Introduction
The majority of cancer mortalities are caused by
metastasis. Hematogenous metastasis includes multiple steps. First,
invasive cancer cells escape from the primary site into nearby
blood vessels (intravasation) and disseminate around the body
through the host circulation. Following this, the circulating
cancer cells exit the vessels to cross the endothelia and invade
secondary organ tissue (extravasation), where they proliferate to
form metastases (1). It is vital
to elucidate the detailed processes of these steps to prevent
metastasis, however, at present, the molecular mechanisms
underlying each step remain poorly understood.
In order to invade surrounding tissues, invasive
cancer cells form invadopodia, the filamentous actin
(F-actin)-based membrane protrusions required for degradation of
the extracellular matrix (ECM) and migration through the tissues
(2–4). It has been reported that invadopodia
play an essential role in the intravasation of cancer cells from
the primary site into the blood vessels (5). However, the importance of invadopodia
formation in extravasation from the blood vessels into the target
organs during metastasis, remains poorly understood.
In patients with aggressive bladder cancer, the most
common site of hematogenous metastasis is the lungs (6). The circulating bladder cancer cells
exit the lung microvessels by transendothelial invasion, to enter
the lung tissue. The present study assessed whether invadopodia
formation is involved in extravasation by analyzing lung metastasis
of aggressive bladder cancer cells.
Materials and methods
Cells, reagents and antibodies
A human invasive and high-grade bladder cancer cell
line, YTS-1, was provided by Dr H. Kakizaki (Yamagata University,
Yamagata, Japan). YTS-1 cells were maintained in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; PAA Laboratories GmbH, Pasching, Austria) with
5% CO2 at 37°C. Primary human lung microvascular
endothelial cells (HMVEC-L) were purchased from Lonza
(Walkersville, MD, USA) and maintained in EGM-2MV medium (Lonza).
All biochemical reagents were purchased from Sigma-Aldrich unless
otherwise noted. Anti-cortactin monoclonal antibody (clone,
EP1922Y) and anti-actin polyclonal antibody were purchased from
Epitomics Inc. (Burlingame, CA, USA) and Sigma-Aldrich,
respectively.
Stable transfectants
YTS-1 cells with reduced expression of cortactin
were generated by short hairpin (sh) RNA technology as previously
described (7). An shRNA expression
plasmid was constructed using pBAsi-hU6 Neo DNA (Takara Bio, Inc.,
Shiga, Japan). The shRNA sequence for cortactin was: GATC
CGCACGAGTCACAGAGAGATCTGTGAAGCCACAGATG
GGATCTCTCTGTGACTCGTGCT TTTTTA, the small interfering (si) RNA
sequence for cortactin is underlined. A human non-targeting siRNA
sequence (Accell Control siRNA kit, Thermo Fisher Scientific,
Waltham, MA, USA) was used to prepare the control cells expressing
non-targeting shRNA. The shRNA expression plasmids (knockdown and
control constructs), together with pTK-HyB, were introduced into
YTS-1 cells in a 10:1 molar ratio using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). Drug-resistant
colonies were selected in the presence of 200 μg/ml hygromycin B.
Two knockdown clones (designated cortKD-1 and -2) were selected
based on the cortactin expression levels. cortKD-1 and -2, and one
control clone (designated YTS control), were used for the assays
described in this study.
Western blot analysis
Total lysates of cancer cells were prepared by
solubilization in 50 mM Tris-HCl buffer (pH 7.5) containing 1%
Igepal CA-630, 150 mM NaCl and proteinase inhibitors. The lysates
were resolved by SDS-PAGE on an 8–16% gradient gel (Invitrogen Life
Technologies) and transferred to polyvinylidene fluoride membrane.
Western blotting was performed using specific primary antibodies
and a horseradish peroxidase-conjugated secondary antibody. Signals
were visualized using the ECL PLUS detection system (GE Healthcare,
Amersham, UK).
