Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of neoplasm worldwide, as well as the third most common
cause of cancer-related mortality (1). Patients usually succumb to metastases
rather than primary HCC. Thus, understanding the molecular
mechanisms of metastasis is of crucial importance for HCC therapy.
It is well known that cancer cells have to accomplish a series of
sequential steps to establish distant metastases, including
detachment from the primary tumor, invasion into and survival in
the vasculature, extravasation into the stroma of different organs,
and proliferation in the secondary site. Each of these steps can be
potentially targeted for treatment, but limited knowledge regarding
the molecular mechanisms of the metastatic steps renders the
majority of therapeutic strategies largely inefficient (2). Therefore, it is important to
investigate the molecular mechanisms of each metastatic step. The
majority of metastasis studies have focused on the primary tumor
and the organ to which it has metastasized (3), known as the first microenvironment
and secondary microenvironment, respectively; the importance of the
circulatory system, known as the third microenvironment, in tumor
metastasis has only recently been recognized (4). Although certain studies have focused
on metastatic mechanisms of cells in the third microenvironment
(5–7), and other studies have demonstrated
that metastatic tumor cells are able to survive for a long time by
forming homotypic multicellular aggregates (8–10),
it is largely unknown how these aggregated cells survive in the
anchorage-independent third microenvironment.
Anoikis, a Greek word meaning ‘loss of home’ or
‘homelessness’, was first coined by Frisch and Francis in 1994 to
describe a type of apoptosis that occurs when adherent cells are
deprived of anchorage (11); it
was later recognized as a potentially important element in tumor
angiogenesis and metastasis (12–14).
Resistance to anoikis enables malignant cells to survive in an
anchorage-independent manner and increase their survival time, as
well as facilitating their eventual reattachment and colonization
at secondary sites. Acquisition of anoikis resistance is known to
be critical for cancer metastasis. However, the mechanisms leading
to aberrant survival vary greatly among cell types (5). Understanding the mechanisms of
anoikis resistance may greatly benefit the development of
efficacious treatments for cancer. Our previous study reported that
hepatoma cells were able to resist anoikis through novel
synoikis-like survival (8).
Synoikis describes the process in which cancer cells form a
multicellular aggregate to maintain survival and proliferation
after detachment. We also reported that hepatoma cells acquired
greater metastatic potential when they were suspended in
extracellular matrix (ECM) and acquired the ability for anoikis
resistance (15).
The phosphatidylinositol 3 kinase (PI-3K)/AKT and
mitogen-activated protein kinase (MAPK) signaling pathways have
been well investigated. They are the most dominant proliferation
and survival signaling pathways. These two survival pathways are
known to be inhibited by a loss of adhesion (16,17).
One of the predominant changes observed in circulating tumor cells
is the loss of adherence to neighboring cells and the basal
membrane. The AKT pathway has been shown to be important in
mediating signals that lead to anchorage-independent survival, and
inhibition of the PI-3K/AKT pathway has been shown to lead to
anchorage-independent cell death, or anoikis (18,19).
AKT and extracellular signal-regulated kinase (ERK) protein kinases
are important in signaling pathways that respond to growth factors
and other extracellular stimuli. They regulate several cellular
functions, including nutrient metabolism, differentiation, cell
growth, proliferation, apoptosis and survival. One study has
reported that treatment with ERK and PI-3K inhibitors significantly
inhibited the invasiveness of hepatocellular carcinoma cells
(20).
Whilst a number of studies investigating
anchorage-independent survival mechanisms have focused on the
PI-3K/AKT and MAPK pathways in different types of malignancy
(21–24), there is little information
regarding hepatoma cells. Therefore, in the present study, the
protective effect of the PI-3K/AKT and MAPK pathways upon anchorage
removal in the synoikis-like hepatoma cells was specifically
examined. The molecular mechanisms underlying resistance to anoikis
in metastatic hepatoma cells were investigated using protein kinase
inhibition.
Materials and methods
Cell culture
BEL7402 human hepatoma cells were routinely cultured
and maintained in RPMI 1640 medium supplemented with 10% fetal calf
serum in the presence of 5% CO2 at 37°C. To obtain the
metastatic cell model, the cells were seeded into plates with a
poly-2-hydroxyethyl-methacrylate (HEMA; Sigma-Aldrich, St. Louis,
MO, USA) coating, as described previously (15). A trypan blue exclusion assay
(Beyotime, Nantong, China) was used to count the number of dead
cells and a cell counting kit-8 (CCK8) assay (Dojindo, Kunamoto,
Japan) was used to determine the cell viability as described
previously (8). All the
experiments described in the current study were performed in
triplicate and repeated three times.
