’s instructions.
Prior to gene transfer, the plasmid was linearized by digestion
with HindIII and XbaI (Fermentas, Vilnius,
Lithuania). The 2.287-kb DNA fragment containing OSP1, TVA and MP-1
cDNA was purified prior to injection.
The TVA sequence was as follows: gtacctaaaa
caagttgagt ggaagattcc actcaaggac aaaaaaagaa aatgggggaa tgaaagaccc
cctcccagaa agggaattgt acaagcccaa gtccctcaaa tatagaagta aaaagtaagt
ttttagatat gtgggacaag ctgacccatc atttagacga aacagaccag aaaagaaaac
aaaatgcaga aaccagacta aattatgggc actgctccat gggccacctg acaggaaaaa
gaaaaagtcc ctgacccccc ggaagccctg accaccatgt actttttgct aactcactgt
tatgattata gccaaaatag gtaacataca cgtatgcctc atttactcca ccaatcacat
ccctgtaacc atgcatctgc ttctgtaagc ctgcttctgc tccccaaaat actataaaaa
gcccaccctg gctctgctag gcgcgccagg atcc.
The OSP1 sequence was as follows: c agaggtacct
aaaacaagtt gagtggaaga ttccactcaa ggacaaaaag aaaatggggg aatgaaagac
cctctcccag aaagggaatt gtacaagccc caaggccctc aactatagaa gtaataaata
agtgttagat atgtggggca agctgaccca tcatttaggc ggaacaggcc agaaaaggaa
ccccaaatgc agaaaccaga ctaaattatg ggccctgctc catgggccac ctggcaggaa
aaagaaaaag tccctgaccc cccggaagcc ctgaccacca tgtacttttt gctaactcac
tgttatgatt atagcccaaa taggtaacat acacgtatgc ctcatttact ccaccaatca
catccctgta accattcatc tgcttctgta agcctgcttc tgctccccca aatactataa
aaagcccacc ctggctctgc taggcgcgcc agtct.
Preparation of gene suspensions
N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate
(DOTAP) liposomal transfection reagent (Roche Diagnostics,
Mannheim, Germany) was used to deliver the foreign DNA. The
DNA-DOTAP solution was prepared by mixing solution A [20 μl
(1 g/l) DOTAP diluted in HBS (Dingguo Changsheng Biotech Co., Ltd.,
Beijing, China; 20 mmol/l Hepes and 150 mmol/l NaCl, pH 7.4) to
obtain 30 μl] with solution B (10 μg DNA diluted with HBS to obtain
30 μl). The mixture was incubated for 20 min at room temperature
(24).
Preparation of TMGT males
Two mature male ICR mice, aged 6–8 weeks, were
anesthetized with pentobarbital (New Asia Pharmaceutical Co., Ltd.,
Shanghai, China) at 0.1 mg/g intra-peritoneal injection (IP), and
60 μl DOTAP-DNA solution was directly injected via the scrotum into
the two testes of each mouse with a 31G needle attached to a 0.5-ml
disposable syringe. Half of the DNA-DOTAP solution(30 μl) was
applied to each testis in an injection. Each testis was injected at
three to four different random but separated sites. Following
injection, the needle was slowly removed from the testis and
ketorolac (Pharmacy of Peking University People’s Hospital,
Beijing, China) was used at 0.5 mg/kg IP once daily to ease pain.
Each mouse received three sets of injections of the DNA-DOTAP
solution, and the interval between each set of injections was 3–5
days.
Production of transgenic mice
At 4–7, 7–10 and 10–13 weeks following the final
testis injection, each injected male mouse was mated with two
wild-type estrus females to produce F1 offspring. Once the F1
offspring matured, F2 offspring were produced. The matings for the
production of F2 offspring occurred between F1 transgenic positive
(P) individuals and either F1 transgenic positive (PxP) individuals
or non-transgenic (W) mice (PxW). Such matings occurred when the F1
offspring were 6–8 weeks and 5–6 months old. In total, four groups
of F2 offspring were produced.
