Introduction
Mesenchymal stem cells (MSCs) are multipotent
stromal cells that can differentiate into a variety of cell types
(1) and exert immunomodulatory
functions (2). In recent years,
studies have demonstrated that MSCs are promising for therapeutic
use in inflammatory bowel disease (IBD) (3–5).
Azathioprine (AZA) is a purine analog immunosuppressive drug. It is
used to treat a vast array of autoimmune diseases, including
rheumatoid arthritis, IBD, multiple sclerosis and autoimmune
hepatitis (6–10). Its active metabolite,
6-mercaptopurine hampers DNA synthesis and inhibits the
proliferation of fast-growing cells, including T lymphocytes
(11). The use of AZA and
6-mercaptopurine has been the mainstay of long-term therapy for the
majority of IBD patients for numerous years. Their role as steroid
sparing agents and in the maintenance of remission is well
recognized particularly in those with recommitting recurrence
(12,13). An in vivo study indicated
that IBD patients treated with AZA have more apoptotic lamina
propria mononuclear cells compared with the untreated controls
(11). As certain IBD patients may
be eligible for MSC transplantation, we examined if the ongoing
treatment with AZA will affect the cell proliferation, cell cycle
and apoptosis of MSCs.
Infliximab is a human-mouse chimeric monoclonal
antibody against tumor necrosis factor-α (TNF-α) that is used in a
variety of autoimmune diseases, including psoriasis, rheumatoid
arthritis, Crohn’s disease, ulcerative colitis and ankylosing
spondylitis (14–17). It functions primarily by binding to
TNF-α and prevents it from binding to its receptor. However, its
potent anti-inflammatory effect has been demonstrated to function
through causing programmed cell death of activated T lymphocytes,
an important cell type mediating inflammation in Crohn’s disease
(18). The inflamed tissue often
releases TNF-α (19), which
stimulates the adherence of MSCs to the endothelium and attracts
the homing of MSCs to injured sites (20). Nevertheless, there was only limited
information available concerning the interaction between the
monoclonal anti-TNF-α antibody, infliximab and MSCs (21).
In the present study, we investigated the effects of
various concentrations of AZA and infliximab on the cell
proliferation, cell cycle and apoptosis of the MSCs derived from
the bone marrow of Sprague-Dawley (SD) rats in vitro, in
order to provide the preliminary data for optimizing the
microenvironment of patients with IBD for the potential use of MSC
transplantation.
Materials and methods
Reagents
The low-glucose Dulbecco’s modified Eagle’s medium
(DMEM) and 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) was
purchased from Invitrogen Life Technologies (Guangzhou, Guangdong,
China). The fetal bovine serum (FBS) was purchased from Hangzhou
Sijiqing Biological Engineering Materials Company (Hangzhou,
Zhejiang, China). The monoclonal fluorescein isothiocyanate (FITC)
anti-rat CD29 Armenian hamster immunoglobulin G (IgG) and FITC
anti-rat CD45 mouse IgG were purchased from BioLegend (San Diego,
CA, USA). The monoclonal FITC anti-rat CD34 mouse IgG and FITC
anti-rat CD44 mouse IgG were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). AZA was purchased from
Shanghai Pharmaceuticals (Shanghai, China). Infliximab was
purchased from Janssen Pharmaceuticals (Janssen Cilag AG, Baar,
Switzerland).
Ethics statement
SD rats with specific pathogen-free grade were
provided by the Animal Center of Sun Yat-sen University (Guangzhou,
Guangdong, China). All experiments were conducted in accordance
with the institutional guidelines of Sun Yat-sen University for the
care and use of experimental animals.
Preparation and culture of MSCs from the
bone marrow of rats
MSCs were obtained from the bone marrow of
3-week-old SD rats. Following euthanasia, whole bone marrow was
flushed with DMEM from the tibia and femur of the SD rat. The
marrow was pooled and collected in fresh tubes. The marrow
suspension was then centrifuged at 157 × g for 10 min. The
supernatant was removed and the pellet was resuspended with
low-glucose DMEM containing 10% FBS. Cells were plated in a 25
cm2 flask and incubated in a humidified atmosphere with
5% CO2 at 37°C. The medium was changed after 2 days and
the nonadherent cells were removed. The medium was then changed
every 3 days. When the cells were at 80–90% confluence, the
adherent cells were detached with 0.25% trypsin EDTA and replated
at a 1:2 ratio. The cells were further purified with passages.
The MSCs at passage 4 were trypsinized and
harvested. The cells were washed with phosphate-buffered saline
(PBS) twice and 1×105 cells were used to identify the
surface markers. The antibodies against CD29 (0.2 μg/105
cells), CD34 (0.5 μg/105 cells), CD44 (0.5
μg/105 cells) and CD45 (0.2 μg/105 cells)
were incubated with the MSCs for 20 min at room temperature
followed by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ,
USA).
To evaluate the effects of AZA and infliximab on the
MSCs derived from the bone marrow of SD rats, the MSCs at passage 4
were used. The cells were grown in various concentrations of
AZA-supplemented (0, 0.05, 0.10, 0.20 and 0.30 mg/ml) or
infliximab-supplemented (0, 0.10, 0.20, 0.30 and 0.40 mg/ml) DMEM
with 10% FBS, respectively. The MSCs were incubated in a humidified
atmosphere with 5% CO2 at 37°C.
Cell proliferation assay
The MSCs at passage 4 were cultured with AZA- or
infliximab-supplemented DMEM as described above in 6-well culture
dishes with 5×104 cells/well for three replicate wells
for each treatment and each day. The cells were trypsinized and
counted every 24 h for eight consecutive days, respectively. The
growth curves of the MSCs from each group were obtained from three
independent experiments.