Microarray
RNA extraction and cDNA synthesis were performed as
described previously (15). Human
oligonucleotide probe arrays (CapitalBio Corporation, Beijing,
China) were applied to detect the mRNA expression levels of 22,000
transcripts. cDNA samples from attached and detached groups were
labeled with Cy3 and Cy5 fluorescent dyes (Amersham Pharmacacia
Biotech, Piscataway, NJ, USA), respectively. DNA microarray
scanning was performed using a LuxScan 10KA Microarray Scanner
(CapitalBio Corporation). The images were analyzed using GenePix
Pro 4.0 software (Molecular Devices, Sunnyvale, CA, USA) and saved
as Excel files.
microRNA array
miRNAs were labeled using the miRCURYTM Array
Labeling kit (Exiqon, Vedbaek, Denmark) and the labeled sample was
concentrated using the RNeasy Mini kit (Qiagen, Dusseldorf,
Germany). miRNA array hybridization was performed using the miRCURY
LNA™ microRNA Array kit (Exiqon, Vedbaek, Denmark). Images were
acquired by scanning the slide using the Genepix 4000B (Molecular
Devices). The data were analyzed by Genepix Pro 6.0 software
(Molecular Devices) and saved as Excel files.
Analysis of the array results
Pathway analysis was used to identify significant
pathways of the differentially expressed genes according to the
Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/), BioCarta (BioCarta LLC,
San Diego, CA, USA; http://www.biocarta.com/) and Reactome (http://www.reactome.org/). Fisher’s exact test and
χ2 test were used to select the significant pathways,
and the threshold of significance was defined by P-value and the
false discovery rate. The enrichment was calculated according to
published studies (25–27). The pathway-network was the
interaction net of the significant pathways of the differentially
expressed genes built according to the interaction among pathways
of the KEGG database to find the interaction among the significant
pathways directly and systemically. It was able to summarize the
pathway interactions of differentially expressed genes in certain
diseases and identify why certain pathways were activated (26). The networks of genes in different
signaling pathways (Signal-net) were analyzed using the KEGG
database to build a network of genes according to associations
among the genes, proteins and compounds in the database (28–32).
The association of the microRNA and genes was calculated using
their differential expression values, and the MicroRNA-Gene-Network
was built according to the interactions of microRNA and genes in
the Sanger microRNA database (Wellcome Trust Sanger Institute,
Hinxton, UK). The adjacency matrix of the microRNA and genes,
A=[ai,j], was constructed as determined by the attribute
associations among genes and microRNA; ai,j represents the relation
weight of gene i to microRNA j.
AKT or ERK inhibition
BEL7402 cells were seeded in 96-well or 6-well
plates with or without a poly-HEMA coating as detached or attached
cells, as described above. The detached and attached cells were
treated with the PI-3K/AKT pathway-specific inhibitor Wortmannin
(Cell Signaling Technology, Inc., Danvers, MA, USA) or the ERK
pathway specific inhibitor PD98059 (Cell Signaling Technology,
Inc.). The cells in either adherent or suspension conditions were
treated in triplicate with either 1 μM wortmannin, 50 μM PD98059 or
both for 24 h. The cell viability, cell death rate and protein
expression level were then determined using the CCK8 kit, trypan
blue exclusion assay and western blot analysis, respectively.
Western blot analysis
Cells were washed three times in phosphate buffered
saline and incubated in lysis buffer (10 mmol/l TrisCl, 8 M urea,
4% CHAPs, 1% DTT, 0.3 mg/ml EDTA and 35 μg/ml PMSF) at 4°C for 20
minutes. Equal quantities of protein from each lysate were
subjected to 10% SDS-PAGE and transferred to a nitrocellulose
membrane. Subsequent to blocking for 1.5 h in 5% non-fat dried milk
containing 0.1% Tween 20, the membrane was incubated at 4°C
overnight in the presence of phospho-p44/42 MAP Kinase
(Thr202/Tyr204) antibody (Cell Signaling Technology, Inc.), p44/42
MAPK antibody (Cell Signaling Technology, Inc.) or β-actin antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
membranes were washed and further incubated for 1 h at 20°C with
horseradish peroxidase-conjugated secondary antibodies (ZSGB-BIO,
Beijing, China). Following washing, the immunoreactive bands were
visualized using a 3,3′-diaminobenzidine kit (ZSGB-BIO). The
results were analyzed by the UVP System (UVP, Upland, California,
USA).
