Introduction
Small ubiquitin-like modifier (SUMO)ylation is a
post-translational modification that involves the covalent, but
reversible, binding of SUMO (1).
Four isoforms of SUMO have been identified, SUMO-1, -2, -3 and -4.
These proteins are ~12 kDa in size and are structurally similar to
ubiquitin (2–5). Similar to ubiquitination, the
SUMOylation cascade contains a SUMO-activating enzyme (E1), a
SUMO-conjugating enzyme (E2) and different SUMO ligases (E3s). All
SUMO isoforms are expressed as immature precursors with a variable
C-terminus comprising 2–11 residues, following an essential GG
motif. Maturation of SUMO involves the removal of this C-terminal
tail by sentrin-specific proteases (SENPs) and exposing the
diglycine motif required for conjugation. SUMO is then activated in
an ATP-dependent manner by conjugation to the E1 heterodimer,
activator of SUMO-1 (Aos1) and ubiquitin-like modifier activating
enzyme 2 (Uba2). Activated SUMO is then transferred to the unique
E2 enzyme Ubc9, which covalently attaches the modifier to the
ɛ-amino group of a target lysine residue in the presence of an E3
SUMO ligase (1,6,7).
Cleavage after the diglycine motif by SENPs then removes the
conjugated SUMO moieties from the target protein.
Due to its regulation of protein activity,
post-translational modifications may have profound effects on
carcinogenesis. SUMOylation has been shown to regulate various
cellular pathways, including DNA replication and repair, chromosome
packing and dynamics, genome integrity, nuclear transport, signal
transduction and cell proliferation. These functions suggest that
abnormal SUMOylation may affect cancer progression and metastasis
(8–15).
Hepatocellular carcinoma (HCC) is the sixth most
common type of malignancy worldwide. It is the fifth most common
type of malignant disease in males, the eighth most common type of
malignant disease in females and the third most common cause of
cancer-related mortality following lung and stomach cancer
(16). Systemic chemotherapy
represents a palliative treatment following resection and/or
transplant surgery for inoperable patients. However, intrinsic or
acquired multidrug resistance (MDR) may interfere with the efficacy
of chemotherapy in HCC. MDR, which results in increased drug efflux
from cells, is associated with increased DNA repair and drug
metabolism, as well as decreased apoptosis, all of which affect the
tumor microenvironment (17–20).
However, the role of SUMOylation in MDR or chemotherapy remains
largely unknown.
The aim of this study was to analyze the resistance
mechanisms of HCC. A novel multidrug resistant-HCC cell line
(HepG2/R) was developed based on HepG2 cells, a model for
chemosensitive HCC cells. We investigated the levels of SUMOylation
in these cells and analyzed the specific role in the development of
drug resistance in HCC cells.
Materials and methods
Chemotherapeutic drugs and
antibodies
The chemotherapeutic drugs 5-fluorouracil (5-FU,
catalog no. F6627-1G), paclitaxel (catalog no. O9512-5MG) and
oxaliplatin (catalog no. O9512-5MG) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Mouse antibodies against SUMO-1
(catalog no. sc-5308), Aos1 (catalog no. sc-271592), Uba2 (catalog
no. sc-136359) and SENP1 (catalog no. sc-271360) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell lines
The HepG2 human HCC cell line was provided by Dr H
Tang (Tianjin Life Science Research Center and Basic Medical
School, Tianjin Medical University, Tianjin, China). The MDR
sub-line HepG2/R was established by culturing HepG2 cells in the
presence of increasing concentrations of 5-FU (0.1, 0.2, 0.4, 0.8
and 1.6 μg/ml). HepG2/R cells were cultured in the presence of 1.6
μg/ml (12.3 mM) 5-FU to maintain their phenotype. All cells were
cultured in Dulbecco’s modified Eagle’s medium supplemented with
10% fetal bovine serum, 100 μg/ml penicillin and 100 μg/ml
streptomycin at 37°C in 5% CO2.