Identification of transgenic
individuals
Extraction of genomic DNA
Genomic DNA was isolated from three-week-old
offspring by dissolving tail tissue in a mixture of 0.5 ml of 10
mmol/l EDTA, 0.5% SDS and 50 μl of 10 g/l proteinase K (Tiangen
Biotech Co., Ltd.). The mixture was incubated at 56°C overnight
with gentle shaking. Once the undigested materials were removed
with a low-speed centrifuge at 9,660 × g for 30 sec, the mixture
was extracted with phenol/chloroform and precipitated with ethanol,
and the DNA was dissolved in double-distilled H2O.
PCR analysis
The primers used were as follows: Antisense: 5′-CTG
CTG CCC GGT AAC GTG ACC GG-3′ and sense: 5′-GCC CTG GGG AAG GTC CTG
CCC-3′ for TVA cDNA. The 20 μl PCR system contained 1 μg genomic
DNA, 200 μmol/l dNTPs, 2 mmol/l MgCl2, 1.2 U Taq DNA
polymerase (Tiangen Biotech Co., Ltd.) and 10 pmol of each primer.
The PCR involved denaturing at 94°C for 5 min, then 30 cycles of
94°C for 30 sec, 73°C for 30 sec and 72°C for 50 sec, with
elongation at 72°C for 5 min. The anticipated size of the amplified
fragment was 477 bp for TVA cDNA. The PCR products were
electrophoresed on a 1% agarose gel.
Southern blot analysis
Southern blot hybridization of genomic DNA was
performed to determine the rate of occurrence of transgenic
positive individuals. Genomic DNA (20 μg) isolated from F1
transgenic individuals was digested to completion with
HindIII and XbaI restriction enzymes, resolved on
0.6% agarose gel and transferred to a nylon membrane. The nylon
membrane was maintained at 80°C for 2 h to fix the DNA, then
prehybridized at 42°C for 30 min in a prehybridization solution
(Roche Diagnostics), followed by hybridization with
digoxigenin-11-dUTP (Roche Diagnostics) -labeled TVA cDNA at 42°C
for 16 h, according to the manufacturer’s instructions.
Semi-quantitative RT-PCR
Total RNA was extracted from the ovaries of F1 mice
using the total RNA extraction kit (Tiangen Biotech Co., Ltd.).
First-strand cDNA was synthesized from 1 μg total RNA treated with
DNase (Tiangen Biotech Co., Ltd.) that was isolated from the
ovaries of the TVA-positive mice. An aliquot of the first-strand
synthesized cDNA was then utilized for TVA PCR amplification. The
primers used were the same as listed previously. The sample loading
and cycling conditions used for the subsequent semi-quantitative
RT-PCR reactions were as described previously. GAPDH was amplified
as a housekeeping gene control. The aforementioned TVA primers were
used, and those used for GAPDH cDNA were as follows: Antisense:
5′-AAT CCC ATC ACC ATC TTC C-3′ and sense: 5′-CAT CAC GCC ACA GTT
TCC-3′.
Statistical analysis
The χ2-test was used to statistically
analyze the data. Categorized variables were described as
percentages and analyzed using the r by c χ2 test. The
statistical analyses were performed with SPSS for Windows (version
13.0; SPSS Inc., Chicago, IL, USA). A two-tailed P<0.05 was
considered to indicate statistically significant differences.