Cell cycle and apoptosis analysis
The MSCs at passage 4 were plated at 6-well culture
dishes with 2×105 cells/well for three replicate wells
of each group. When the cells were at 70–80% confluence, the
low-glucose DMEM with 10% FBS was replaced by AZA- (0, 0.05, 0.10,
0.20 and 0.30 mg/ml) or infliximab-supplemented (0, 0.10, 0.20,
0.30 and 0.40 mg/ml) DMEM with 10% FBS, respectively. Cell cycle
and apoptosis analysis was performed at 24, 48 and 72 h using flow
cytometry, respectively. The data were obtained from three
independent experiments.
For cell cycle analysis, 5×105 MSCs were
fixed with 70% ethanol overnight at −20°C and washed twice with
PBS. For each reaction, the cells were incubated with 50 μg of
RNase (Sigma-Aldrich, St. Louis, MO, USA) and 9 μg of propidium
iodide (Invitrogen Life Technologies, Carlsbad, CA, USA) for 30 min
at 4°C in the dark. Cell cycle analysis was then performed using a
FACSCalibur flow cytometer (Becton-Dickinson).
To identify the apoptotic MSCs, 1×105
cells were harvested and washed with PBS followed by incubation
with 5 μl Annexin V conjugated to FITC 488 (Molecular Probes,
Eugene, OR, USA) and 0.2 μg of propidium iodide (Invitrogen Life
Technologies, Carlsbad, CA, USA) for 15 min at room temperature in
the dark. Flow cytometry analysis was carried out using a
FACSCalibur flow cytometer (Becton-Dickinson).
Statistical analysis
Values are presented as the mean ± standard
deviation. Comparisons of the means between two groups were
performed using the Student’s t-test. Comparisons of the means
among multiple groups were performed using one-way ANOVA. P<0.05
was considered to indicate a statistically significant
difference.
Results
Morphological features of MSCs derived
from the bone marrow of SD rats
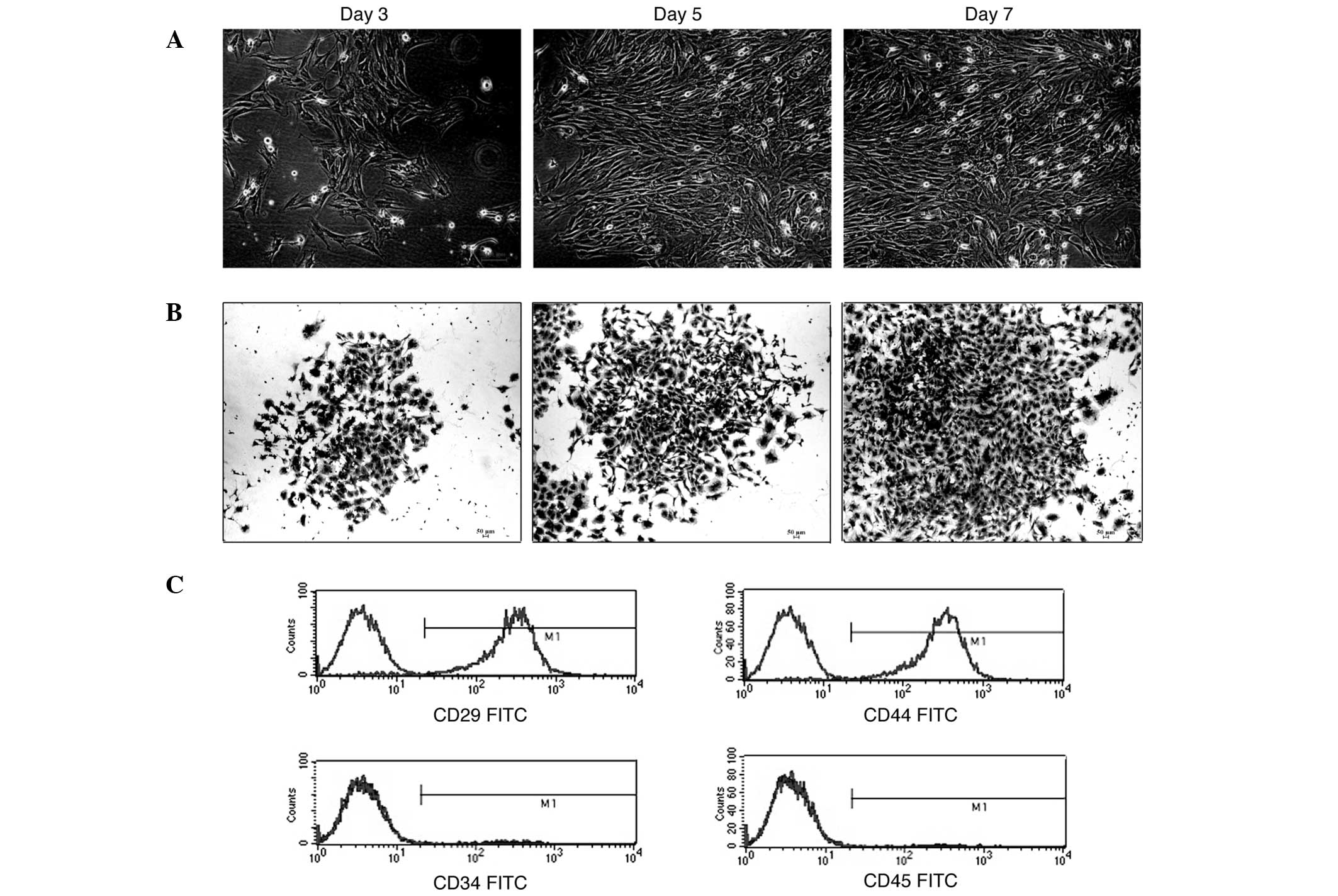
The primary culture of MSCs was obtained as
described. A small fraction of cells from the marrow suspension
attached and grew as fibroblastic cells that developed into visible
symmetric colonies at ~3 days after initial plating (Fig. 1A). The hematopoietic stem cells and
nonadherent cells were removed with changes of medium. The attached
cells dispersed widely and appeared as small cell bodies with few
long and thin cell processes. The cell body contained a large and
round nucleus with a prominent nucleolus (Fig. 1A and B). The cells were detached
with 0.25% trypsin EDTA at ~10–12 days after initial plating. The
cells at passage 2 attached within 48 h and demonstrated long
fusiform. The cells grew vigorously and demonstrated a ‘swirling
growth’ pattern. Homogeneity was attained following 3 passages.