Immunofluorescence microscopy
Cells seeded on coverslips were fixed in 4%
paraformaldehyde and permeabilized with phosphate-buffered serum
(PBS) containing 0.1% saponin and 1% bovine serum albumin. Cells
were stained with Alexa Fluor 568-labeled phalloidin (Invitrogen
Life Technologies), together with the monoclonal antibody against
cortactin, and Alexa Fluor 488-labeled secondary antibody
(Invitrogen Life Technologies) was used for primary antibody
detection. Cell staining was examined under an Olympus IX-71
fluorescence microscope (Olympus Inc., Tokyo, Japan) and LSM 710
Laser Scanning confocal microscope (Carl Zeiss, Oberkochen,
Germany).
Gelatin zymography
Gelatin zymography was performed in a 10% Novex
Zymogram pre-cast SDS-PAGE gel (Invitrogen Life Technologies) in
the presence of 0.1% gelatin under nonreduced conditions. Cells
(1×106) in 2 ml RPMI-1640 containing 10% FBS were placed
in a single well (6-well dish) and grown to 80% confluence. Cells
were washed with PBS and incubated with 2 ml Opti-MEM (Invitrogen
Life Technologies) for 24 h. Conditioned media were collected and
subjected to SDS-PAGE. Gels were washed in 2.5% Triton X-100 for 30
min at room temperature to remove SDS and incubated at 37°C
overnight in substrate buffer containing 50 mM Tris-HCl (pH 8.0)
and 5 mM CaCl2. Following this, cells were stained with
0.5% Coomasie Brilliant Blue R-250 in 50% methanol and 10% acetic
acid for 1 h. The bands of matrix metalloprotease-2 (MMP-2) appear
as clear bands against dark background due to its gelatin
degradation activity.
Matrigel and transendothelial invasion
assays
The two invasion assays were performed using a
conventional Transwell system (BD Biosciences, San Jose, CA, USA).
For the Matrigel invasion assay, the bottom surface of the upper
chamber filter (8-μm pore size) was coated with 100 μg/ml
fibronectin and the top surface was covered with 1 mg/ml Matrigel
matrix (BD Biosciences). The lower chamber was filled with
serum-free RPMI-1640 medium. Bladder cancer cells
(5×104) were labeled with the Vybrant CFDA-SE Cell
Tracer kit (Invitrogen Life Technologies) and placed in the upper
chamber. Following incubation at 37°C for 24 h, non-migrated cells
remaining at the top surface of the filter were carefully removed
with cotton swabs. Migrated cells on the bottom surface were fixed
with 4% paraformaldehyde and counted under a fluorescence
microscope (Olympus IX-71). For transendothelial invasion assay,
HMVEC-L cells (1×105) were placed onto a collagen
I-coated upper chamber (8-μm pore size) and cultured over 2 days to
form a monolayer. Carboxyfluorescein diacetate-succinimidyl
ester-labeled bladder cancer cells were re-suspended in EGM-2MV
medium and cells (5×104) were added onto the HMVEC-L
monolayer. Following incubation at 37°C for 24 h, non-migrated
cells were removed and migrated cells were fixed and counted.
Lung metastasis assay in nude mice
Bladder cancer cells (2×106) were
suspended in 0.1 ml serum-free RPMI-1640 medium and injected into
the tail vein of 6- to 8-week-old nude mice (BALB/cAJcI-nu/nu; Clea
Japan, Inc., Tokyo, Japan). After 3 weeks, lungs were harvested and
fixed with formalin. The left lung was removed and trisected. The
lung blocks were cut further into 4-μm sections and stained with
hematoxylin and eosin (HE). Lung metastatic foci were identified by
HE staining based on the histopathological findings, for example
abnormally high cell density and atypical nuclear morphology. The
degree of metastasis was evaluated by the proportion (%) of the
tumor area occupying the total lung area of the section. Images of
the lung were captured with a scanner (Epson Perfection 4990;
Epson, Tokyo, Japan) and the proportion (%) of the metastasized
tumor area was calculated using DP2-BSW software (Olympus). The
experiments were approved by the committee for animal experiments
of Oyokyo Kidney Research Institute (Hirosaki Hospital, Hirosaki,
Japan).