Results
Detection of TVA expression in F1 mice by
PCR and southern blotting
The group of TMGT-treated mice, two TGMT males and
four wild-type females, produced a total of 131 pups. The
transgenic individuals in the F1 cohort were identified based on
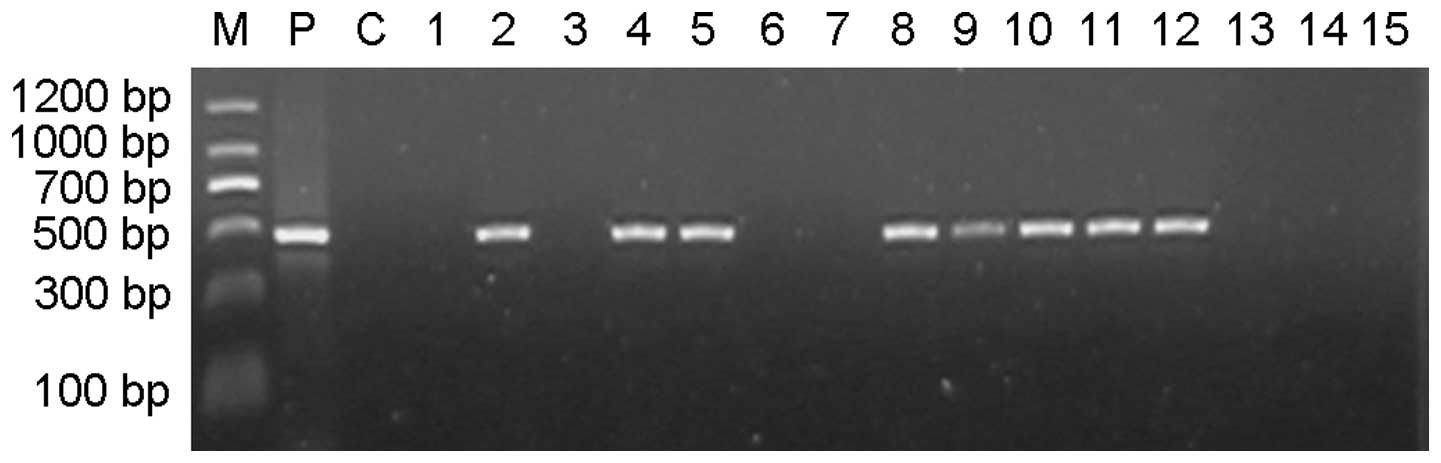
PCR amplification of a 477-bp TVA product (Fig. 2). Analysis of the results indicated
that the TVA-positive rates in the F1 offspring produced following
parental mating at three stages (4–7, 7–10 and 10–13 weeks
following the final testis injection) were 55.71 (39/70), 30.77
(4/13) and 18.75% (9/48), respectively (Table I). There was a statistically
significant difference between the positive rates in the first and
third stages (P<0.05). The average positive rate in the F1 group
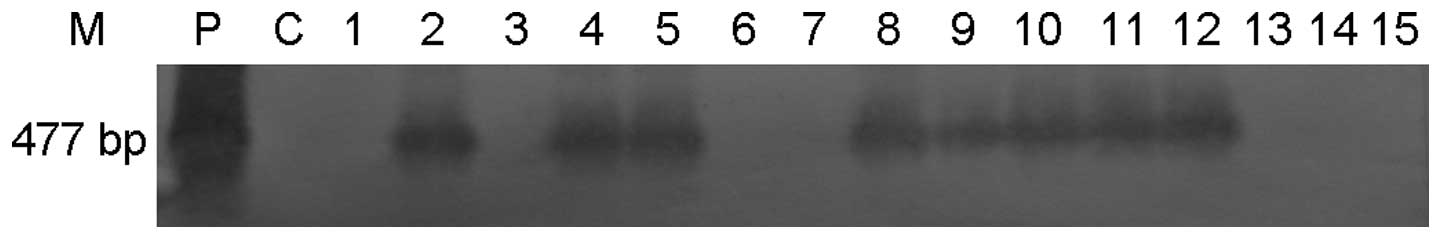
was 39.69% (52/131). The results of the southern blot hybridization
were consistent with those of the PCR analysis (Fig. 3). The male to female ratio in the
transgenic progeny was ~1:1 and the mean number of pups born per
litter was 10.08 (131/13), with a newborn pup (three weeks old)
survival rate of 93.13% (122/131).