Human samples
Samples of paraffin-embedded HCC tissues and their
adjacent non-neoplastic controls (n=20) were obtained from patients
who underwent surgical resection at the Department of Hepatobiliary
Surgery, Tianjin Medical University Cancer Institute and Hospital.
HCC in these samples was confirmed by pathological diagnosis. This
study was approved by the Committee for the Protection of Human
Subjects of Tianjin Medical University Cancer Institute and
Hospital and informed consent was obtained from all patients.
Immunohistochemical analysis
The expression of SUMO-1 was demonstrated by
immunohistochemistry (IHC) according to the manufacturer’s
instructions (pv-6000; ZSGB-BIO, Beijing, China). Sections
(4μm-thick) were deparaffinized in xylene and endogenous peroxidase
activity was blocked with 3% hydrogen peroxide in 50% methanol for
10 min at room temperature. The slides were rehydrated in a series
of alcohol washes, and washed with phosphate-buffered saline (PBS)
and pretreated with citrate buffer for 20 min at 95°C. Following
blocking of nonspecific binding sites with 10% normal goat serum in
PBS for 20 min at 37°C, the sections were incubated overnight at
4°C with anti-SUMO-1 (dilution, 1:200). Sections were rinsed with
PBS and incubated with HRP-goat anti-mouse immunoglobulin G (IgG)
for 20 min at 37°C. Subsequently, the slides were incubated with
3,3′-diaminobenzidine chromogen for 5–10 min at room temperature
and washed with distilled water. Finally, sections were
counterstained with hematoxylin for 1 min, dehydrated and mounted
on coverslips. PBS without the primary antibody was used as a
negative control.
Western blot analysis
Protein lysate was separated by SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (GE Healthcare,
Piscataway, NJ, USA). Membranes were blocked in TBST (50 mM Tris pH
7.5, 0.15 M sodium chloride, 0.5% Tween-20) containing 5% non-fat
milk. Immunoblotting was performed using indicated antibodies
diluted in TBST. The membranes were washed and incubated with goat
anti-mouse IgG-horseradish peroxidase conjugate (Bio-Rad, Hercules,
CA, USA). Specific complexes were visualized using an enhanced
chemiluminescence detection system (GE Healthcare, Little Chalfont,
UK).
3-(4,5-dimethylthiahiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) assay
MTT assays were used to evaluate rates of cell
growth (Cell Growth Determination kit; Sigma-Aldrich, catalog no.
CGD1). Cells were trypsinized into a single-cell suspension and
seeded into 96-well plates at a density of 1×103
cells/well. At the indicated time points, 10% of the culture volume
(20 μl) MTT was added to each well and incubated for 4 h at 37°C.
Cultures were removed from the incubator and the resultant MTT
formazan crystals were dissolved in 180 μl MTT solvent (0.1 N HCl
in anhydrous isopropanol). Absorbance values were measured using a
microplate absorbance reader (Bio-Rad Laboratories, Richmond, CA,
USA) at a wavelength of 570 nm. To measure the effect of the
chemotherapeutic agents on growth, the inhibitory rate was
calculated as follows: Cell growth inhibition (%) =
[A570(control) −
A570(experiment)]/A570(control) × 100.
Statistical analysis
All experiments were performed at least in
triplicate. Statistical significance was evaluated using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment and characterization of the
HCC MDR cell line HepG2/R
To obtain multidrug resistant-cells, HepG2 cells
were treated with increasing concentrations of 5-FU between 0.1 and
1.6 μg/ml. The drug-resistant subclone HepG2/R was established
almost one year following initiation of treatment. HepG2/R cells
were morphologically distinct from their parental cell line in that
they demonstrated increased pseudopodia formation (Fig. 1A). Cell proliferation did not
differ significantly between the HepG2 and HepG2/R cells (Fig. 1B). Next, the sensitivity of the two
cell lines to specific concentrations of chemotherapeutic drugs was
investigated. Following treatment with 0.8–6.4 μg/ml 5-FU, the
inhibitory ratios of HepG2/R were markedly lower compared with
those of the parental HepG2 cells (Fig. 1C). The cross-resistance to other
chemotherapeutic drugs, including paclitaxel and oxaliplatin was
also investigated. HepG2/R cells were observed to develop
cross-resistance to paclitaxel and oxaliplatin to varying degrees
(Fig. 1C).
