Introduction
Lung cancer is the most common form of cancer, and
the leading cause of cancer-induced mortality in humans. Non-small
cell lung cancer (NSCLC) accounts for 80% of all lung cancer
diagnoses, with lung adenocarcinoma (AC) representing one of the
most predominant histological types of NSCLC and accounting for
20–30% of all cases (1,2). The majority of patients with NSCLC
are diagnosed in the late stage, when the tumor has become
unresectable; therefore, chemotherapy remains the first treatment
plan for advanced NSCLC. Clinically, cisplatin (DDP) in combination
with other antitumor agents, including paclitaxel, gemcitabine and
vinorelbine, is the first-line therapeutic strategy for treatment
of advanced NSCLC (3). Recently,
certain small molecule agents have shown favorable results for lung
AC treatment; however, improvements are often insignificant with
regard to the extension of the life of the patient with NSCLC
(4). For example, certain patients
with the epidermal growth factor receptor (EGFR) mutation benefit
from gefitinib and erlotinib, whilst others with the echinoderm
microtubule-associated protein-like 4-anaplastic lymphoma kinase
(EML4-ALK) mutation benefit from crizotinib. However, numerous
patients remain who are unable to benefit from such targeted
therapy, due to the lack of effective gene-specific agents
(4–6). Therefore, the identification of new
and effective therapeutic agents for the treatment of lung AC is
required.
Hypoxia-inducible factor 1 (HIF-1) is a
heterodimeric transcription factor consisting of a constitutively
expressed β-subunit and an oxygen-regulated α-subunit, and has a
critical role in the adaptive response to hypoxic environments.
HIF-1 regulates >60 downstream genes involved in energy
metabolism, angiogenesis, tumor growth and progression, metastatic
spread and apoptosis (7).
Overexpression of HIF-1α has been detected in >70% of solid
tumors. While upregulation or activation of HIF-1α promotes tumor
growth, downregulation or loss of HIF-1α activity has been
demonstrated to markedly decrease tumor growth, vascularization and
energy metabolism (8–11). The suppression of HIF-1α expression
by RNA interference (RNAi) has been shown to be an effective method
of cancer therapy (12).
Evidence suggests that HIF-1α is widely expressed in
NSCLC tissue, and is associated with the expression of numerous
biological factors involved in NSCLC pathogenesis, including
vascular endothelial growth factor, platelet-derived endothelial
cell growth factor and basic fibroblastic growth factor (8,11,13–16).
In addition, high levels of HIF-1α are also associated with a poor
prognosis, independent of routinely used clinicopathological
variables. At present, several small molecule inhibitors of HIF-1α
have been identified and are awaiting clinical trial (7). However, none of the presently
available inhibitors exclusively target HIF-1α. To address the
potential of small interfering RNA (siRNA) targeting HIF1α for the
treatment of AC, the effect of siRNA targeting HIF-1α on the growth
of AC A549 cells was investigated. The results showed that siRNA
targeting HIF-1α significantly inhibited the expression of HIF-1α
in A549 cells. The results also indicated that silencing of HIF-1α
was capable of suppressing the growth of AC A549 cells through
induction of apoptosis.
Materials and methods
Cell culture
Human lung AC A549 cells were purchased from the
Shanghai Cell Bank of Chinese Academy of Science (Shanghai, China),
and cultured in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA)
containing 15% calf serum, 100 U/ml penicillin and 100 mg/l
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. For hypoxic treatment, cells were cultured in a
modular incubator chamber (Forma®, Thermo Fisher
Scientific, Waltham, MA, USA) that was infused with 2%
O2, 5% CO2, and 93% N2.
RNAi treatment
siRNA targeting HIF-1α was designed and validated by
Boya Bio-engineering Company (Shanghai, China) (17). The antisense sequence of siRNA
targeting HIF-1α was 5′-AGTTCACCTGAGCCTAATA-3′, and the control
sequence was 5′-CTTAGCCTTCGAATGATCT-3′. Gene silencing was
performed via transfection with the HIF-1α siRNA (25, 50 and 100
nM) and control sequences. siRNA was transfected into A549 cells
using Lipofectamine 2000, according to the manufacturer’s
instructions (Invitrogen Life Technologies, Carlsbad, CA, USA).
Cells were pretreated with siRNA for 4 h at 37°C under normoxic
conditions. Cells were then transferred to hypoxic conditions or
maintained in a normoxic environment for the indicated
durations.