 | Table ITransgene statistics of F1 offspring
following copulation between sexually mature male TMGT-injected
mice and wild-type female mice. |
Table I
Transgene statistics of F1 offspring
following copulation between sexually mature male TMGT-injected
mice and wild-type female mice.
| Time after TMGT
injection when mating (weeks) | No. of offspring | No. of transgenic
offspring | Positive rate
(%) | Average Positive rate
(%) |
|---|
| 4–7 | 70 | 39 | 55.71a | 39.69 |
| 7–10 | 13 | 4 | 30.77 | |
| 10–13 | 48 | 9 | 18.75a | |
Detection of TVA expression in transgenic
mice by semi-quantitative RT-PCR
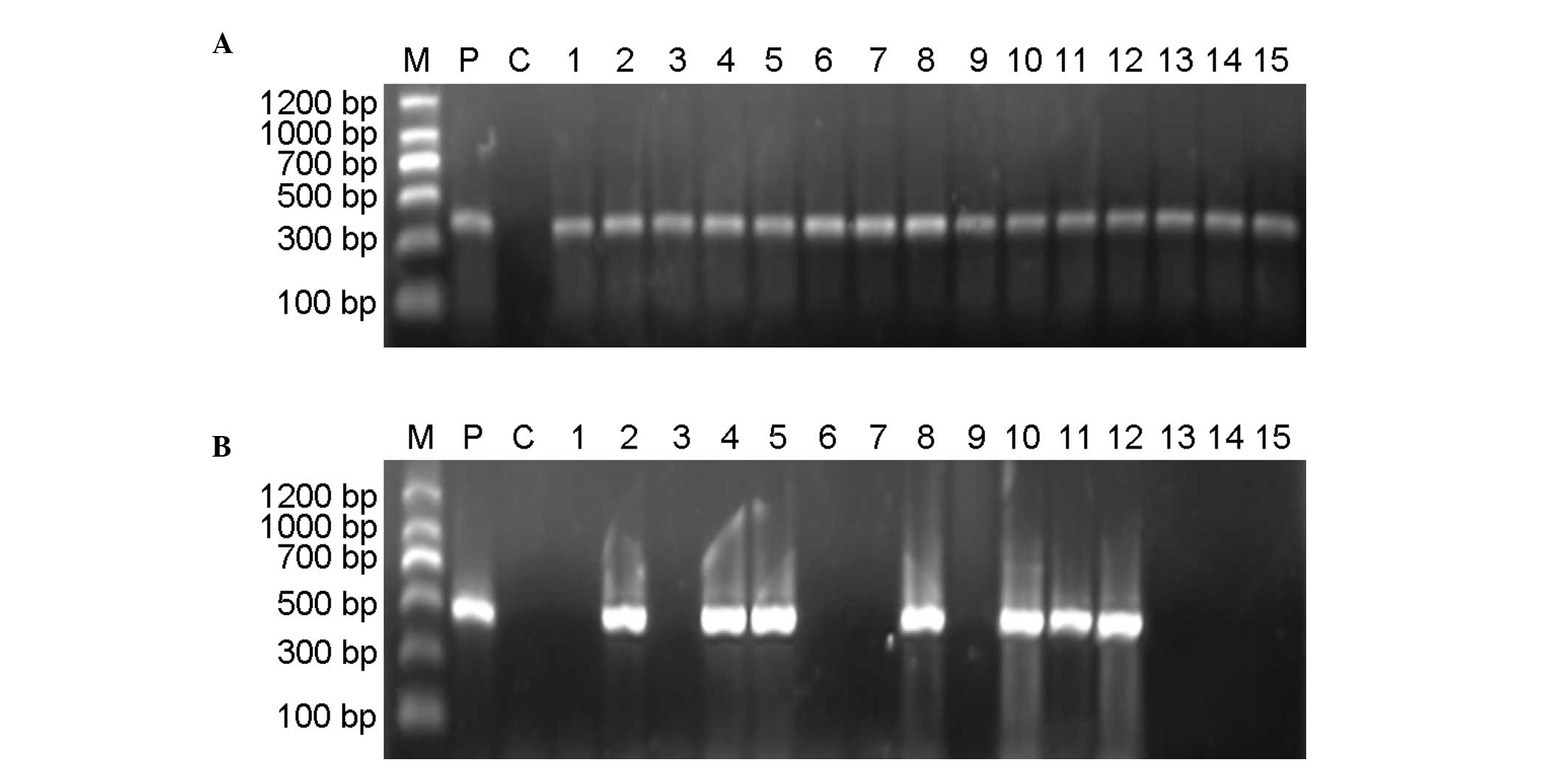
Semi-quantitative RT-PCR analysis of TVA expression
was performed using a GAPDH internal control. In the majority of
the TVA transgenic mice, the predicted 477-bp TVA fragment was
amplified. In each of those cases, the predicted 382-bp fragment
from a GAPDH internal control was amplified (Fig. 4). The transgenic positive rate in
the F1 group by semi-quantitative RT-PCR analysis was 34.35%.
Production of F2 generation mice
To investigate the pattern of foreign gene
transmission into a subsequent generation, F1 transgenic positive
individuals were mated with F1 transgenic positive individuals
(PxP) or with non-transgenic mice (PxW) when they were 6–8 weeks
(early) and 5–6 months (late) old. The transgenic status of the
obtained F2 offspring was analyzed by PCR and southern blot
hybridization.
The analyses indicated that the transgenic positive
rates in the F2 group in the four groups (early PxW, early PxP,
late PxW and late PxP groups) were 9.52 (6/63), 26.67 (4/15), 6.52