Statistical analysis
Results are expressed as the mean ± standard
deviation. The statistical significance of differences between the
groups was determined by one-way analysis of variance using SPSS
13.0 statistical software (SPSS, Inc., Chicago, IL, USA). Fisher’s
exact test and χ2 test were used to select the
significant pathways and P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell aggregates are
proliferation-resistant
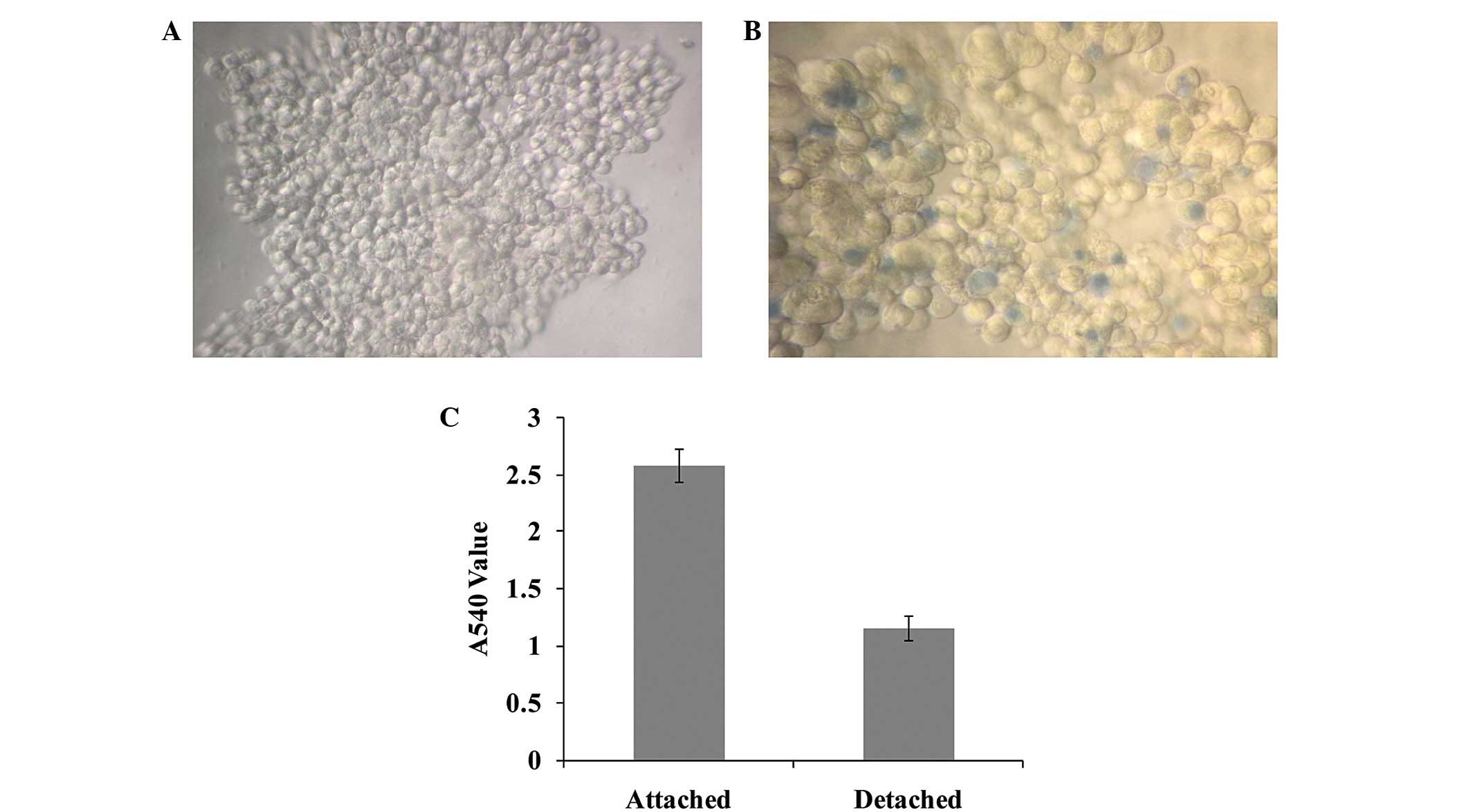
The detached cells gathered to form aggregates
(Fig. 1A). The majority of cells
in aggregates were alive, with the death rate <5% (Fig. 1B). The proliferation ability of
cells in cell aggregates was markedly lower in the detached group
than in the attached control group, as determined by a CCK8 assay
(Fig. 1C).