Quantitative polymerase chain reaction
(qPCR) analysis
A549 cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and harvested using a cell scraper.
Total RNA was isolated using TRIzol reagent, and cDNA was
synthesized according to the manufacturer’s instructions
(Invitrogen Life Technologies). qPCR was performed using a standard
SYBR-Green PCR Master Mix (Toyobo Corp, Osaka, Japan) (18), and PCR-specific amplification was
conducted in a 7900HT Fast Real-Time PCR System from Applied
Biosystems (Forster City, CA, USA). The primers for HIF-1α and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as an
internal normalization control) were as follows: HIF-1α,
5′-CAGCAACCAGGTGACCGTG-3′ (forward) and
5′-TGCTGCCTTGTATGGGAGCATT-3′ (reverse); GAPDH,
5′-CGGAGTCAACGGATTTGGTGGTAT-3′ (forward) and
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse).
Western blot analysis
Protein extracts were equally loaded on a 10% sodium
dodecyl sulfate-polyacrylamide gel, electrophoresed and transferred
to a nitrocellulose membrane. The blots were stained with 0.2%
Ponceau S red to ensure equal protein loading. Following blocking
with 5% non-fat milk in PBS, membranes were then probed with
primary antibodies against HIF-1α or caspase-3 (Invitrogen Life
Technologies) at 4°C overnight. Membranes were then probed with an
alkaline phosphatase-linked secondary antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at 37°C. Signals
were detected using a chemiluminescence
Phototope®-horseradish peroxidase (HRP) kit, according
to the manufacturer’s instructions (Cell Signaling Technology Inc.,
Beverly, MA, USA). Where necessary, β-actin (Invitrogen Life
Technologies) was used as an internal control.
MTT assay
To evaluate the effect of HIF-1α siRNA on lung AC
cells, the MTT assay was performed as previously described
(18,19). Cells were grown in monolayer
culture to 60% confluency, harvested using Trypsin and plated at a
density of 4×104 cells/well into separate wells of a
96-well plate (Costar; Corning Inc., Corning, NY, USA). Incubation
was continued in 1% O2 (hypoxia) conditions. Following
incubation for the indicated times, 20 μl 5 mg/ml MTT (Sigma, St.
Louis, MO, USA) solution in PBS was added to each well for an
additional 4 h. Subsequently, the supernatant was discarded and 150
μl dimethylsulfoxide (DMSO) was added to each well. Following MTT
incubation, the absorbance of the samples was determined using a
microplate reader at 490 nm (Sunrise™; Tecan Group Ltd., Männedorf,
Switzerland). All experiments were performed at least three times
for each experimental condition.
Annexin-V assay
The Annexin-V assay was performed using the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
according to the manufacturer’s instructions (Becton Dickinson, San
Diego, CA, USA) (17). Briefly,
2×105 cells were collected, washed twice with cold PBS,
suspended in 100 μl binding buffer, and incubated with Annexin
V-FITC at room temperature for 10 min. Subsequently, 5 μl propidium
iodide (PI, 20 μg/ml) was added and cells were incubated away from
the light at room temperature for 5 min. Cells were then directly
analyzed by FACScan™ (Beckton Dickinson) and evaluated using the
Cell Quest program (Becton Dickinson).