(3/46) and 15.94% (11/69), respectively (Table II). The positive rates in the
early and late groups were 12.82 (10/78) and 12.17% (14/115),
respectively (P>0.05). The positive rates in PxW groups and PxP
groups were 8.26 (9/109) and 17.86% (15/84), respectively
(P<0.05).
 | Table IITransgene statistics of F2
offspring. |
Table II
Transgene statistics of F2
offspring.
| Group | Age of the mice
when mating | No. of
offspring | No. of transgenic
offspring | Positive rate
(%) | No. of
offspring | No. of transgenic
offspring | Average positive
rate (%) |
|---|
| PxW | 6–8 weeks | 63 | 6 | 9.52a | 109 | 9 | 8.26c |
| 5–6 months | 46 | 3 | 6.52b | | | |
| PxP | 6–8 weeks | 15 | 4 | 26.67a | 84 | 15 | 17.86c |
| 5–6 months | 69 | 11 | 15.94b | | | |
The positive rate in the entire F2 group was 12.44%
(24/193), while that in the entire F1 group was 39.69% (52/131).
The F2 group’s positive rate was significantly lower than that of
the F1 group (P<0.05). The results of the southern blot
hybridization analysis were consistent with the PCR-based
results.
Discussion
During the past few decades, TMGT has been performed
using surgical incisions to expose testis tubules. Foreign DNA was
then injected into the animal’s seminiferous tubules using
microinjection. Injected foreign DNA may be transferred into eggs
via sperm and subsequently expressed in the progeny (25,26).
In the present study, the foreign DNA was injected by syringe, on
three occasions at multiple sites, into the male mice testes
without incision. This renders the procedure easier and decreases
the potential for injury to the mice.