Immunophenotyping of the MSCs
The MSCs at passage 4 were used to identify the
surface markers, including CD29, CD34, CD44 and CD45. Flow
cytometry analysis indicated that 98.25±0.58% of the cells were
CD29 positive, 98.97±0.53% were CD44 positive, only 1.11±0.34% of
these were CD34 positive and 0.99±0.53% were CD45 positive
(Fig. 1C). We considered the
isolated cells to be bone marrow-derived MSCs (BMSCs) from SD
rats.
Effects of AZA and infliximab on the
morphology of BMSCs from SD rats
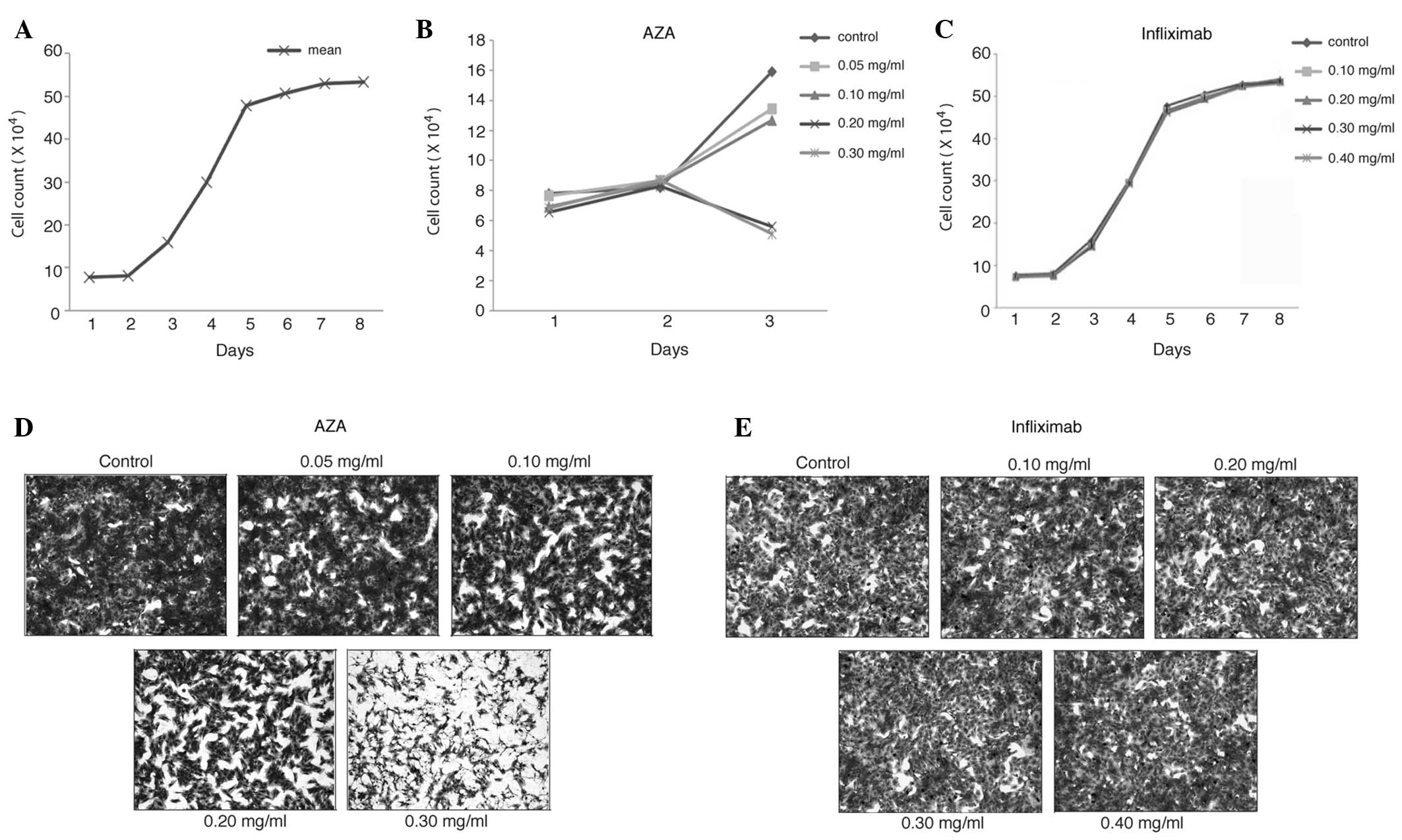
The MSCs at passage 4 were grown in various
concentrations of AZA- or infliximab-supplemented medium,
respectively. On day 3, a decreased number and diminished size of
the BMSC colonies were observed in those cultured in 0.20 and 0.30
mg/ml AZA supplemented medium (Fig.