Statistical analysis
The data were analyzed using the SPSS 15.0 software
package (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) followed by post hoc least significant difference
(LSD) or Dunnett’s tests was performed to determine the
significance of the differences in multiple comparisons. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
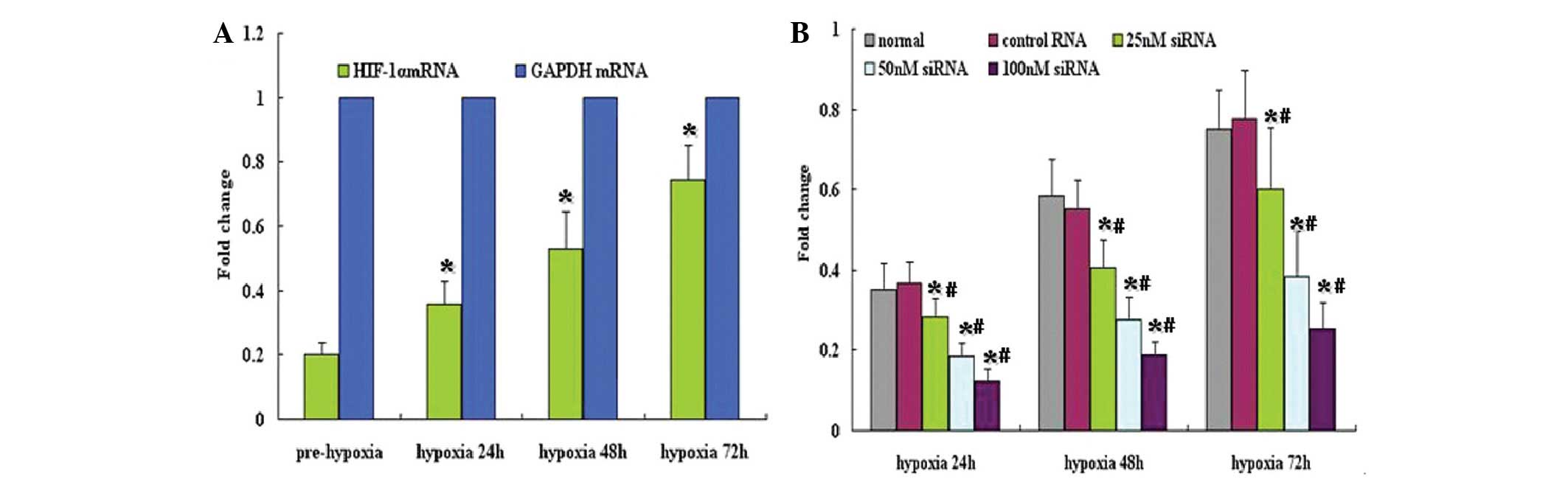
HIF-1α siRNA downregulates HIF-1α mRNA
expression
To assess the potential for HIF-1α siRNA to block
HIF-1α expression, HIF-1α mRNA expression levels in A549 cells were
analyzed using qPCR. Under normoxic conditions, HIF-1α mRNA was
expressed at low levels in A549 cells; however, under hypoxic
conditions, HIF-1α mRNA expression was significantly increased
(Fig. 1A). HIF-1α siRNA
significantly inhibited the expression of HIF-1α mRNA in HIF1α
siRNA-treated cells, (P<0.05), particularly at concentrations of
50 and 100 nM, compared with control cells (Fig. 1B).
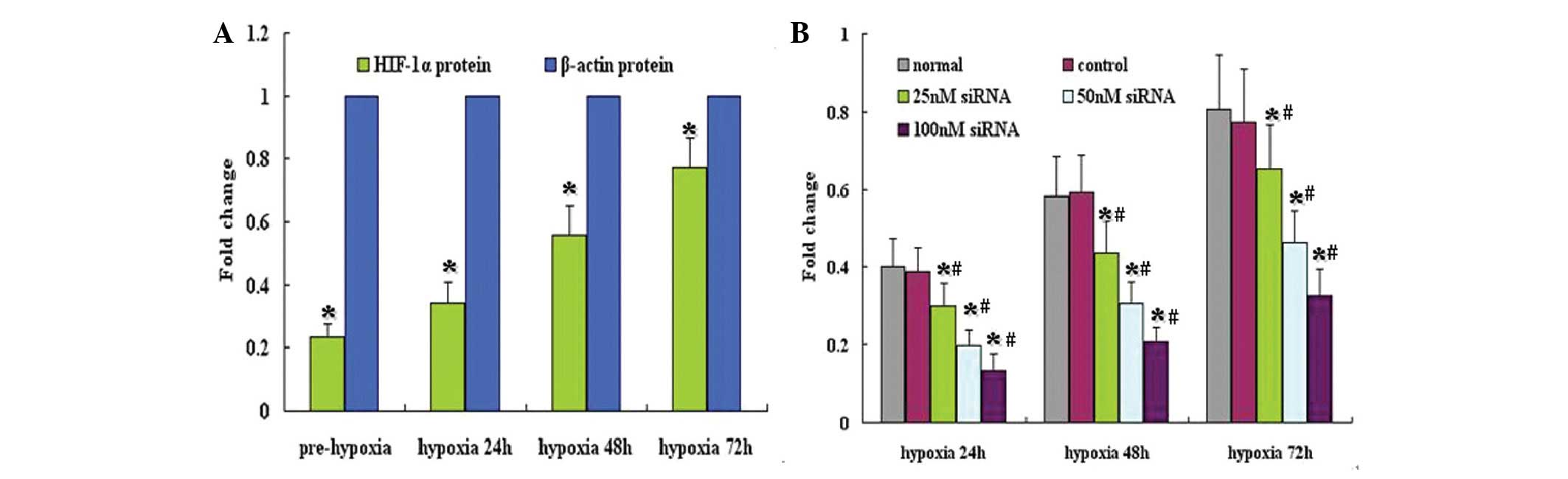
HIF-1α siRNA reduces HIF-1α protein
expression
To investigate the effect of HIF-1α siRNA on HIF-1α
protein expression, western blot analysis was performed. The
results are shown in Fig. 2. Under
normoxic conditions, HIF-1α protein expression in A549 cells was
low; however, expression was markedly induced under hypoxia
(Fig. 2A). Treatment with control
siRNA did not affect HIF-1α protein expression; however, HIF-1α
siRNA significantly downregulated HIF-1α protein expression under
hypoxic conditions (Fig. 2B).