In the study, the transgenic rates of F1 offspring
decreased with the time elapsed following TMGT treatment. The same
phenomenon was observed in the F2 offspring, with the F2 transgenic
rate decreasing with the age of the F1 parents. The observed
overall F1 transgenic positive rate of 39.69% is concordant with
the result of 41% obtained by He et al (24) in a study on F1 offspring. However,
our F2 transgenic positive rate was 12.44%, which is significantly
lower than the rate of 37% obtained by He et al in a study
on F2 offspring (24). Sato et
al (27) also obtained F2
transgenic-positive animals. However, Shen et al (26) and Valenzuela et al (28) did not identify transgene-positive
individuals in an F2 generation. We hypothesize that the transgenic
positive rate in F2 offspring is associated with the age at which
the F1 parents are mated; the younger the F1 parent, the higher the
F2 transgenic positive rate; when the F1 parent ages, the F2
transgenic positive rate decreases.
A number of studies investigating TMGT have
suggested a possible TMGT mechanism: When foreign DNA is injected
directly into a testis, it is rapidly transferred to epididymal
spermatozoa, which subsequently transfer the DNA to oocytes through
fertilization (29). In addition,
the foreign gene is transferred to sperm during the spermatogenic
processes from spermatogonia to mature spermatozoa (25,26,30).
Moreover, the foreign DNA may exist as integrated and/or
non-integrated types in sperm cells (5). Regardless, the TMGT mechanism has not
been investigated extensively. Sato et al (27) suggested that TMGT via epididymal
spermatozoa was a possible method for an in vivo gene
transfer system. A number of studies have reported that the TMGT
method allows the foreign gene to be expressed over a long period
of time, as intra-testis cells and sexually mature sperm are able
to carry a large number of foreign genes (31–33).
Spermatozoa are produced in the seminiferous tubules
of the testes, where they emerge initially as spermatogonia and
then undergo mitosis to become type A or type B spermatogonia. Type
B spermatogonia become primary spermatocytes that undergo one
meiotic division to become secondary spermatocytes and another to
become spermatids. Type A spermatogonia are the only immortalized
diploid cells that are reproduced in adult male animals, including
mice, and they have a high self-renewal capacity and pluripotent
potential (34,35). On that basis, and following
consideration of the results of the present study along with those
of Shen et al (26) and
Valenzuela et al (28), it
was suggested that the TMGT mechanism may include additional
components. Once a foreign DNA and liposome (DOTAP) complex is
injected directly into the testis, all cells that have contact with
that complex, not only spermatids at different development stages,
but also the two types of spermatogonia are considered to be
transfected with foreign DNA. Following this transfection, the
original genome returns to stability by clearing the foreign DNA
step-by-step; thus, the transgenic rate decreases with time.
It was proposed that the reasons transgenic rates
decrease with time are associated with the state of foreign DNA.
The foreign DNA may exist as episomal and integrated genes.
Following mitosis, novel cells inherit the integrated foreign DNA
but the episomal foreign DNA is diluted and lost. Thus, this may be
the reason for the low transmission rates in the F2 offspring of
the present study. It may be that the level of episomal foreign DNA
is markedly greater than the integrated DNA in the present study.
It is also hypothesized that the integrated gene may be unstable as
it is not possible to control the integration position in the
chromosome. This will accelerate the rate at which the integrated
gene is lost from the genome. We aim to use the transposons to
improve the affection of gene transfer. It has been proposed that
the integrated gene may be more stable when using the transposons
at the special integration position in the chromosome. The
mechanism and transgenic affection of TMGT are complex.
Considerable investigation is required to reveal the exact
mechanism.
The results of the present study demonstrate that
the TMGT method does not require expensive equipment or specialized
skills. It is also simpler, less costly and more convenient than
the PM and OMGT methods and techniques involving in vitro
transfection of embryonic cells, stem cells and embryonic stem
cells. For the method of the present study, a needle and a plastic
syringe were sufficient for delivery of the foreign DNA.
In the present study, total RNA was extracted from
transgenic mice ovaries. Following semi-quantitative RT-PCR of the
RNA, TVA-positive strands were observed. Thus, TVA is capable of
being expressed in the ovary under the control of OSP1; a result
that is in concurrent with those of Hamilton et al (22,23).