MAPK pathway is upregulated to promote
the survival of cells in cell aggregates
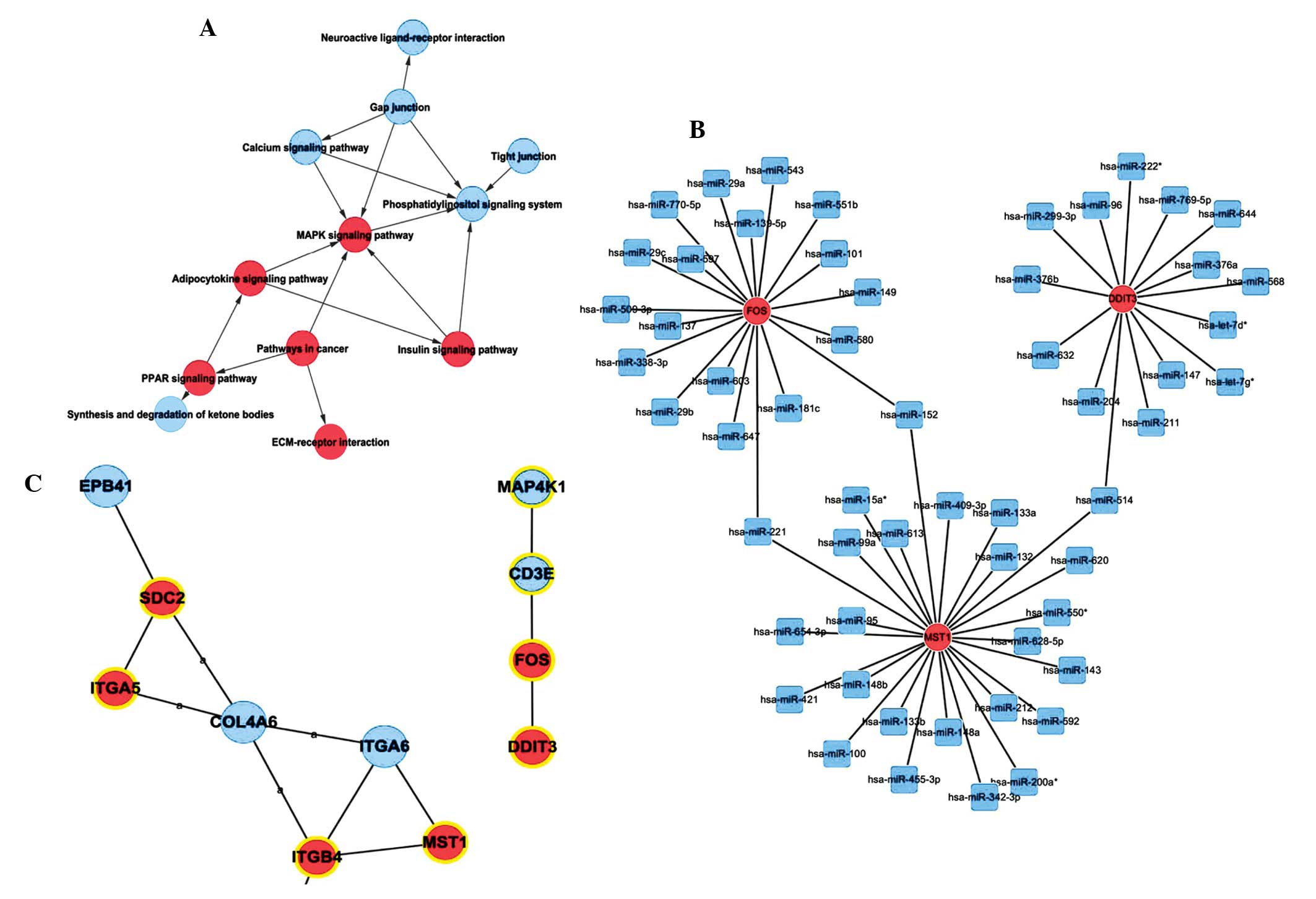
The MAPK signaling pathway was important in the
different expression pathway networks (Fig. 2A). The indegree and outdegree of
MAPK signaling pathway were 5 and 1, respectively. It was one of
the most important pathways in the entire pathway network. Five
genes in this pathway, including FOS, DDIT3 and MST1, were
upregulated when the cells were detached and gathered to form
aggregates. In the conjoint analysis of Signal-Net and
microRNA-Gene-Network, FOS, DDIT3 and MST1 in the MAPK signaling
pathway existed in the two networks (Fig. 2B). The microRNA-Gene-Network of
differentially expressed genes in the MAPK pathway, revealed that
FOS, DDIT3 and MST1 were regulated by several microRNAs (Fig. 2C). For example, miR-221 regulated
FOS and MST1, while miR-514 regulated MST1 and DDIT3.