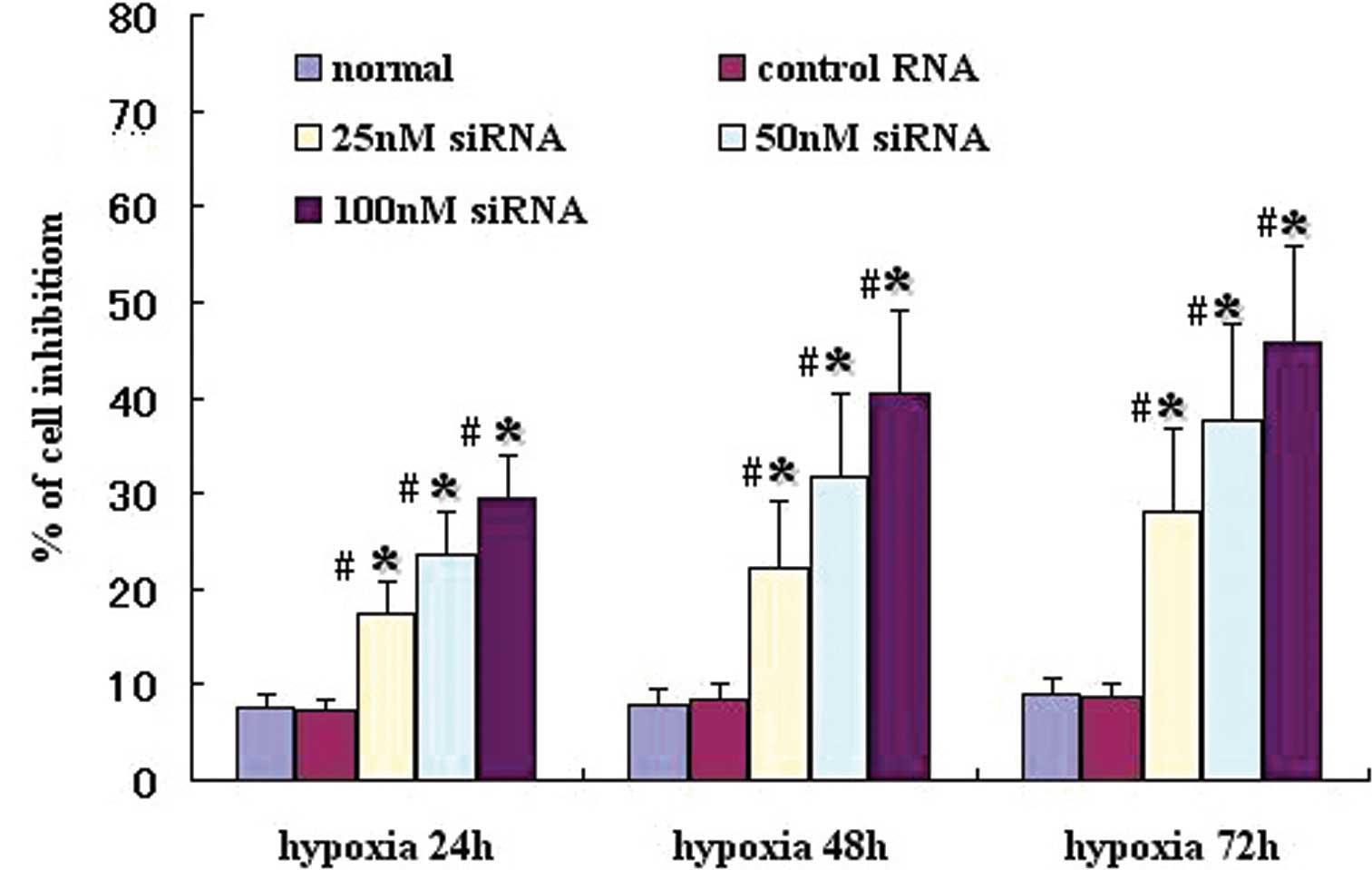
HIF-1α siRNA inhibits the growth of A549
cells
HIF-1α has a critical role in cell growth in a
number of tumor cell lines; therefore the effect of HIF-1α
silencing on A549 cell growth was assessed in vitro. As
shown in Fig. 3, under hypoxia,
HIF-1α siRNA significantly inhibited growth of A549 cells,
particularly at concentrations of 50 and 100 nM, compared with
control siRNA. Moreover, the suppression of growth in the HIF-1α
siRNA-transfected cells was more efficient under 72 h hypoxia,
compared with 24 h
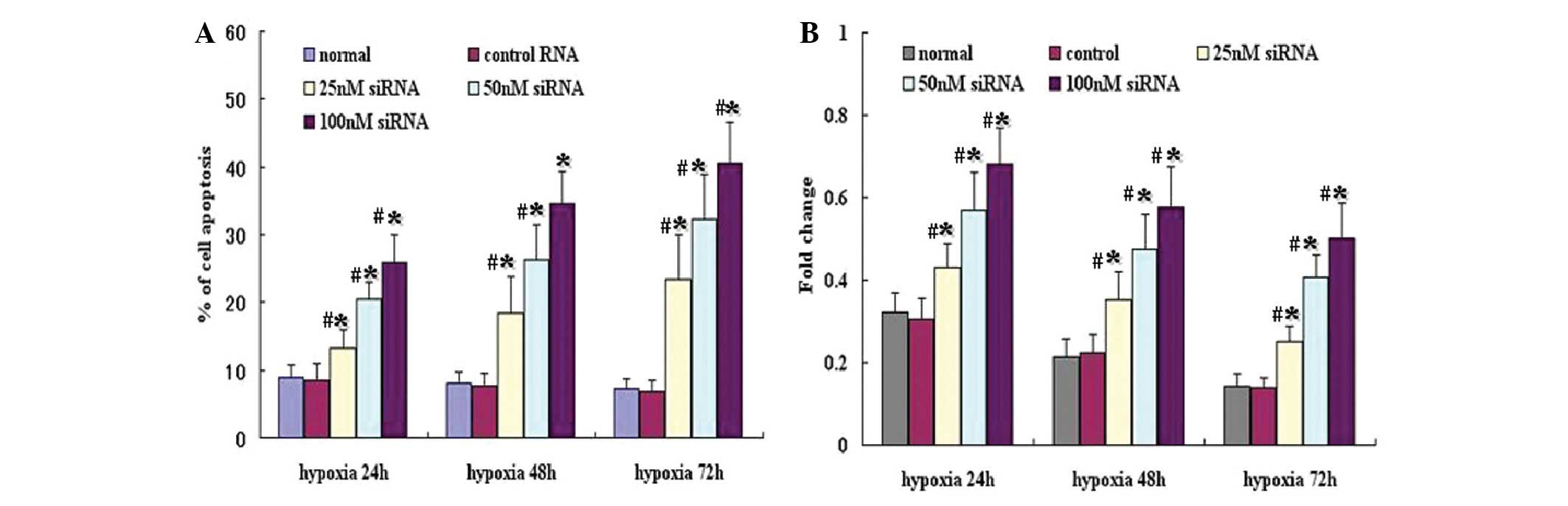
HIF-1α siRNA induces apoptosis in A549
cells
Flow cytometry experiments were performed to
determine whether the growth inhibitory effect observed in A549
cells treated with HIF-1α siRNA, was associated with apoptosis.
Annexin-V and PI staining were used to detect apoptotic cells,
including early- (FITC+/PI−) and late-stage
apoptosis or necrotic cells (FITC+/PI+). As
shown in Fig. 4A, under hypoxic
conditions, HIF-1α siRNA caused a significant induction of
apoptosis in A549 cells compared with the control (P<0.05),
particularly at concentrations of 50 or 100 nM.