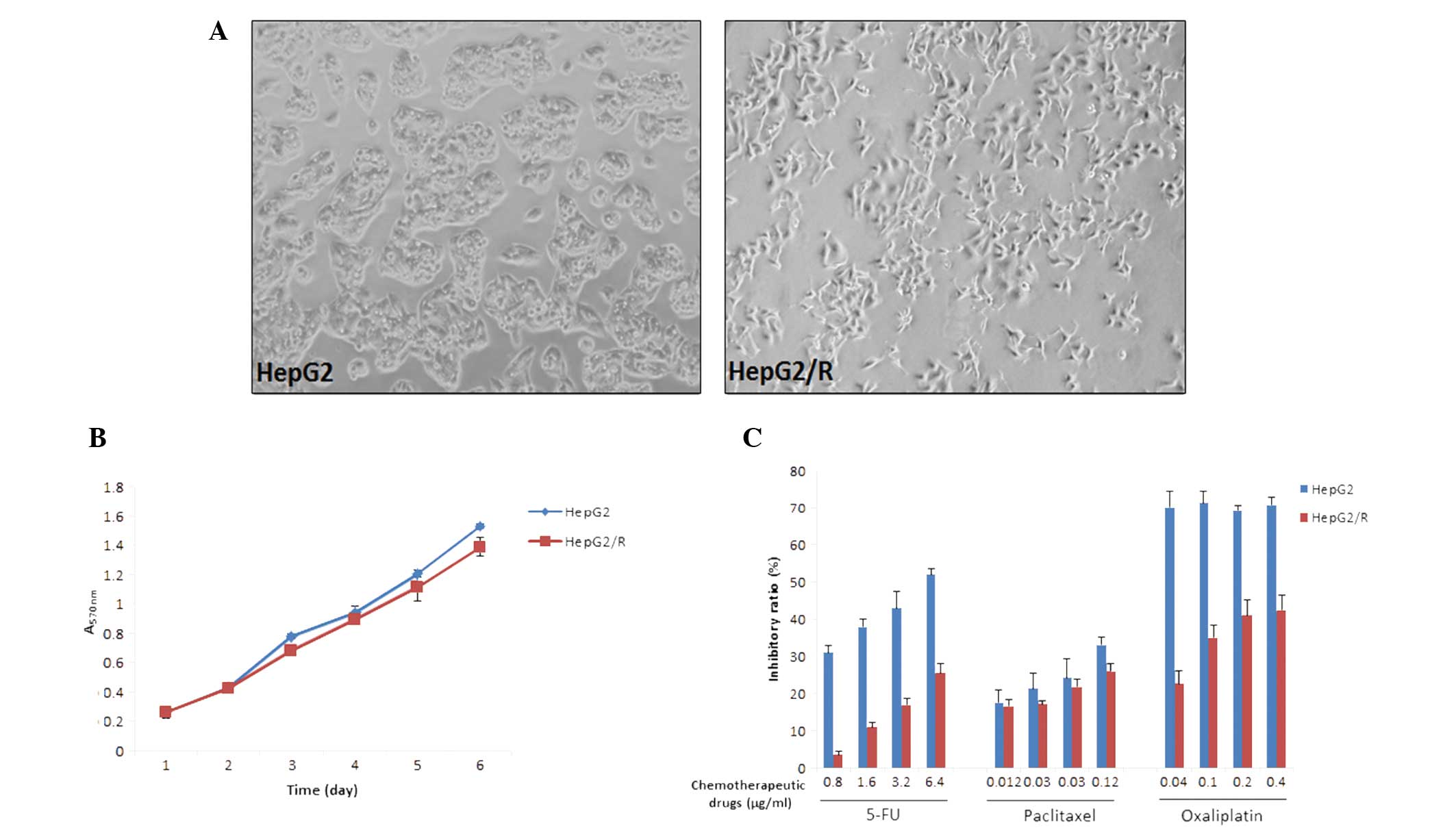 | Figure 1Characterization of the hepatocellular
carcinoma multidrug resistance cell line, HepG2/R. (A) HepG2/R
cells are morphologically distinct from their parental HepG2 cells,
showing increased pseudopodia formation. (B) The MTT assay was used
to measure cell growth over a six-day period. Proliferation rates
of the HepG2 cells are similar to those of the HepG2/R cells. The
results are expressed as the mean ± SD of three assays. (C) Cells
were treated with various concentrations of chemotherapeutic drugs,
5-FU, paclitaxel and oxaliplatin, and inhibitory ratios were