Statistical analysis
The SPSS 12.0 statistical program (SPSS, Chicago,
IL, USA) was used. Statistically significant differences were
determined using the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
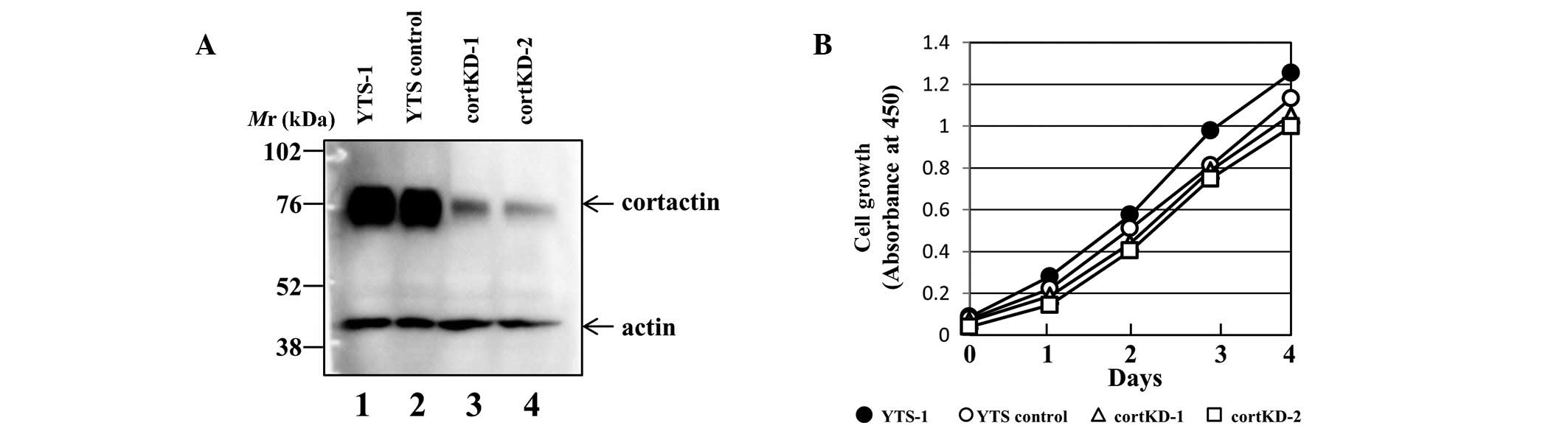
Establishment of cortactin knockdown
bladder cancer cells
To determine the contribution of invadopodia
formation to extravasation, the effect of a reduction of
invadopodia formation on cancer cell extravasation was analyzed. To
reduce invadopodia formation, the expression of cortactin in
invasive bladder cancer cells was silenced, as cortactin is an
invadopodium marker and one of the most important regulators for
invadopodia formation. Several stable cortactin knockdown cell
lines were established using an invasive bladder cancer cell line,
YTS-1. A YTS-1 cell line expressing non-targeting siRNA as a
control (designated YTS control) was also prepared. Western
blotting revealed that cortactin expression was reduced in two of
the knockdown cell lines (designated cortKD-1 and -2) compared with
parent YTS-1 and YTS controls (Fig.
1A). However, there was no significant difference in the growth
rate between these cell lines (Fig.
1B). The results from the assays using cortKD-1 are shown and
the two knockdown cell lines yielded almost identical results in
all assays.