In conclusion, the present study showed that an
avian TVA gene may be introduced into ICR mice by TMGT. The
positive transgenic rate was high in the F1 offspring (39.69%);
however, in the F2 offspring, the positive transgenic rate was
12.44%, indicating that the foreign DNA may be partially lost. The
TMGT method is suitable for creating transgenic animals (24,36)
and is a reliable method for transgenesis. The results of the
present study show that female transgenic mice are able to express
TVA under the control of OSP1, indicating the importance of the
RCAS-TVA technique for ovarian tumorigenesis research.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172454), Research and
Development Fund, Peking University People’s Hospital (grant no.
RDB2008-16). The authors would like to thank Professor Li Yi of
Baylor College of Medicine (Houston, TX, USA), for kindly providing
the plasmid pUC18-MMTV-TVA-MP-1.
References
|
1
|
Gordon JW, Scangos GA, Plotkin DJ, Barbosa
JA and Ruddle FH: Genetic transformation of mouse embryos by
microinjection of purified DNA. Proc Natl Acad Sci USA.
77:7380–7384. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AW: Transgenic animals: current and
alternative strategies. Cloning. 1:25–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mullins LJ and Mullins JJ: Transgenesis in
the rat and larger mammals. J Clin Invest. 97:1557–1560. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wall RJ: New gene transfer methods.
Theriogenology. 57:189–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu Y and Liang S: Progress in gene
transfer by germ cells in mammals. J Genet Genomics. 35:701–714.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreira PN, Pozueta J, Pérez-Crespo M,
Valdivieso F, Gutiérrez-Adán A and Montoliu L: Improving the
generation of genomic-type transgenic mice by ICSI. Transgenic Res.
16:163–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ivics Z, Izsvák Z, Medrano G, Chapman KM
and Hamra FK: Sleeping Beauty transposon mutagenesis in rat
spermatogonial stem cells. Nat Protoc. 6:1521–1535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhup S and Majumdar SS: Transgenesis via
permanent integration of genes in repopulating spermatogonial cells
in vivo. Nat Methods. 5:601–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SY, Wang JG, Cui HX, et al: Efficient
generation of transgenic mice by direct intraovarian injection of
plasmid DNA. Biochem Biophys Res Commun. 358:266–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bu W, Chen J, Morrison GD, et al: Keratin
6a marks mammary bipotential progenitor cells that can give rise to
a unique tumor model resembling human normal-like breast cancer.
Oncogene. 30:4399–4409. 2011. View Article : Google Scholar
|
|
12
|
Du Z and Li Y: RCAS-TVA in the mammary
gland: an in vivo oncogene screen and a high fidelity model for
breast transformation? Cell Cycle. 6:823–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Z, Podsypanina K, Huang S, et al:
Introduction of oncogenes into mammary glands in vivo with an avian
retroviral vector initiates and promotes carcinogenesis in mouse
models. Proc Natl Acad Sci USA. 103:17396–17401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hambardzumyan D, Amankulor NM, Helmy KY,
Becher OJ and Holland EC: Modeling adult gliomas using RCAS/t-va
technology. Transl Oncol. 2:89–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis BC, Klimstra DS and Varmus HE: The
c-myc and PyMT oncogenes induce different tumor types in a somatic
mouse model for pancreatic cancer. Genes Dev. 17:3127–3138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loftus SK, Larson DM, Watkins-Chow D,
Church DM and Pavan WJ: Generation of RCAS vectors useful for
functional genomic analyses. DNA Res. 8:221–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu Y and Liang SL: Mammalian models based
on RCAS-TVA technique. Zoological Res. 3:335–345. 2008.
|
|
18
|
Reddy JP and Li Y: The RCAS-TVA system for
introduction of oncogenes into selected somatic mammary epithelial
cells in vivo. J Mammary Gland Biol Neoplasia. 14:405–409. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sausville J, Molinolo AA, Cheng X, et al:
RCAS/SCL-TVA animal model allows targeted delivery of polyoma
middle T oncogene to vascular endothelial progenitors in vivo and
results in hemangioma development. Clin Cancer Res. 14:3948–3955.