measured using the MTT assay. Results are expressed as the mean ±
SD from three assays. SD, standard deviation; MTT,
3-(4,5-dimethylthiahiazol-2-yl)-2,5-diphenyl tetrazolium bromide;
5-FU, 5-fluorouracil. Magnification, ×200. |
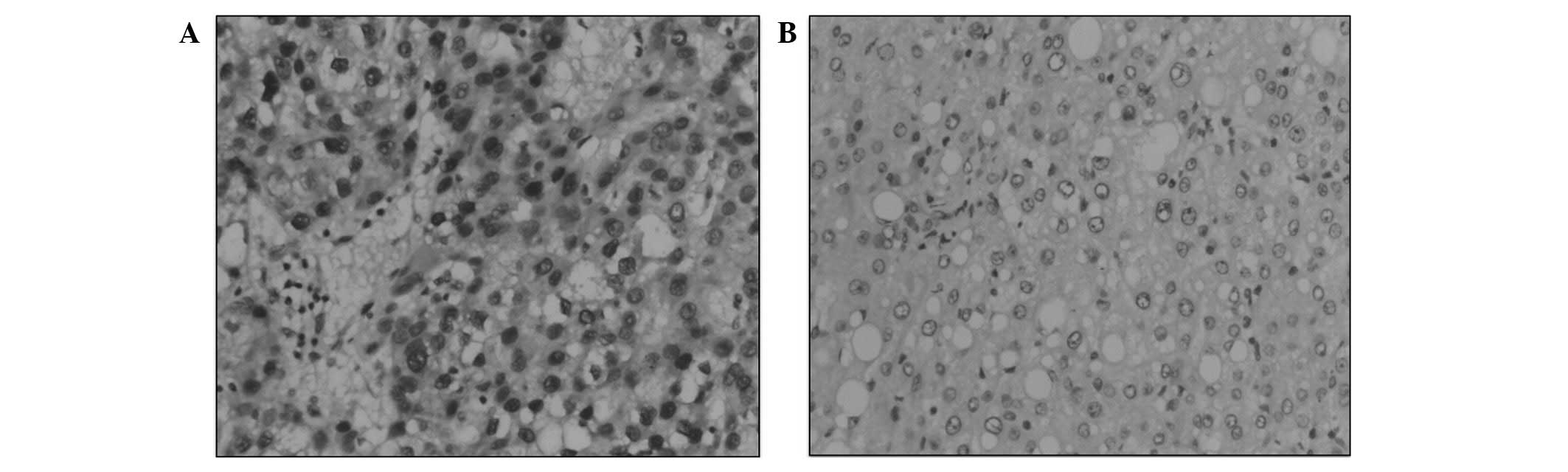
SUMO-1 is overexpressed in clinical HCC
samples
Immunostaining was used to evaluate the SUMO-1
expression in tumor cells from HCC tissues and normal cells from
the adjacent non-neoplastic liver tissue. Nuclear SUMO-1 expression
was detected in HCC neoplastic cells from all samples (100%).
However, in the matched non-neoplastic controls, little or no
SUMO-1 expression was observed (Fig.
2A and B).