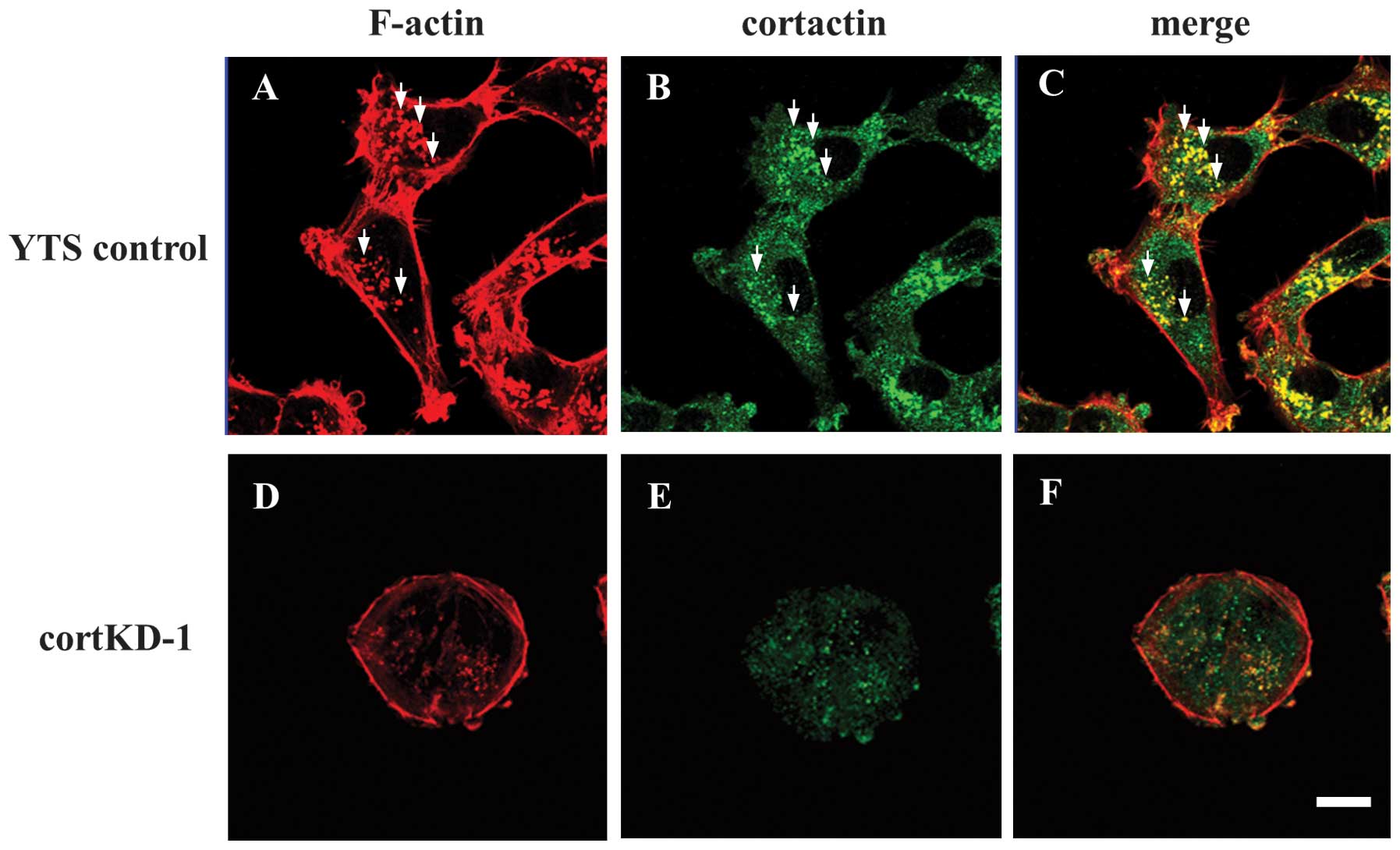
Reduction of invadopodia formation in
cortactin knockdown bladder cancer cells
Cortactin promotes invadopodia formation and
maturation in a number of cancer cells, and is also an invadopodium
marker (8–10). Cells were double-stained with
phallodin and anti-cortactin monoclonal antibody. Invadopodia in
YTS control cells were visualized by Alexa Fluor 568-labeled
phalloidin staining as F-actin-rich puncta (Fig. 2A). Several typical invadopodia were
indicated, as shown in Fig. 2A–C.