2008. View Article : Google Scholar
|
|
20
|
Orsulic S: An RCAS-TVA-based approach to
designer mouse models. Mamm Genome. 13:543–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing D and Orsulic S: A genetically
defined mouse ovarian carcinoma model for the molecular
characterization of pathway-targeted therapy and tumor resistance.
Proc Natl Acad Sci USA. 102:6936–6941. 2005. View Article : Google Scholar
|
|
22
|
Bao R, Selvakumaran M and Hamilton TC:
Targeted gene therapy of ovarian cancer using an ovarian-specific
promoter. Gynecol Oncol. 84:228–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selvakumaran M, Bao R, Crijns AP, Connolly
DC, Weinstein JK and Hamilton TC: Ovarian epithelial cell
lineage-specific gene expression using the promoter of a
retrovirus-like element. Cancer Res. 61:1291–1295. 2001.PubMed/NCBI
|
|
24
|
He X, Qi B, Liu G, Yu W and Chen Q: A
novel method to transfer gene in vivo system. Prog Biochem Biophys.
33:685–690. 2006.
|
|
25
|
Chang KT, Ikeda A, Hayashi K, et al:
Production of transgenic rats and mice by the testis-mediated gene
transfer. J Reprod Dev. 45:29–36. 1999. View Article : Google Scholar
|
|
26
|
Shen W, Li L, Pan Q, Min L, Dong H and
Deng J: Efficient and simple production of transgenic mice and
rabbits using the new DMSO-sperm mediated exogenous DNA transfer
method. Mol Reprod Dev. 73:589–594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato M, Ishikawa A and Kimura M: Direct
injection of foreign DNA into mouse testis as a possible in vivo
gene transfer system via epididymal spermatozoa. Mol Reprod Dev.
61:49–56. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valenzuela M, Relloso M and Esponda P: In
vivo transfection of the mouse vas deferens. J Exp Zool.
293:532–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Celebi C, Auvray P, Benvegnu T,
Plusquellec D, Jégou B and Guillaudeux T: Transient transmission of
a transgene in mouse offspring following in vivo transfection of
male germ cells. Mol Reprod Dev. 62:477–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yonezawa T, Furuhata Y, Hirabayashi K,
Suzuki M, Takahashi M and Nishihara M: Detection of transgene in
progeny at different developmental stages following testis-mediated
gene transfer. Mol Reprod Dev. 60:196–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coward K, Kubota H, Hibbitt O, McIlhinney
J, Kohri K and Parrington J: Expression of a fluorescent
recombinant form of sperm protein phospholipase C zeta in mouse
epididymal sperm by in vivo gene transfer into the testis. Fertil
Steril. 85(Suppl 1): 1281–1289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hibbitt O, Coward K, Kubota H, et al: In
vivo gene transfer by electroporation allows expression of a
fluorescent transgene in hamster testis and epididymal sperm and
has no adverse effects upon testicular integrity or sperm quality.
Biol Reprod. 74:95–101. 2006. View Article : Google Scholar
|
|
33
|
Kubota H, Hayashi Y, Kubota Y, Coward K
and Parrington J: Comparison of two methods of in vivo gene
transfer by electroporation. Fertil Steril. 83(Suppl 1): 1310–1318.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gilbert SF: Developmental Biology. 8th
edition. Sinauer Associates; Sunderland, MA: pp. 613–646. 2006
|
|
35
|
Schlatt S: Spermatogonial stem cell
preservation and transplantation. Mol Cell Endocrinol. 187:107–111.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ju H, Bai L, Ren H, Mu Y, Yang S and Li K:
Production of transgenic mice by type-A spermatogonia-mediated gene
transfer. Agri Sci Chin. 10:431–437. 2011. View Article : Google Scholar
|