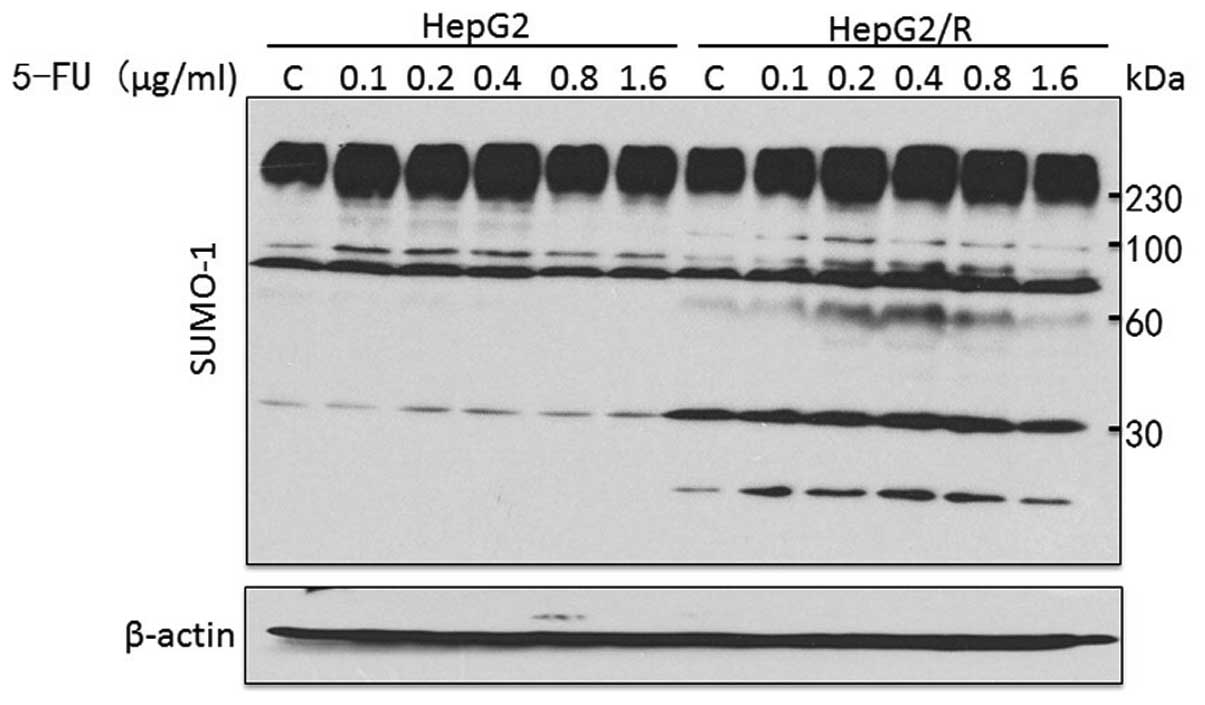
SUMO-1-conjugated proteins differ between
HepG2 and HepG2/R cells
To investigate the pattern of SUMOylation in HepG2
and HepG2/R cells treated with 5-FU at different concentrations,
whole-cell lysates were probed with a SUMO-1 antibody. The total
levels of SUMOylation were significantly enhanced in HepG2/R cells.
As shown in Fig. 3, distinct
SUMO-1-conjugated and free SUMO-1 proteins were detected in HepG2/R
cells at ~60 and 12 kDa, respectively. The levels of
SUMO-1-conjugated proteins at 30 and 100 kDa were also markedly
increased.