Anoikis-resistant suspended cells are
less sensitive to AKT and ERK inhibitors
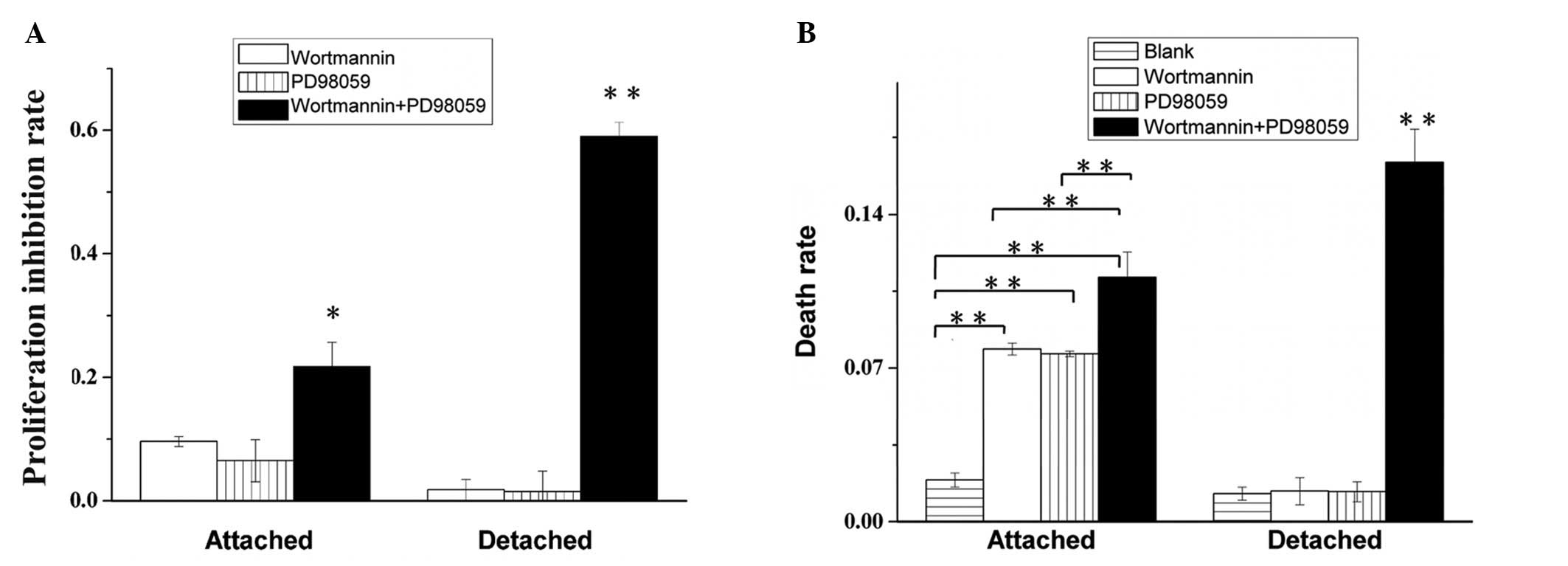
The growth of attached BEL7402 cells was greatly
inhibited by the effect of AKT or ERK inhibition (Fig. 3A). However, the detached BEL7402
cells were markedly more resistant to AKT or ERK inhibition.
Notably, when the AKT and ERK pathways were inhibited together, the
viability of the detached cells decreased markedly, much more than
in the attached groups. The death rates of attached and detached
BEL7402 hepatoma cells were detected by a trypan blue assay
(Fig. 3B). The cell death rate of
detached cells treated with Wortmannin or PD98059 separately was
markedly lower than in the attached control. However, when the
inhibitors were used together, the death rate of detached cells
increased markedly, to a significantly greater degree than that in
the attached control group.