2D). The BMSCs became thinner and smaller while cultured in the
high AZA concentration (0.20 and 0.30 mg/ml) medium compared with
those in the low AZA concentration (0.05 and 0.10 mg/ml) medium.
However, the number and the morphology of the BMSC colonies in
various concentrations of infliximab were very similar to those in
the control medium on day 3 (Fig.
2E) through to day 7 (data not shown).
Effects of AZA and infliximab on the
proliferation of BMSCs from SD rats
As demonstrated in Fig.
2A, the BMSCs grew in the exponential phase from day 3 to day 6
in the blank control medium (0 mg/ml AZA), however, they reached
the plateau phase following day 6. Although the proliferation of
the BMSCs cultured in 0.05 and 0.10 mg/ml AZA-supplemented medium
was inhibited compared with that in the blank control medium on day
3, the difference was not statistically significant (P>0.05;
Table I). While the AZA
concentration was increased to 0.20 and 0.30 mg/ml, the
proliferation of the BMSCs was inhibited by 66% and 67% compared
with that in the blank control medium on day 3, respectively
(P<0.05; Fig. 2B and Table I). Nevertheless, the proliferation
of BMSCs was not significantly affected by infliximab for up to 8
days in the testing range of concentrations (Fig. 2C and Table II).
 | Table IEffects of AZA on the growth of MSCs
(cell count, ×104; n=3). |
Table I
Effects of AZA on the growth of MSCs
(cell count, ×104; n=3).
| Group | Day 1 | Day 2 | Day 3 |
|---|
| A | 7.80±0.18 | 8.20±0.23 | 15.88±0.51 |
| B | 7.63±0.07 | 8.64±0.47 | 13.45±0.47 |
| C | 6.90±0.09 | 8.50±0.37 | 12.66±0.20 |
| D | 6.53±0.39 | 8.29±0.06 | 5.58±0.12a |
| E | 6.81±0.03 | 8.69±0.36 | 5.12±0.10a |
 | Table IIEffects of infliximab on the growth of
MSCs (cell count, ×104; n=3). |
Table II
Effects of infliximab on the growth of
MSCs (cell count, ×104; n=3).
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|
| A | 7.80±0.18 | 8.20±0.23 | 15.88±0.51 | 29.87±0.23 | 47.83±0.27 | 50.60±0.53 | 52.96±0.65 | 53.37±0.55 |
| B | 7.12±0.20 | 7.41±0.38 | 14.46±0.22 | 29.67±0.37 | 46.56±0.33 | 49.95±0.03 | 52.26±0.06 | 53.27±0.21 |
| C | 7.37±0.22 | 7.50±0.38 | 14.67±0.26 | 29.68±0.26 | 46.75±0.24 | 49.49±0.31 | 52.71±0.20 | 54.05±0.58 |
| D | 7.34±0.18 | 7.99±0.90 | 14.32±0.16 | 29.10±0.09 | 46.08±0.04 | 49.19±0.03 | 52.22±0.06 | 53.47±0.26 |
| E | 7.49±0.32 | 7.89±0.06 | 14.86±0.06 | 29.42±0.49 | 46.27±0.26 | 49.42±0.39 | 52.52±0.40 | 53.08±0.06 |
Effects of AZA and infliximab on
apoptosis in BMSCs from SD rats
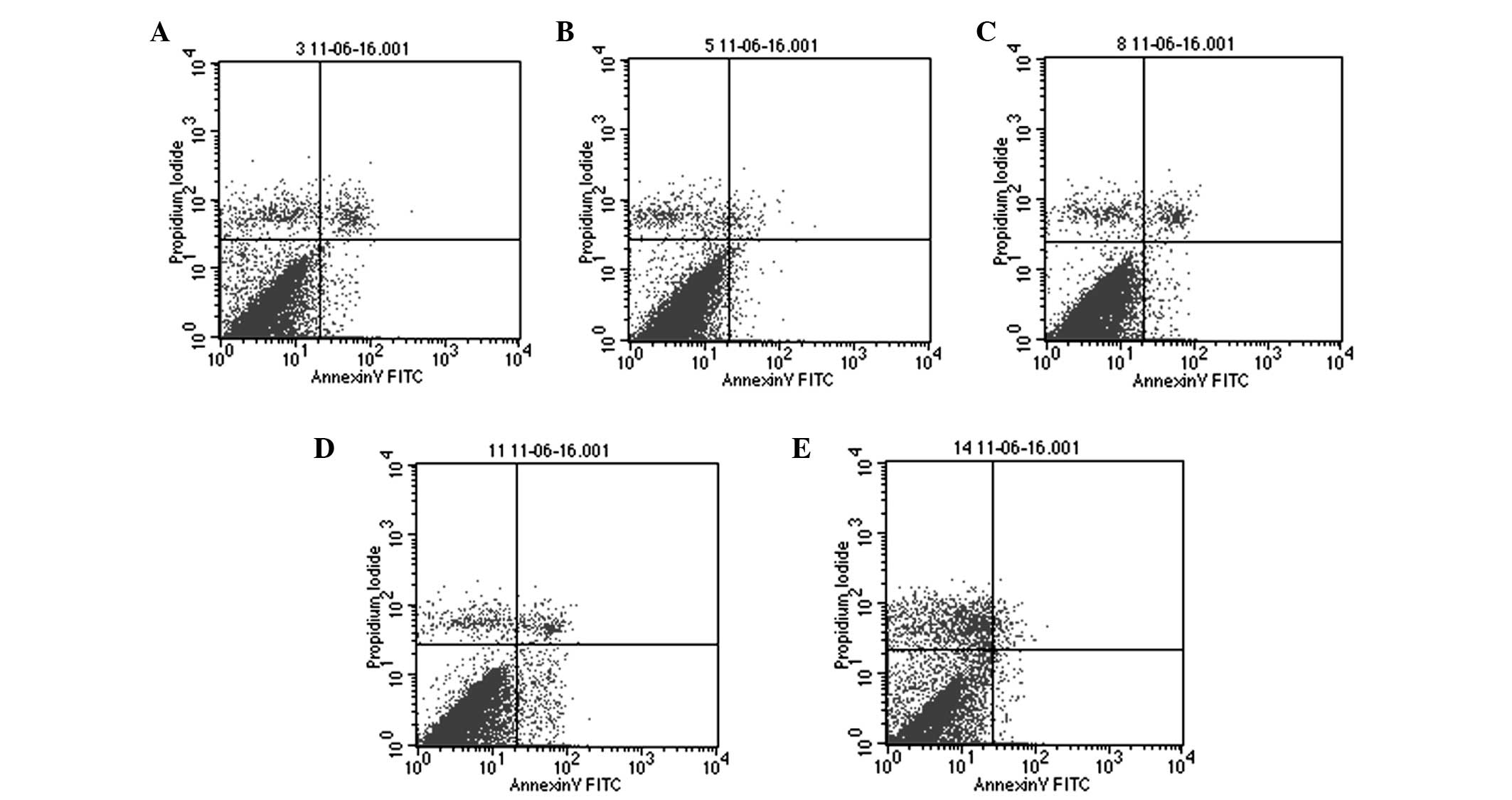
At 24 h of AZA treatment, no significant difference
in the apoptotic rate was observed across all groups of AZA-treated
BMSCs (P>0.05; Table III).