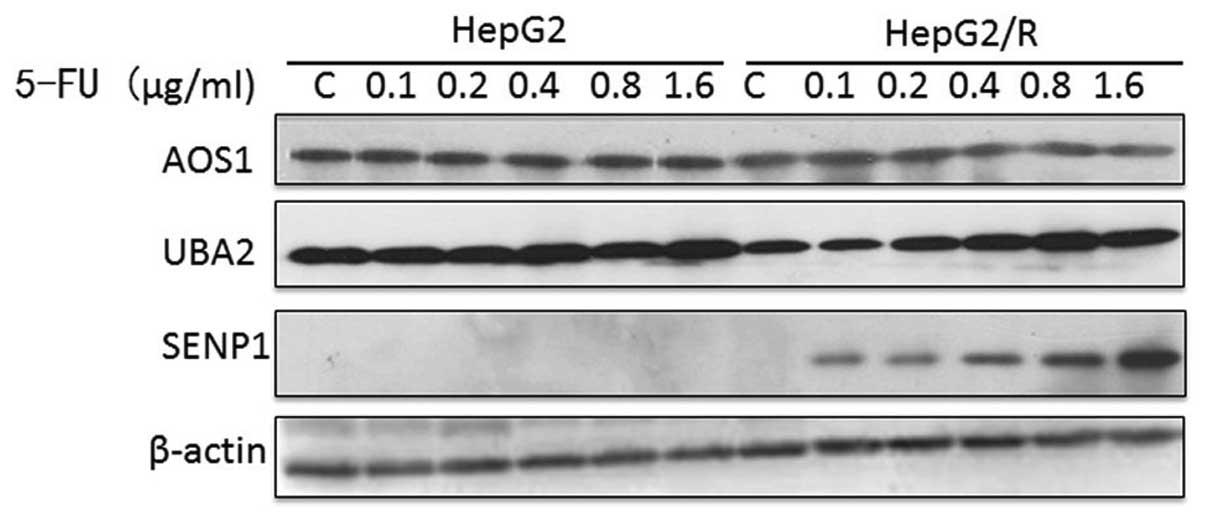
Uba2 and SENP1 are upregulated in the
HepG2/R cell line
In addition, the levels of SUMOylation enzymes in
the HepG2 and HepG2/R cells were compared to determine whether the
SUMOylation pathway differed. Although Aos1 levels were unaffected
by the 5-FU treatment, Uba2 and SENP1 levels increased in a
dose-dependent manner in HepG2/R cells (Fig. 4).
Discussion
Drug resistance is a major obstacle for the
successful chemotherapeutic treatment of HCC. Often following
chemotherapy, tumors fail to reduce in size or the cancer recurs
following an initial response. MDR is particularly problematic as
it involves the simultaneous resistance to numerous
chemotherapeutic drugs of different classes. However, the mechanism
of MDR remains unclear. To elucidate the molecular mechanisms in
HCC a multidrug-resistant cell model is required. 5-FU has been
used alone or in combination with other chemotherapeutic drugs for
HCC and other malignant tumors (21,22).
Thus, an MDR HCC cell line, HepG2/R, was established by exposing
HepG2 cells to increasing concentrations of 5-FU over one year.
HepG2/R cells exhibited a strong resistance to 5-FU and also
demonstrated cross-resistance to multiple, structurally diverse
chemotherapeutic agents, including paclitaxel and oxaliplatin.
SUMOylation is essential for the maintenance of
protein stability, transcriptional regulation and modification of
particular transcription factors. SUMOylation is also involved in
cellular processes, including mitosis, differentiation, senescence
and apoptosis (8,23–26).
Guo et al (27) reported
that SUMO-1 was overexpressed in all HCC cell lines and clinical
HCC samples that were tested. In agreement with this observation,
the current study showed an increase in the expression of SUMO-1 in
clinical HCC samples compared with the adjacent non-neoplastic
liver tissue controls. This suggests that SUMOylation may be
important in HCC. In addition, total levels of SUMO-1-conjugated
proteins are increased in HepG2/R cells compared with parental
HepG2 cells. This demonstrates that higher levels of SUMOylation
are associated with MDR in HCC, possibly by affecting pathways that
induce MDR.