A portion of cortactin staining also exhibited a punctate pattern
(Fig. 2B) and co-localization of
cortactin with F-actin puncta indicated that the puncta were
invadopodia (Fig. 2C). In cortKD-1
cells, cortactin knockdown resulted in a marked cell-morphological
change to a round shape, and no invadopodia were observed (Fig. 2D–F). These results indicate that
invadopodia formation is impaired in cortactin knockdown bladder
cancer cells.
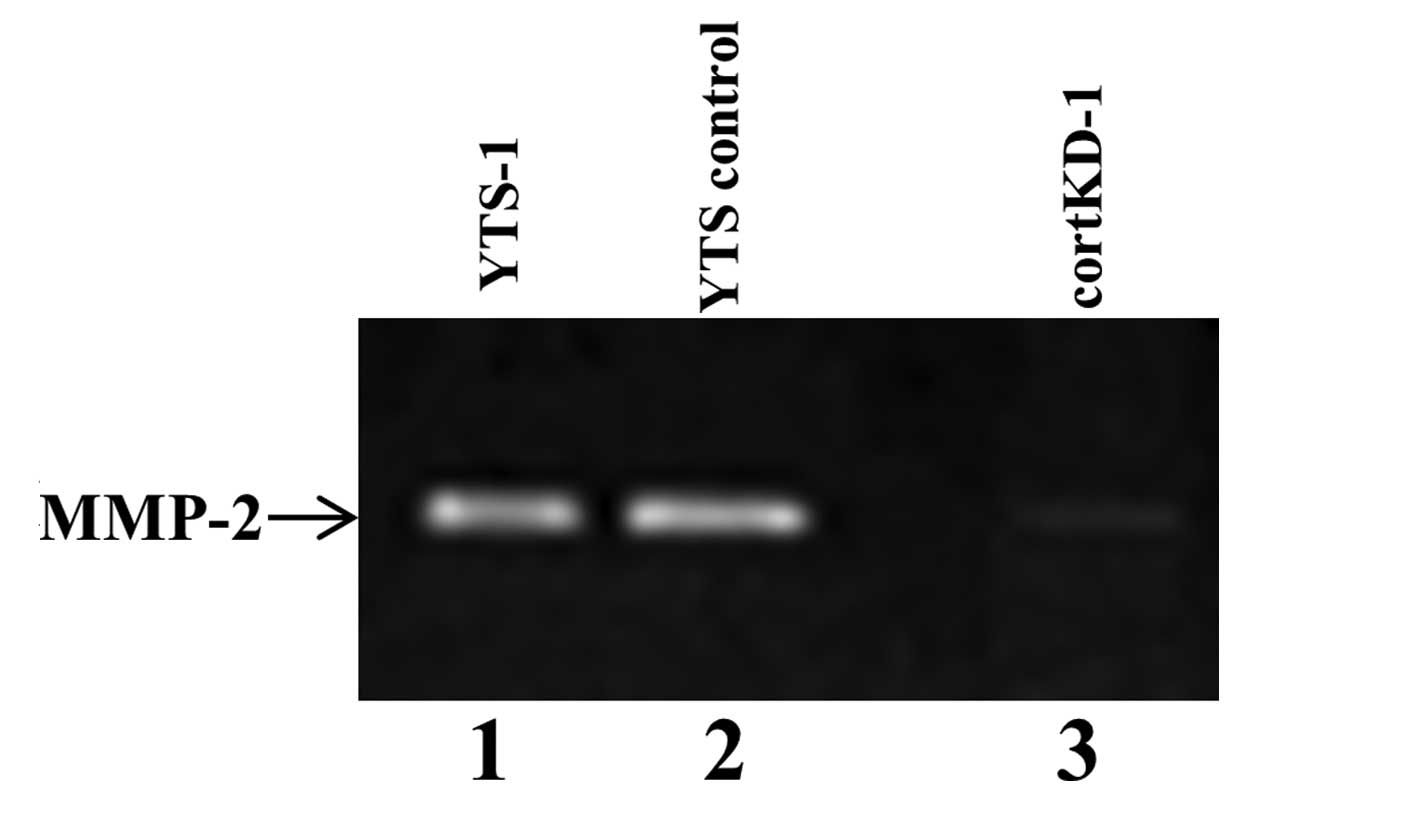
Reduced secretion of metalloproteinase in
cortactin knockdown cells
To examine if cortactin knockdown affects
invadopodia functions, the cells were assayed for the secretion of
matrix metalloproteinase (MMP)-2, one of the most important
functions of invadopodia. Gelatin zymography revealed that large
amounts of MMP-2 were secreted by YTS-1 parent cells and YTS
control cells (Fig. 3, lanes 1 and
2). By contrast, the secretion of MMP-2 by cortactin knockdown was
markedly lower than that of YTS control cells (Fig. 3, lane 3), indicating that the
ability to secrete MMP-2 was markedly reduced in cortactin
knockdown cells.