However, the number of the apoptotic BMSCs increased significantly
at 48 and 72 h in the 0.20 mg/ml AZA group compared with the blank
control group (P<0.05; Table
III). In the 0.30 mg/ml AZA group, the number of apoptotic
BMSCs increased at 48 h, however decreased at 72 h due to necrosis
of the cells (P<0.05; Fig. 3
and Table III). In all groups of
the infliximab-treated BMSCs, the apoptotic rates were not
statistically different from that of the blank control group on the
respective days (P>0.05; Table
IV).
 | Table IIIEffects of AZA on apoptosis and the
cell cycle of MSCs (%, n=3). |
Table III
Effects of AZA on apoptosis and the
cell cycle of MSCs (%, n=3).
| Group | Time (h) | Apoptosis | Necrosis | G0–G1 | G2-M | S |
|---|
| A | 24 | 3.80±0.41 | 1.64±0.22 | 91.63±0.61 | 6.30±0.44 | 2.07±0.42 |
| 48 | 1.79±0.91 | 3.95±0.77 | 86.53±0.66 | 9.51±0.67 | 3.95±0.29 |
| 72 | 3.53±0.39 | 5.37±0.88 | 74.34±4.20 | 15.11±4.69 | 10.56±0.49 |
| B | 24 | 3.74±0.83 | 1.71±0.47 | 90.85±1.28 | 7.25±0.86 | 1.91±0.43 |
| 48 | 2.42±0.96 | 3.70±0.82 | 86.99±0.60 | 9.31±0.33 | 3.83±0.58 |
| 72 | 4.08±3.13 | 5.80±0.21 | 74.04±3.47 | 15.28±3.03 | 10.67±0.47 |
| C | 24 | 4.78±1.01 | 1.79±0.74 | 91.97±1.50 | 6.09±0.76 | 1.94±0.78 |
| 48 | 2.46±0.43 | 3.85±0.32 | 86.63±0.51 | 8.86±1.54 | 4.21±1.18 |
| 72 | 3.74±1.08 | 5.04±0.26 | 74.26±3.43 | 14.46±3.23 | 11.28±0.26 |
| D | 24 | 4.10±1.57 | 1.87±0.67 | 91.04±1.39 | 7.03±1.32 | 1.93±0.10 |
| 48 | 4.68±1.15a | 3.29±0.68 | 87.90±0.51 | 9.34±0.37 | 4.03±0.35 |
| 72 | 11.27±1.96a | 5.74±0.441 | 88.16±0.33a | 10.27±0.82 | 1.57±0.51a |
| E | 24 | 4.55±1.97 | 1.73±0.63 | 90.36±1.63 | 7.58±1.22 | 2.06±0.49 |
| 48 | 5.09±0.71a | 3.40±0.68 | 88.90±0.72a | 8.60±0.30 | 2.50±0.51a |
| 72 | 1.67±0.68a | 14.58±0.51a | 92.94±1.26a | 6.31±0.86a | 0.75±0.24a |
 | Table IVEffects of infliximab on the
apoptosis and cell cycle of MSCs (%, n=3). |
Table IV
Effects of infliximab on the
apoptosis and cell cycle of MSCs (%, n=3).