To analyze the apoptosis induced by HIF-1α siRNA
under hypoxic conditions, levels of caspase-3, a critical indicator
of apoptosis, were examined. Under hypoxia, the level of caspase-3
was significantly increased following HIF-1α siRNA transfection,
particularly with 50 or 100 nM of HIF-1α siRNA (Fig. 4B).
Discussion
In the majority of types of human cancer, including
lung AC, overexpression of HIF-1 promotes cell proliferation,
apoptosis and metastasis (15,16,20,21).
In the present study, the results indicated that silencing HIF1-α
with siRNA may represent a therapeutically beneficial strategy for
treatment of AC. Hypoxia markedly increased HIF-1α mRNA and protein
expression in A549 cells; however, silencing HIF-1α with siRNA
effectively inhibited HIF-1α mRNA and protein expression.
Furthermore, under hypoxia, HIF-1α siRNA significantly inhibited
A549 cell growth, and promoted apoptosis through upregulation of
caspase-3 expression.
Tissue hypoxia is a fundamental characteristic of
solid tumors, including types of lung cancer, and promotes
biological processes involved in tumor progression. Based on the
central role of HIF-1α in the adaptive response to hypoxia,
targeting HIF-1α may serve as a potential anticancer strategy for
the clinic (7). Consistent with
results from previous studies, the present results demonstrated
that in A549 cells, HIF-1α mRNA and protein were expressed at low
levels under normoxic conditions; however, mRNA and protein levels
increased markedly under hypoxia (13,15).
Previous evidence has suggested that HIF-1α is important in the
regulation of cell growth and development, as well as invasion of
AC (11,16). It was therefore hypothesized that
the downregulation of HIF-1α was likely to lead to growth
inhibition of AC. At present, a number of small molecule
inhibitors, such as 17-AAG, temsirolimus and rapamycin, have been
demonstrated to decrease HIF-1α expression; however, none of these
drugs have been shown to directly and specifically target HIF-1α
(7). In the present study, it was
observed that HIF-1α silencing by siRNA not only significantly
inhibited the expression of HIF-1α mRNA and protein, but also
effectively suppressed the proliferation of AC cells under hypoxic
conditions.
Apoptosis is important in the regulation of tumor
cell growth, metastasis and invasion. Numerous studies have
reported that HIF-1α regulates cancer cell growth and apoptosis;
however, HIF-1α possesses dual functions in mediating apoptosis of
tumor cells, i.e, anti- and pro-apoptotic function (13,14,22,23).
The overall effect of HIF-1α on cell apoptosis remains incompletely
understood, but may depend on tumor microenvironment, particularly
hypoxia (21). As a fast-growing
cancer, AC often exhibits a hypoxic microenvironment and metabolic
disturbance. Evidence suggests that HIF-1α is widely expressed in
NSCLC tissue, including lung AC. The present study demonstrated
that, under hypoxia, silencing HIF-1α using siRNA induced A549 cell
apoptosis in vitro, in a caspase-dependent manner. Caspases
are crucial mediators of programmed cell death (apoptosis), with
caspase-3 representing a frequently activated death protease that
catalyzes the specific cleavage of key cellular proteins, and is
required for certain hallmarks of apoptosis (24). In AC cells, it was observed in the
present study that HIF-1α siRNA effectively upregulated the level
of caspase-3 expression under hypoxic conditions, in line with the
increase in A549 cell apoptosis induced by HIF-1α siRNA.
In conclusion, the present study showed that siRNA
technology may be used to specifically inhibit HIF-1α expression in
AC cell lines. Silencing of HIF-1α not only induced apoptosis in AC
A549 cells, but also suppressed growth of AC. However, achieving
the specific efficiency of HIF-1α siRNA in vivo remains a
challenge. There is a requirement for further in-depth studies and
trials to verify the antitumor activity of HIF-1α siRNA.
Acknowledgements
This study was supported by the Science and
Technology Foundation of Guangdong Province (no.
2008B030301315).
References
|
1
|
Kadara H, Kabbout M and Wistuba II:
Pulmonary adenocarcinoma: a renewed entity in 2011. Respirology.
17:50–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Noguchi M, et al;
American Thoracic Society. International Association for the Study
of Lung Cancer/American Thoracic Society/European Respiratory
Society: international multidisciplinary classification of lung
adenocarcinoma: executive summary. Proc Am Thorac Soc. 8:381–385.