Compensatory activation of ERK in
anoikis-resistant hepatoma cells upon inhibition of the PI-3K/AKT
pathway
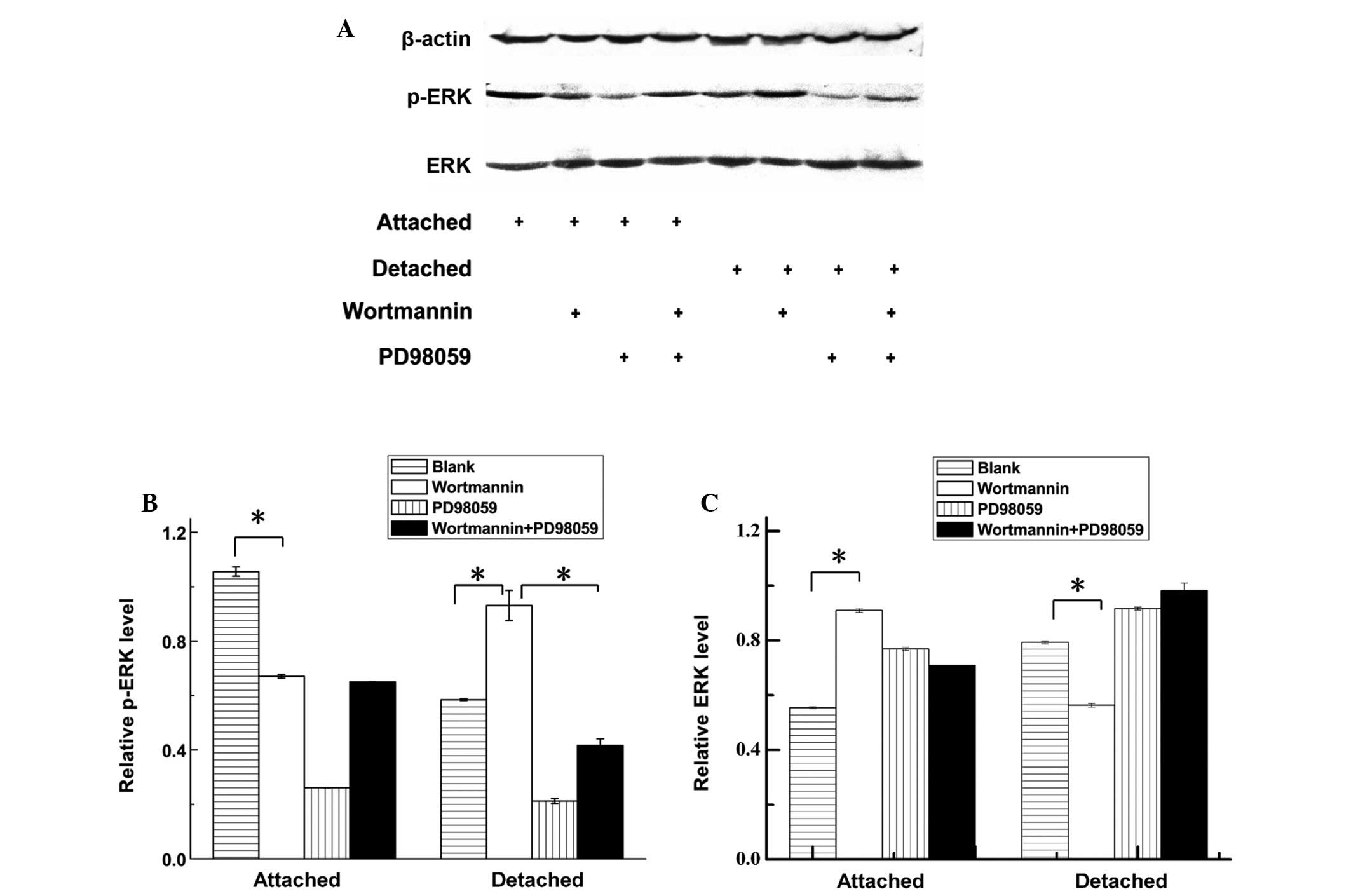
The ERK compensatory activation of the PI-3K/AKT
pathway inhibited the detached cell group and was confirmed by
western blot analysis. The ERK and phospho (p)-ERK western blotting
results in different groups are shown in Fig. 4A. The relative p-ERK protein level
of attached and detached hepatoma cells indicates that the p-ERK
protein level was upregulated in detached cells when Wortmannin was
used to inhibit the AKT pathway (Fig.
4B). The relative ERK protein level of attached and detached
hepatoma cells indicates that ERK protein was downregulated in
detached cells but upregulated in attached cells when Wortmannin
was used to inhibit the AKT pathway (Fig. 4C).
Discussion
Metastasis is an important factor in malignancy and
is commonly responsible for the failure of cancer treatment. How
cancer cells manage to survive during the entire metastatic process
and succeed in localizing to a secondary location remains
controversial. Our previous study revealed that hepatoma cells
prevented anoikis through synoikis-like survival (8). Acquisition of anoikis resistance
caused the detached hepatoma cells to develop further malignant
properties and acquire the metastatic potential to form metastatic
cancer at the secondary microenvironment (15).
In the present study, microarray was used to
elucidate the molecular mechanism that may be involved in the
proliferation inhibition of detached hepatoma cells. The results
indicated that the MAPK signaling pathway is upregulated in
anoikis-resistant cells. MAPK signaling pathway upregulation
usually leads to cell proliferation activity, which is contrary to
the proliferation inhibition of detached hepatoma cells, a process
validated in the current and in previous studies (8). This contradiction is noteworthy and
further investigation is required.