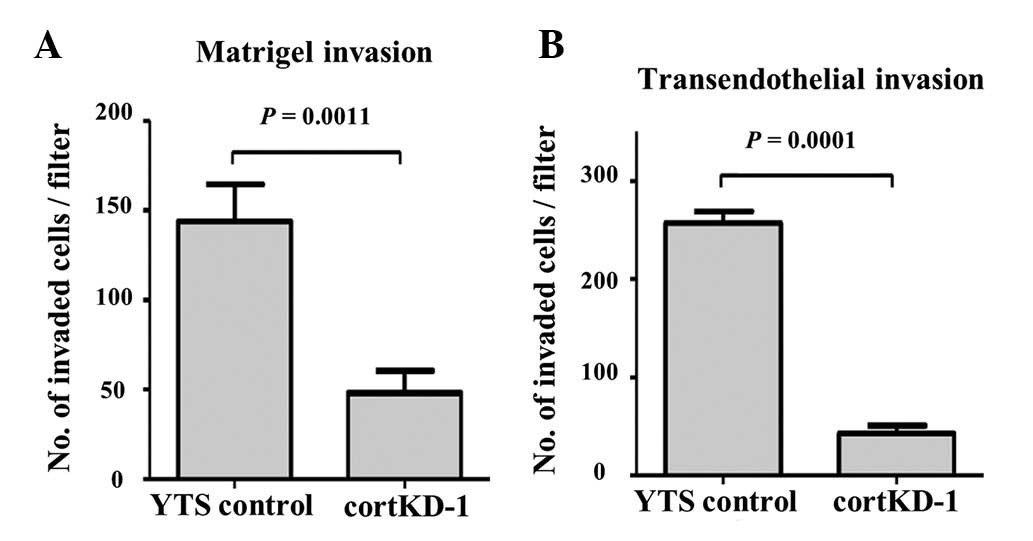
In vitro invasion capacity of cortactin
knockdown cells
Cortactin knockdown was found to impair invadopodia
formation and secretion of MMP-2 (Figs. 2 and 3). To examine whether defective formation
of invadopodia affects the invasion capacity of invasive cancer
cells, a Matrigel invasion assay was performed. cortKD-1 cells
exhibited a significantly lower invasion capacity through the
Matrigel matrix (Fig. 4A). This
result, taken together with Fig.
3, suggests that impaired invadopodia have a reduced capacity
to degrade and migrate through the ECM.
In order for bladder cancer cells to metastasize in
the lung, the cancer cells need to exit the circulation by
migrating through an endothelial monolayer of the lung
microvasculature. To determine the ability of cancer cells to
invade and migrate through a cellular endothelial barrier, the
transendothelial invasion capacity was measured. A transendothelial
invasion assay was performed, as previously described (11), in which cortKD-1 cells exhibited a
significantly lower transendothelial invasion capacity through the
endothelial monolayer of the lung microvascular vessels (Fig. 4B). These results indicate that
defective invadopodia formation by cortactin knockdown reduces the
invasion capacity through a Matrigel matrix and the endothelial
monolayer.