SUMOylation is a dynamic process involving the
conjugation (Aos1/Uba2, Ubc9 and different E3 ligases) and
de-conjugation machinery (SENPs) (1). Notably, these enzymes have also been
observed to affect tumorigenesis. Inactivation of Uba2 leads to
mitotic catastrophe and cell death following Myc
hyperactivation. Uba2 inhibition switches a transcriptional
subprogram of Myc from activated to repressed (28). SENP1 is highly expressed in
prostate cancer and correlates with hypoxia-inducing factor 1
(HIF-1) expression, which is associated with an increase in
P-glycoprotein expression and the occurrence of MDR in tumor cells
(29,30). In the current study, high
concentrations of 5-FU were found to increase Uba2 expression in
HCC MDR cells. This increase may affect cellular processes
regulated by oncogenic Myc. 5-FU treatment also increased SENP1
expression in a dose-dependent manner, potentially implicating
HIF-1 in the development of MDR in HCC.
In conclusion, the development of MDR in HCC was
observed to be associated with increased SUMOylation, likely due to
the increased expression of Uba2 and SENP1. These results suggest
that the SUMOylation pathway may be a novel therapeutic target for
preventing the development of MDR in HCC, thus, increasing the
efficacy of chemotherapy for this widespread disease.
Acknowledgements
This study was supported by scientific research
grants from Tianjin Medical University (grant nos. 2010ky04 and
2011ky42).
Abbreviations:
|
Aos1
|
activator of SUMO-1
|
|
HCC
|
hepatocellular carcinoma
|
|
MDR
|
multidrug resistance
|
|
SENP1
|
sentrin-specific protease 1
|
|
SUMO
|
small ubiquitin-like modifier
|
|
Uba2
|
ubiquitin-like modifier activating
enzyme 2
|
References
|
1
|
Geiss-Friedlander R and Melchior F:
Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol.
8:947–956. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meluh PB and Koshland D: Evidence that the
MIF2 gene of Saccharomyces cerevisiae encodes a centromere
protein with homology to the mammalian centromere protein CENP-C.
Mol Biol Cell. 6:793–807. 1995.PubMed/NCBI
|
|
3
|
Shen Z, Pardington-Purtymun PE, Comeaux
JC, Moyzis RK and Chen DJ: UBL1, a human ubiquitin-like protein
associating with human RAD51/RAD52 proteins. Genomics. 36:271–279.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okura T, Gong L, Kamitani T, Wada T, Okura
I, Wei CF, Chang HM and Yeh ET: Protection against Fas/APO-1- and
tumor necrosis factor-mediated cell death by a novel protein,
sentrin. J Immunol. 157:4277–4281. 1996.PubMed/NCBI
|
|
5
|
Seeler JS and Dejean A: Nuclear and
unclear functions of SUMO. Nat Rev Mol Cell Biol. 4:690–699. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wadosky KM and Willis MS: The story so
far: post-translational regulation of peroxisome
proliferator-activated receptors by ubiquitination and SUMOylation.
Am J Physiol Heart Circ Physiol. 302:H515–H526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gong L, Li B, Millas S and Yeh ET:
Molecular cloning and characterization of human AOS1 and UBA2,
components of the sentrin-activating enzyme complex. FEBS Lett.
448:185–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muller S, Berger M, Lehembre F, Seeler JS,
Haupt Y and Dejean A: c-Jun and p53 activity is modulated by SUMO-1
modification. J Biol Chem. 275:13321–13329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G and Warbrick E: The p66 and p12
subunits of DNA polymerase delta are modified by ubiquitin and
ubiquitin-like proteins. Biochem Biophys Res Commun. 349:360–366.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka K, Nishide J, Okazaki K, Kato H,
Niwa O, Nakagawa T, Matsuda H, Kawamukai M and Murakami Y:
Characterization of a fission yeast SUMO-1 homologue, pmt3p,
required for multiple nuclear events, including the control of
telomere length and chromosome segregation. Mol Cell Biol.