| Group | Time (day) | Apoptosis | G0–G1 | G2-M | S |
|---|
| A | 1 | 2.82±0.56 | 91.60±0.09 | 5.73±0.35 | 2.68±0.37 |
| 3 | 3.22±0.75 | 74.50±3.28 | 15.22±2.87 | 10.28±0.72 |
| 7 | 5.25±0.52 | 93.47±0.09 | 4.86±0.41 | 1.67±0.34 |
| B | 1 | 2.37±0.51 | 92.34±0.29 | 5.57±0.19 | 2.09±0.43 |
| 3 | 3.63±1.86 | 74.57±4.06 | 15.81±3.66 | 9.61±0.41 |
| 7 | 5.11±0.23 | 94.08±0.62 | 4.17±0.28 | 1.75±0.23 |
| C | 1 | 2.85±0.85 | 91.90±1.00 | 5.24±0.05 | 2.51±0.46 |
| 3 | 2.66±0.77 | 74.42±4.59 | 14.42±3.07 | 11.16±1.60 |
| 7 | 5.03±0.35 | 94.29±0.62 | 4.16±0.40 | 1.55±0.31 |
| D | 1 | 3.08±0.73 | 91.66±1.11 | 5.34±0.53 | 2.99±0.43 |
| 3 | 2.65±0.64 | 79.34±1.70 | 11.06±1.20 | 9.60±1.59 |
| 7 | 4.87±0.50 | 93.36±0.81 | 4.75±0.37 | 1.88±0.72 |
| E | 1 | 2.64±1.42 | 91.93±0.68 | 5.20±0.29 | 2.61±0.28 |
| 3 | 3.18±1.03 | 72.00±3.97 | 12.64±1.30 | 10.18±2.88 |
| 7 | 5.01±0.42 | 93.81±0.79 | 4.53±0.37 | 1.65±0.36 |
Effect of AZA and infliximab on the cell
cycle in BMSCs from SD rats
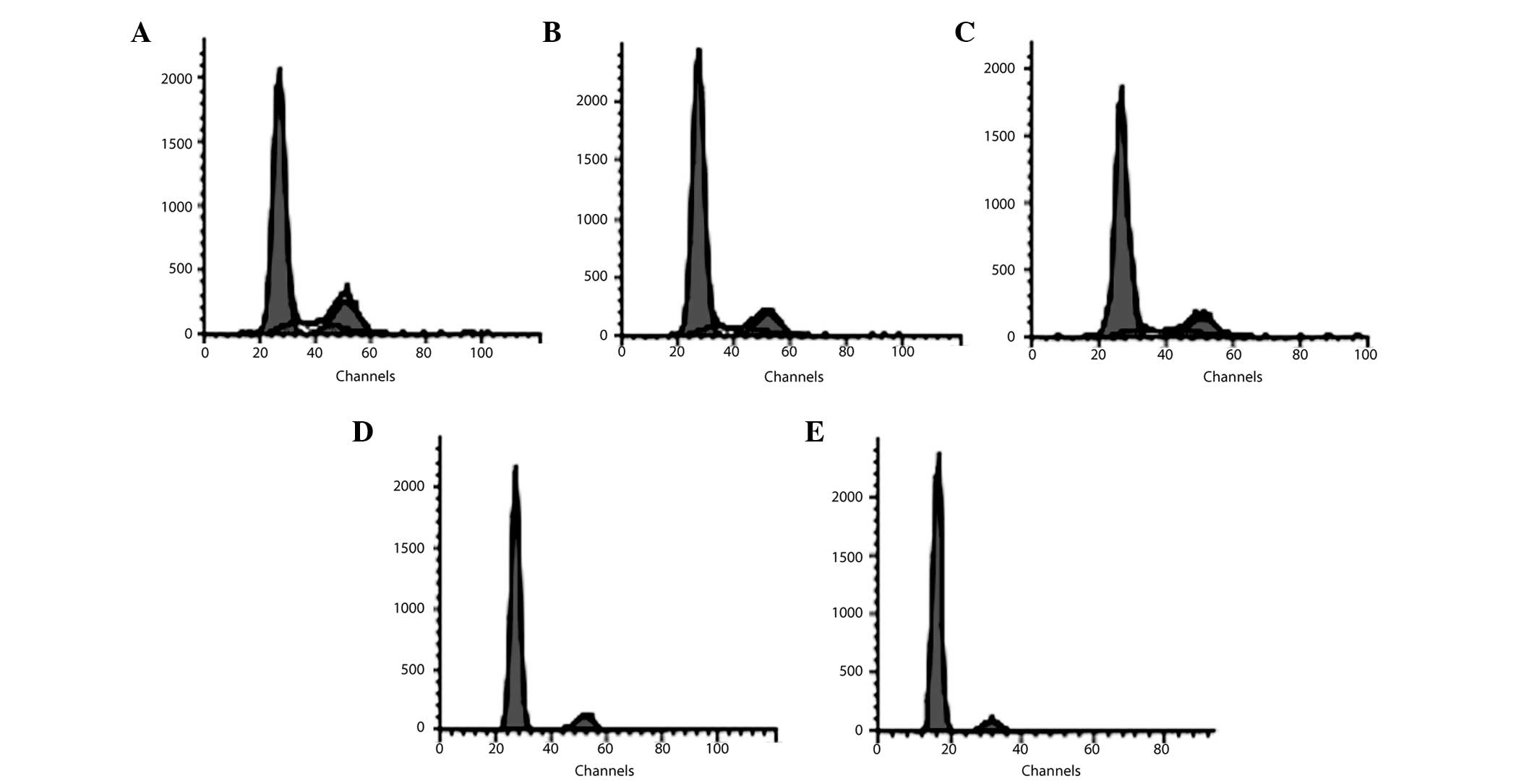
At 24 h of AZA treatment, no significant effect of
AZA on the cell cycle was observed in all groups of the AZA-treated
BMSCs (P>0.05; Table III). At
48 h, the percentage of BMSCs in the G0–G1 phase increased and that
in the S phase reduced in the 0.30 mg/ml AZA group compared with
the blank control group (P<0.05; Table III). At 72 h, the percentage of
cells in the G0–G1 phase increased and that in the S phase reduced
in the 0.20 and 0.30 mg/ml AZA-treated group compared with the
blank control group, respectively (P<0.05; Fig. 4 and Table III). The percentage of BMSCs in
the G2-M phase also significantly decreased in the 0.30 mg/ml
AZA-supplemented group at 72 h (P<0.05; Fig. 4 and Table III). On the other hand,
infliximab did not affect the cell cycle distribution of BMSCs on
the respective days compared with the blank control group (Table IV).