Tumor formation by cortactin knockdown
cells
To evaluate the role of invadopodia in cancer cell
extravasation for metastasis, the YTS control and cortKD-1 cells
were subjected to a lung metastasis assay, as this type of assay
closely mimics hematogenous metastasis (7,12).
Three weeks after cancer cell injection into mice, the lungs were
harvested and lung sections were examined for tumorous regions. A
large tumor area was observed on the lung section three weeks after
YTS control cells were injected (Fig.
5A). However, no tumor area was observed on the lung section
from the mice injected with cortKD-1 cells (Fig. 5B). When YTS control cells were
injected, all the mice exhibited lung metastases (n=6). By
contrast, none of the mice injected with cortKD-1 cells exhibited
detectable metastases (n=10) (Fig.
5C). The degree of metastasis of YTS control cells was 44.8%,
based on the proportion (%) of the tumor area occupying the total
lung area examined at lower magnification (x40; Fig. 5D). In order for invasive bladder
cancer cells to form metastatic foci in the lung, cancer cell
extravasation and proliferation in the lung are required. There was
no marked difference in the growth rate between YTS control and
cortKD-1 cells (Fig. 1B). However,
cortKD-1 cells exhibited a markedly lower degree of metastasis
compared with YTS control cells (Fig.
5). These results, taken together with the lower
transendothealial invasion capacity of cortKD-1 cells (Fig. 4B), suggests that the lower degree
of lung metastasis is due to reduced extravasation in the lung
tissues.
Discussion
Tyrosine phosphorylation of cortactin by
non-receptor type tyrosine kinases, including Src and Arg, is a key
regulatory step for invadopodia formation (10,13,14).
It has been demonstrated that cortactin regulates invadopodia
formation in several types of cancer cell. Cortactin is also known
to regulate MMP secretion and matrix degradation, contributing to
the invasive character of cancer cells (15,16).
The present study demonstrated that cortactin plays a critical role
in invadopodia formation and MMP-2 secretion in invasive bladder
cancer cells (Figs. 2 and 3). Invadopodia formation regulated by
cortactin was also shown to be necessary for matrix degradation and
invasion by invasive bladder cancer cells (Fig. 4A).
Cancer cell extravasation is a decisive process for
metastasis whereby cancer cells exit the blood vessels to cross
endothelia and invade secondary organ tissues. Gligorijevi et
al previously demonstrated that invadopodia formation is
involved in cancer cell intravasation, using an in vivo
system (17). This study knocked
down the expression of neural Wiskott-Aldrich syndrome protein
(N-WASP) in MTLn3 rat mammary gland adenocarcinoma cells. The
N-WASP knockdown MTLn3 cells demonstrated a markedly decreased
invadopodia formation, as N-WASP is an essential component of
invadopodia. When the N-WASP knockdown cells were implanted into
SCID mice, in vivo invadopodia formation and matrix
degradation were reduced in the area of intravasation. The present
study also demonstrated that invadopodia formation is involved in
cancer cell extravasation, using an in vivo system. The
invadopodia formation in invasive bladder cancer cells was reduced
by silencing cortactin, which is another essential component of
invadopodia. Subsequently, cortactin knockdown cells were subjected
to the in vivo tumor formation assay, which included cancer
cell extravasation. The lower rate of lung metastasis of cortKD-1
cells (Fig. 5) indicates that the
extravasation of cortKD-1 cells is impaired due to reduced
invadopodia formation.