2011. View Article : Google Scholar
|
|
3
|
Hodkinson PS and Sethi T: Advances in the
prevention and treatment of lung cancer. J R Coll Physicians Edinb.
41:142–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar
|
|
5
|
de Mello RA, Marques DS, Medeiros R and
Araújo AM: Epidermal growth factor receptor and K-Ras in non-small
cell lung cancer-molecular pathways involved and targeted
therapies. World J Clin Oncol. 2:367–376. 2011.PubMed/NCBI
|
|
6
|
Moran C: Importance of molecular features
of non-small cell lung cancer for choice of treatment. Am J Pathol.
178:1940–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patiar S and Harris AL: Role of
hypoxia-inducible factor-1alpha as a cancer therapy target. Endocr
Relat Cancer. 13(Suppl 1): S61–S75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savai R, Schermuly RT, Voswinckel R, et
al: HIF-1α attenuates tumor growth in spite of augmented
vascularization in an A549 adenocarcinoma mouse model. Int J Oncol.
27:393–400. 2005.
|
|
9
|
Scortegagna M, Martin RJ, Kladney RD,
Neumann RG and Arbeit JM: Hypoxia-inducible factor-1alpha
suppresses squamous carcinogenic progression and
epithelial-mesenchymal transition. Cancer Res. 69:2638–2646. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinha I, Null K, Wolter W, et al:
Methylseleninic acid downregulates hypoxia-inducible factor-1α in
invasive prostate cancer. Int J Cancer. 130:1430–1439.
2012.PubMed/NCBI
|
|
11
|
Wan J, Chai H, Yu Z, et al: HIF-1α effects
on angiogenic potential in human small cell lung carcinoma. J Exp
Clin Cancer Res. 30:772011.
|
|
12
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Volm M and Koomägi R: Hypoxia-inducible
factor (HIF-1) and its relationship to apoptosis and proliferation
in lung cancer. Anticancer Res. 20:1527–1533. 2000.PubMed/NCBI
|
|
14
|
Swinson DE, Jones JL, Cox G, et al:
Hypoxia-inducible factor-1 alpha in non small cell lung cancer:
relation to growth factor, protease and apoptosis pathways. Int J
Cancer. 111:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shyu KG, Hsu FL, Wang MJ, Wang BW and Lin
S: Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma
cell invasion. Exp Cell Res. 313:1181–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo F, Liu X, Yan N, et al:
Hypoxia-inducible transcription factor-1alpha promotes
hypoxia-induced A549 apoptosis via a mechanism that involves the
glycolysis pathway. BMC Cancer. 6:262006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao HY, Wang GP, Gu LJ, et al: HIF-1α
siRNA and cisplatin in combination suppress tumor growth in a nude
mice model of esophageal squamous cell carcinoma. Asian Pac J
Cancer Prev. 13:473–477. 2012.
|
|
18
|
Wang G, Ye Y, Yang X, Liao H, Zhao C and
Liang S: Expression-based in silico screening of candidate
therapeutic compounds for lung adenocarcinoma. PLoS One.
6:e145732011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu K, Ding Q, Fang Z, et al: Silencing of
HIF-1alpha suppresses tumorigenicity of renal cell carcinoma
through induction of apoptosis. Cancer Gene Ther. 17:212–222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurokawa T, Miyamoto M, Kato K, et al:
Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha) in
oesophageal squamous cell carcinoma correlates with lymph node
metastasis and pathologic stage. Br J Cancer. 89:1042–1047. 2003.
View Article : Google Scholar
|
|
21
|
Theodoropoulos GE, Lazaris AC,
Theodoropoulos VE, et al: Hypoxia, angiogenesis and apoptosis
markers in locally advanced rectal cancer. Int J Colorectal Dis.
21:248–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flamant L, Notte A, Ninane N, Raes M and
Michiels C: Anti-apoptotic role of HIF-1 and AP-1 in paclitaxel
exposed breast cancer cells under hypoxia. Mol Cancer. 9:1912010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yook YJ, Seo YJ, Kang HJ, et al: Induction
of hypoxia-inducible factor-1α inhibits drug-induced apoptosis in
the human leukemic cell line HL-60. Korean J Hematol. 45:158–163.
2010.
|
|
24
|
Olsson M and Zhivotovsky B: Caspases and
cancer. Cell Death Differ. 18:1441–1449. 2011. View Article : Google Scholar
|