Discussion
It is considered that IBD is associated with
genetic, immunological, infectious and psychological factors. IBD
patients suffer a long term and recurrent course of the disease,
which significantly affects their quality of life and occasionally
leads to a poor prognosis. The conventional medication for IBD
includes aminosalicylates, corticosteroids and immunosuppressants.
However, these medications are not able to alter the natural
history of IBD and may add up to insurmountable side effects with
long-term use. In recent years, stem cell transplantation has
become a promising strategy for the treatment of IBD (4,5).
MSCs are able to colonize at the intestinal mucosa and
differentiate into fibroblasts, myofibroblasts and epithelial
cells. MSCs also provide a variety of growth factors to support
tissue repair and reconstruction (2). In addition, MSCs are likely to
communicate with other cells to exert immunomodulating effects on
countering the mucosal damage induced by the chronic inflammation
of IBD (2). All these suggest an
essential role of MSCs in IBD treatment.
As MSCs potentially contribute to the repair of the
mucosa in IBD (5), the importance
of optimizing the microenvironment for the colonization and
differentiation of MSCs needs to be emphasized (22). The majority of MSCs were
distributed into the lung after they were intravenously injected
into the experimental animals (23–25).
Therefore, it is assumed that only a small fraction of the MSCs are
able to eventually colonize to the injured intestinal mucosa in the
IBD scenario. Factors, including the intestinal epithelium, the
cytokines produced by the epithelium and drugs are able to affect
the proliferation and differentiation of MSCs, which directly
affect the outcome of MSC transplantation.
AZA is a purine analog immunosuppressive drug. There
are few studies investigating the effect of AZA on the
proliferation and differentiation of MSCs. Lazebnik et al
(26) reported that 72.7% of
patients with ulcerative colitis who were receiving
5-aminosalicylate, prednisone, AZA and methotrexate responded to
MSC transplantation in a two-year follow-up study. Nevertheless,
they did not ascertain whether AZA was associated with the
treatment failure for the remaining 27.3% of patients. Duijvestein
et al (21) demonstrated in
an in vitro study that the phenotype and function of MSCs
were not affected by the therapeutic concentrations of drugs
generally used in IBD treatment, including AZA. In the present
study, we demonstrated that there were no significant effects of
AZA on the cell proliferation, cell cycle and apoptosis of the
BMSCs derived from SD rats when the concentration was <0.10
mg/ml. The proliferation of the BMSCs was inhibited by >66% and
the percentage of apoptotic BMSCs increased while the concentration
of AZA exceeded 0.20 mg/ml and the treatment lasted for 72 h
compared with the blank control group. In the 0.30 mg/ml AZA group,
apoptosis of the BMSCs reduced, however more necrotic cells were
observed at 72 h. These results suggest that AZA was able to induce
apoptosis of the BMSCs at certain concentrations with prolonged
exposure time, which lead to cell death of the BMSCs at high doses.
We also demonstrated that the percentage of BMSCs increased in the
G0–G1 phase and decreased in the S phase when the concentration of
AZA exceeded 0.20 mg/ml with prolonged exposure time. Those in the
G2-M phase also decreased at 72 h in the 0.30 mg/ml AZA group.
These results indicated that AZA affects the proliferation of BMSCs
through the synthesis of DNA and the mitosis of cells.
In the last decade, biological therapy was
introduced as a novel strategy for IBD treatment, however the
response rate was only 38–68% with certain cases flaring up
following termination of the use of biological therapy (17). This is associated with the
development of antibodies against the biological agents and this
problem has yet to be resolved (27,28).
Therefore, gastroenterologists and investigators are seeking novel
strategies for the treatment of IBD. MSC transplantation is one of
these resolutions. Duijvestein et al (21) demonstrated that the therapeutic
concentration of infliximab did not affect the survival, phenotype
and function of human MSCs in vitro. Our data demonstrated
that infliximab (0.10–0.40 mg/ml) had a minimal effect on the cell
proliferation, apoptosis and cell cycle of the MSCs derived from
the SD rats. These results provide useful information on the
interaction between the two treatment methods of IBD in
vitro. The in vivo effects of infliximab on the
biological features of MSCs and on the homing of MSCs to the
injured mucosa in the inflammatory microenvironment are yet to be
determined. Further studies are also necessary to investigate the
immunoregulatory effect of MSCs in vivo, particularly on
improving the autoantibody-related events of biological treatment
in IBD.
In conclusion, AZA was able to affect the
proliferation and induce apoptosis, or cause death of MSCs at high
doses. Infliximab has no observed effect on the cell proliferation,
apoptosis and cell cycle of MSCs derived from the SD rats in
vitro.
Acknowledgements
This study was supported by the Beijing Medical
Award Foundation, China Medical Hand in Hand Project (Y. Zhong, no.
XHYSGZZSDX-001) and the National Natural Science Foundation of
China (Y. Zhong, no. 81370499). This study was also supported by
the Yat-sen Scholarship for Young Scientist (Y. Lin).
References
|
1
|
Beyer Nardi N and da Silva Meirelles L:
Mesenchymal stem cells: isolation, in vitro expansion and
characterization. Handb Exp Pharmacol. 174:249–282. 2006.
|
|
2
|
Li YP, Paczesny S, Lauret E, et al: Human
mesenchymal stem cells license adult CD34+ hemopoietic
progenitor cells to differentiate into regulatory dendritic cells
through activation of the Notch pathway. J Immunol. 180:1598–1608.