19:8660–8672. 1999.PubMed/NCBI
|
|
11
|
Bachant J, Alcasabas A, Blat Y, Kleckner N
and Elledge SJ: The SUMO-1 isopeptidase Smt4 is linked to
centromeric cohesion through SUMO-1 modification of DNA
topoisomerase II. Mol Cell. 9:1169–1182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma Y, Arnaoutov A and Dasso M: SUMO-2/3
regulates topoisomerase II in mitosis. J Cell Biol. 163:477–487.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu N, Kong X, Ji Z, Zeng W, Potts PR,
Yokomori K and Yu H: Scc1 sumoylation by Mms21 promotes sister
chromatid recombination through counteracting Wapl. Genes Dev.
26:1473–1485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McAleenan A, Cordon-Preciado V,
Clemente-Blanco A, Liu IC, Sen N, Leonard J, Jarmuz A and Aragón L:
SUMOylation of the α-kleisin subunit of cohesin is required for DNA
damage-induced cohesion. Curr Biol. 22:1564–1575. 2012.
|
|
15
|
Luo K, Zhang H, Wang L, Yuan J and Lou Z:
Sumoylation of MDC1 is important for proper DNA damage response.
EMBO J. 31:3008–3019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: Hepatocellular carcinoma (HCC): a global perspective. J Clin
Gastroenterol. 44:239–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Correia AL and Bissell MJ: The tumor
microenvironment is a dominant force in multidrug resistance. Drug
Resist Updat. 15:39–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doublier S, Belisario DC, Polimeni M,
Annaratone L, Riganti C, Allia E, Ghigo D, Bosia A and Sapino A:
HIF-1 activation induces doxorubicin resistance in MCF7 3-D
spheroids via P-glycoprotein expression: a potential model of the
chemo-resistance of invasive micropapillary carcinoma of the
breast. BMC Cancer. 12:42012. View Article : Google Scholar
|
|
19
|
Brökers N, Le-Huu S, Vogel S, Hagos Y,
Katschinski DM and Kleinschmidt M: Increased chemoresistance
induced by inhibition of HIF-prolyl-hydroxylase domain enzymes.
Cancer Sci. 101:129–136. 2010.PubMed/NCBI
|
|
20
|
Fabregat I, Roncero C and Fernández M:
Survival and apoptosis: a dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Homann N, Pauligk C, Luley K, et al:
Pathological complete remission in patients with oesophagogastric
cancer receiving preoperative 5-fluorouracil, oxaliplatin and
docetaxel. Int J Cancer. 130:1706–1713. 2012. View Article : Google Scholar
|
|
22
|
Kitagawa K, Kawada K, Morita S, et al:
Prospective evaluation of corrected QT intervals and arrhythmias
after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil
in women with breast cancer. Ann Oncol. 23:743–747. 2012.
View Article : Google Scholar
|
|
23
|
Nagai S, Davoodi N and Gasser SM: Nuclear
organization in genome stability: SUMO connections. Cell Res.
21:474–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai Q and Robertson ES: Ubiquitin/SUMO
modification regulates VHL protein stability and nucleocytoplasmic
localization. PLoS One. 5:e126362010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding B, Sun Y and Huang J: Overexpression
of SKI oncoprotein leads to p53 degradation through regulation of
MDM2 protein sumoylation. J Biol Chem. 287:14621–14630. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bettermann K, Benesch M, Weis S and
Haybaeck J: SUMOylation in carcinogenesis. Cancer Lett.
316:113–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo WH, Yuan LH, Xiao ZH, Liu D and Zhang
JX: Overexpression of SUMO-1 in hepatocellular carcinoma: a latent
target for diagnosis and therapy of hepatoma. J Cancer Res Clin
Oncol. 137:533–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kessler JD, Kahle KT, Sun T, et al: A
SUMOylation-dependent transcriptional subprogram is required for
Myc-driven tumorigenesis. Science. 335:348–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Xia N, Li T, et al: SUMO-specific
protease 1 promotes prostate cancer progression and metastasis.
Oncogene. 32:2493–2498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wartenberg M, Ling FC, Muschen M, et al:
Regulation of the multidrug resistance transporter P-glycoprotein
in multicellular tumor spheroids by hypoxia-inducible factor
(HIF-1) and reactive oxygen species. FASEB J. 17:503–505. 2003.
|