To determine whether a downstream PI-3K/AKT or MAPK
pathway is required for the survival of metastatic hepatoma cells
in suspension, the response of attached and detached BEL7402 cells
to PI-3K/AKT and MAPK pathway inhibitors was evaluated. The results
revealed that when the PI-3K/AKT and MAPK pathways were inhibited
separately, the effect on the viability of the anoikis-resistant
cells was weak. By contrast, when these two protein kinase pathways
were inhibited simultaneously, extensive cell death ensued. These
data indicate that there is a clear interplay between these two
prosurvival pathways. It is possible that when one of these two
pathways was inhibited, the other pathway was overactivated and
compensated for the loss of this survival signal. Notably, the
western blot analysis revealed that inhibition of the PI-3K/AKT
pathway markedly increased the phosphorylation level of the ERK
protein. This result validates the hypothesis that crosstalk exists
between these two signaling pathways. It was previously reported
that inhibition of AKT activation resulted in a marked increase in
the ERK pathway phosphorylation when apoptosis was induced by
DNA-damaging drugs (33). However,
no report has yet confirmed this compensatory activation in
metastatic hepatoma cells when one of the survival pathways is
inhibited. The marked activation of ERK pathway phosphorylation may
offer further survival opportunities for hepatoma cells following
deprivation of anchorage, which may also contribute to their
metastatic potential. Whether the anoikis resistance is dependent
on AKT or ERK activation remains controversial and the function of
these prosurvival pathways in resisting detachment-induced cell
death remains a matter of debate (34–37).
The MAPK signaling pathway is considered to be
fundamental in the regulation of proliferation in mammalian cells
by sharing a substrate and cross-cascade interaction with other
signal transduction systems. Understanding this pathway is
therefore essential for the rational design of novel
pharmacotherapeutic approaches (38). Notably, modulation of the PI-3K/AKT
and MAPK signaling pathways has also been reported to influence
apoptotic responses to anticancer drugs. The data from the present
study indicate that due to the powerful interplay among cell
signaling pathways, using a combination of kinase inhibitors may
yield a substantial advance in successfully producing a downstream
phenotypic response in anoikis-resistant hepatoma cells. This is in
accordance with the hypothesis that a combination of drugs that
target different aspects of the metastatic process may be a
therapeutic strategy in the future (2). However, the mechanism of this
powerful interplay remains unknown. The functions of FOS, DDIT3,
MST1, miR-221 and miR-514 may be investigated to analyze the
underlying metastatic mechanisms in future studies.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (grant nos. 30700357, 30772031 and
30873025) and the Natural Science Foundation of Shandong Province
(grant no. ZR2010HM033).
Abbreviations:
|
PI-3K
|
phosphatidylinositol 3-kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
CCK8
|
cell counting kit-8
|
|
ECM
|
extracellular matrix
|
|
Poly-HEMA
|
poly(2-hydrocyethyl methacrylate
|
|
FDR
|
false discovery rate
|
|
Re
|
the enrichment
|
|
Path-Net
|
pathway-network
|
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2
|
Mazzocca A and Carloni V: The metastatic
process: methodological advances and pharmacological challenges.
Curr Med Chem. 16:1704–1717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sleeman J and Steeg PS: Cancer metastasis
as a therapeutic target. Eur J Cancer. 46:1177–1180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loberg RD, Fridman Y, Pienta BA, Keller
ET, McCauley LK, Taichman RS and Pienta KJ: Detection and isolation
of circulating tumor cells in urologic cancers: a review.
Neoplasia. 6:302–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frisch SM: Evidence for a function of
death-receptor-related, death-domain-containing proteins in
anoikis. Curr Biol. 9:1047–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derouet M, Wu X, May L, Hoon Yoo B,
Sasazuki T, Shirasawa S, et al: Acquisition of anoikis resistance
promotes the emergence of oncogenic K-ras mutations in colorectal
cancer cells and stimulates their tumorigenicity in vivo.
Neoplasia. 9:536–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Cao L, Li J, Liang X, Liu Y, Liu
H, et al: Acquisition of anoikis resistance reveals a synoikis-like
survival style in BEL7402 hepatoma cells. Cancer Lett. 267:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geiger TR and Peeper DS: Critical role for
TrkB kinase function in anoikis suppression, tumorigenesis, and
metastasis. Cancer Res. 67:6221–6229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Lu H, Dazin P and Kapila Y:
Squamous cell carcinoma cell aggregates escape suspension-induced,
p53-mediated anoikis: fibronectin and integrin alphav mediate
survival signals through focal adhesion kinase. J Biol Chem.