2008.PubMed/NCBI
|
|
3
|
Powell DW, Mifflin RC, Valentich JD, Crowe
SE, Saada JI and West AB: Myofibroblasts. II Intestinal
subepithelial myofibroblasts. Am J Physiol. 277:C183–C201.
1999.PubMed/NCBI
|
|
4
|
Lazebnik LB, Konopliannikov AG, Kniazew
OV, et al: Use of allogeneic mesenchymal stem cells in the
treatment of intestinal inflammatory disease. Ter Arkh. 82:38–43.
2010.(In Russian).
|
|
5
|
Gonzalez-Rey E, Anderson P, Gonzalez MA,
Rico L, Büscher D and Delgado M: Human adult stem cells derived
from adipose tissue protect against experimental colitis and
sepsis. Gut. 58:929–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suarez-Almazor ME, Spooner C and Belseck
E: Azathioprine for treating rheumatoid arthritis. Cochrane
Database Syst Rev. 4:CD0014612000.PubMed/NCBI
|
|
7
|
Sandborn WJ: Azathioprine: state of the
art in inflammatory bowel disease. Scand J Gastroenterol. (Suppl
225): 92–99. 1998. View Article : Google Scholar
|
|
8
|
Patel AA, Swerlick RA and McCall CO:
Azathioprine in dermatology: the past, the present, and the future.
J Am Acad Dermatol. 55:369–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rumbo C, Emerick KM, Emre S and Shneider
BL: Azathioprine metabolite measurements in the treatment of
autoimmune hepatitis in pediatric patients: a preliminary report. J
Pediatr Gastroenterol Nutr. 35:391–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abu-Shakra M and Shoenfeld Y: Azathioprine
therapy for patients with systemic lupus erythematosus. Lupus.
10:152–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tiede I, Fritz G, Strand S, et al:
CD28-dependent Rac1 activation is the molecular target of
azathioprine in primary human CD4+ T lymphocytes. J Clin
Invest. 111:1133–1145. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waters OR and Lawrance IC: Understanding
the use of immunosuppressive agents in the clinical management of
IBD. Curr Drug Targets. 12:1364–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chevaux JB, Peyrin-Biroulet L and Sparrow
MP: Optimizing thiopurine therapy in inflammatory bowel disease.
Inflamm Bowel Dis. 17:1428–1435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh CJ, Das KM and Gottlieb AB: Treatment
with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal
antibody dramatically decreases the clinical activity of psoriasis
lesions. J Am Acad Dermatol. 42:829–830. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maini R, St Clair EW, Breedveld F, et al:
Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal
antibody) versus placebo in rheumatoid arthritis patients receiving
concomitant methotrexate: a randomised phase III trial. ATTRACT
Study Group. Lancet. 354:1932–1939. 1999. View Article : Google Scholar
|
|
16
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar
|
|
17
|
Yamamoto-Furusho JK: Current and future
biological treatments in inflammatory bowel disease. Gene Therapy
Applications. Kang C: InTech; Rijeka: pp. 363–374. 2011
|
|
18
|
Van den Brande JM, Braat H, van den Brink
GR, et al: Infliximab but not etanercept induces apoptosis in
lamina propria T-lymphocytes from patients with Crohn’s disease.
Gastroenterology. 124:1774–1785. 2003.
|
|
19
|
Efron PA and Moldawer LL: Cytokines and
wound healing: the role of cytokine and anticytokine therapy in the
repair response. J Burn Care Rehabil. 25:149–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Böcker W, Docheva D, Prall WC, et al:
IKK-2 is required for TNF-alpha-induced invasion and proliferation
of human mesenchymal stem cells. J Mol Med (Berl). 86:1183–1192.
2008.PubMed/NCBI
|
|
21
|
Duijvestein M, Molendijk I, Roelofs H, et
al: Mesenchymal stromal cell function is not affected by drugs used
in the treatment of inflammatory bowel disease. Cytotherapy.
13:1066–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zan H and Zhong YQ: The progress of
mesenchymal stem cells in the treatment of inflammatory bowel
disease. New Medicine. 4:211–215. 2011.(In Chinese).
|
|
23
|
Fischer UM, Harting MT, Jimenez F, et al:
Pulmonary passage is a major obstacle for intravenous stem cell
delivery: the pulmonary first-pass effect. Stem Cells Dev.
18:683–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schrepfer S, Deuse T, Reichenspurner H,
Fischbein MP, Robbins RC and Pelletier MP: Stem cell
transplantation: the lung barrier. Transplant Proc. 39:573–576.
2007. View Article : Google Scholar
|
|
25
|
Lee RH, Pulin AA, Seo MJ, et al:
Intravenous hMSCs improve myocardial infarction in mice because
cells embolized in lung are activated to secrete the
anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lazebnik LB, Kniazev OV, Konopliannikov
AG, et al: Allogeneic mesenchymal stromal cells in patients with
ulcerative colitis: two years of observation. Eksp Klin
Gastroenterol. 11:3–15. 2010.(In Russian).
|
|
27
|
Baert F, Noman M, Vermeire S, Van Assche
G, D’Haens G, Carbonez A and Rutgeerts P: Influence of
immunogenicity on the long-term efficacy of infliximab in Crohn’s
disease. N Engl J Med. 348:601–608. 2003.
|
|
28
|
Sagynbaeva VÉ, Lazebnik LB, Kniazev OV and
Efremov LI: Antibodies to infliximab and antigens HLA I–II class as
the witnesses of immune response to the biological treatment of
inflammatory bowel disease. Eksp Klin Gastroenterol. 12:7–14.
2011.(In Russian).
|