In order for invasive cancer cells to extravasate,
the endothelial barrier surrounding blood vessels and the basement
membrane must be broken down (18). In the present study, perturbing the
function of cortactin by shRNA was found to reduce MMP-2 secretion,
invadopodia-mediated ECM degradation, transendothelial invasion and
in vivo tumor formation by invasive cancer cells. These
results indicate that cortactin-mediated invadopodia formation is
required for the invasion and extravasation process of bladder
cancer metastasis. In conclusion, the potential of cortactin as a
target for anti-invasion and anti-extravasation therapeutics should
be investigated further.
Acknowledgements
This study was supported by the grants-in-aid for
Scientific Research from the Japanese Society for the Promotion of
Science (nos. 22570131 and B22390301), the Ministry of Education,
Culture, Sports, Science and Technology of Japan (nos. 21791483 and
21791484) and Japan Science and Technology Agency (CREST).
References
|
1
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caldieri G, Ayala I, Attanasio F and
Buccione R: Cell and molecular biology of invadopodia. Int Rev Cell
Mol Biol. 275:1–34. 2009. View Article : Google Scholar
|
|
3
|
Linder S, Wiesner C and Himmel M:
Degrading devices: invadosomes in proteolytic cell invasion. Annu
Rev Cell Dev Biol. 27:185–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011.
|
|
5
|
Yamaguchi H, Lorenz M, Kempiak S, et al:
Molecular mechanisms of invadopodium formation: the role of the
N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol.
168:441–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith SC, Nicholson B, Nitz M, et al:
Profiling bladder cancer organ site-specific metastasis identifies
LAMC2 as a novel biomarker of hematogenous dissemination. Am J
Pathol. 174:371–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuboi S, Sutoh M, Hatakeyama S, et al: A
novel strategy for evasion of NK cell immunity by tumours
expressing core2 O-glycans. EMBO J. 30:3173–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artym VV, Zhang Y, Seillier-Moiseiwitsch
F, Yamada KM and Mueller SC: Dynamic interactions of cortactin and
membrane type 1 matrix metalloproteinase at invadopodia: defining
the stages of invadopodia formation and function. Cancer Res.
66:3034–3043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ayala I, Baldassarre M, Giacchetti G, et
al: Multiple regulatory inputs converge on cortactin to control
invadopodia biogenesis and extracellular matrix degradation. J Cell
Sci. 121:369–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oser M, Yamaguchi H, Mader CC, et al:
Cortactin regulates cofilin and N-WASp activities to control the
stages of invadopodium assembly and maturation. J Cell Biol.
186:571–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugiyama N, Yoneyama MS, Hatakeyama S, et
al: In vivo selection of high-metastatic subline of bladder cancer
cell and its characterization. Oncol Res. 20:289–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatakeyama S, Yamamoto H and Ohyama C:
Tumor formation assays. Methods Enzymol. 479:397–411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mader CC, Oser M, Magalhaes MA, et al: An
EGFR-Src-Arg-cortactin pathway mediates functional maturation of
invadopodia and breast cancer cell invasion. Cancer Res.
71:1730–1741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oser M, Mader CC, Gil-Henn H, et al:
Specific tyrosine phosphorylation sites on cortactin regulate
Nck1-dependent actin polymerization in invadopodia. J Cell Sci.
123:3662–3673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clark ES and Weaver AM: A new role for
cortactin in invadopodia: regulation of protease secretion. Eur J
Cell Biol. 87:581–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirkbride KC, Sung BH, Sinha S and Weaver
AM: Cortactin: a multifunctional regulator of cellular
invasiveness. Cell Adh Migr. 5:187–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gligorijevic B, Wyckoff J, Yamaguchi H,
Wang Y, Roussos ET and Condeelis J: N-WASP-mediated invadopodium
formation is involved in intravasation and lung metastasis of
mammary tumors. J Cell Sci. 125:724–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag
L, Lee A and Muschel RJ: Intravascular origin of metastasis from
the proliferation of endothelium-attached tumor cells: a new model
for metastasis. Nat Med. 6:100–102. 2000. View Article : Google Scholar : PubMed/NCBI
|