279:48342–48349. 2004. View Article : Google Scholar
|
|
11
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christofori G: Changing neighbours,
changing behaviour: cell adhesion molecule-mediated signalling
during tumour progression. EMBO J. 22:2318–2323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
15
|
Cao L, Han L, Zhang Z, Li J, Qu Z, Du J,
et al: Involvement of anoikis-resistance in the metastasis of
hepatoma cells. Exp Cell Res. 315:1148–1156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aplin AE, Howe A, Alahari SK and Juliano
RL: Signal transduction and signal modulation by cell adhesion
receptors: the role of integrins, cadherins, immunoglobulin-cell
adhesion molecules, and selectins. Pharmacol Rev. 50:197–263.
1998.PubMed/NCBI
|
|
17
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Douma S, Van Laar T, Zevenhoven J,
Meuwissen R, Van Garderen E and Peeper DS: Suppression of anoikis
and induction of metastasis by the neurotrophic receptor TrkB.
Nature. 430:1034–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhan M, Zhao H and Han ZC: Signalling
mechanisms of anoikis. Histol Histopathol. 19:973–983. 2004.
|
|
20
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Díaz-Montero CM, Wygant JN and McIntyre
BW: PI3-K/Akt-mediated anoikis resistance of human osteosarcoma
cells requires Src activation. Eur J Cancer. 42:1491–1500.
2006.PubMed/NCBI
|
|
22
|
Horowitz JC, Rogers DS, Sharma V, Vittal
R, White ES, Cui Z and Thannickal VJ: Combinatorial activation of
FAK and AKT by transforming growth factor-beta1 confers an
anoikis-resistant phenotype to myofibroblasts. Cell Signal.
19:761–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collins NL, Reginato MJ, Paulus JK, Sgroi
DC, Labaer J and Brugge JS: G1/S cell cycle arrest provides anoikis
resistance through Erk-mediated Bim suppression. Mol Cell Biol.
25:5282–5291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukazawa H, Noguchi K, Murakami Y and
Uehara Y: Mitogen-activated protein/extracellular signal-regulated
kinase kinase (MEK) inhibitors restore anoikis sensitivity in human
breast cancer cell lines with a constitutively activated
extracellular-regulated kinase (ERK) pathway. Mol Cancer Ther.
1:303–309. 2002.
|
|
25
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:D277–D280. 2004.PubMed/NCBI
|
|
26
|
Yi M, Horton JD, Cohen JC, Hobbs HH and
Stephens RM: WholePathwayScope: a comprehensive pathway-based
analysis tool for high-throughput data. BMC Bioinformatics.
7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, et al: A systems biology approach for pathway
level analysis. Genome Res. 17:1537–1545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jansen R, Greenbaum D and Gerstein M:
Relating whole-genome expression data with protein-protein
interactions. Genome Res. 12:37–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C and Li H: Network-constrained
regularization and variable selection for analysis of genomic data.
Bioinformatics. 24:1175–1182. 2008. View Article : Google Scholar
|
|
30
|
Wei Z and Li H: A Markov random field
model for network-based analysis of genomic data. Bioinformatics.
23:1537–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JD and Wiemann S: KEGGgraph: a graph
approach to KEGG PATHWAY in R and bioconductor. Bioinformatics.
25:1470–1471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spirin V and Mirny LA: Protein complexes
and functional modules in molecular networks. Proc Natl Acad Sci
USA. 100:12123–12128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH,
Kim BW, et al: Interplay between PI3K/Akt and MAPK signaling
pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys
Acta. 1763:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rul W, Zugasti O, Roux P, Peyssonnaux C,
Eychene A, Franke TF, et al: Activation of ERK, controlled by Rac1
and Cdc42 via Akt, is required for anoikis. Ann N Y Acad Sci.
973:145–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishida K, Nagahara H, Kogiso T, Aso T,
Hayashi N and Akaike T: Cell adhesion aside from integrin system
can abrogate anoikis in rat liver cells by down-regulation of FasL
expression, not by activation of PI-3K/Akt and ERK signaling
pathway. Biochem Biophys Res Commun. 300:201–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi EM, Kwak SJ, Kim YM, Ha KS, Kim JI,
Lee SW and Han SJ: COX-2 inhibits anoikis by activation of the
PI-3K/Akt pathway in human bladder cancer cells. Exp Mol Med.
37:199–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zugasti O, Rul W, Roux P, Peyssonnaux C,
Eychene A, Franke TF, et al: Raf-MEK-Erk cascade in anoikis is
controlled by Rac1 and Cdc42 via Akt. Mol Cell Biol. 21:6706–6717.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|