Introduction
Acetaminophen is a commonly used over-the-counter
analgesic and overdose of acetaminophen was the most frequent cause
of acute liver failure worldwide in 2008 (1). Acute acetaminophen intoxication
results in centrilobular hepatic necrosis involving
N-acetyl-p-benzoquinoneimine (NAPQI) and cytochrome P450 (Cyp)
(2). Current treatment protocols
recommend an initial dose of 150 mg/kg N-acetylcysteine (NAC),
infused over a period of 1 h, followed by decreasing quantities of
NAC infused over the subsequent 20 h (3). However, the optimal treatment for
acetaminophen toxicity remains unclear (4).
Schisandra chinensis is a deciduous woody
vine found in northwestern China, far eastern Russia and Korea
(5). As a well-known traditional
medicinal herb and food additive (5), Schisandra chinensis is used
for its antioxidant, tonic and sedative effects (6,7). In
addition to its adaptogenic properties, it is also a
hepatoprotectant (8,9).
Lysosomes have long been recognized as the ‘suicide
bags’ of cells (10). Studies have
indicated that lysosomal disruption occurs subsequent to numerous
types of cellular stresses in hepatocytes and other cell types
(11–13). A breakdown of lysosomes may result
in cell death by necrosis, which is associated with an increase in
cytosolic acidification (14). Kon
et al (15) found that
mobilization of chelatable iron from lysosomes was key in
acetaminophen hepatotoxicity. The formation of reactive oxygen
species (ROS) increases following acetaminophen exposure, and
agents that augment antioxidant defenses and scavenge ROS protect
against acetaminophen toxicity in vitro and in vivo
(16). In the present study, the
mechanism and effect of Schisandra chinensis on
acetaminophen-induced hepatotoxicity and liver failure in mice was
evaluated by observing the extent of lysosomal disruption and ROS
release.
Materials and methods
Preparation of Schisandra chinensis
Dried Schisandra chinensis fruits (500 g),
provided by Zhixin Pharmaceutical Company (Guangdong, China), were
authenticated by the pharmacist at The First Hospital of China
Medical University (Shenyang, China) and macerated in 70% ethanol
for 30 min at room temperature. The fruits were then refluxed three
times (for 1 h each) with 70% ethanol. The combined extract was
filtered and condensed by rotary evaporation (Rotary evaporator,
Shyarong Biochemical Instruments Inc., Shanghai, China) under
reduced pressure. The condensed extract was then freeze-dried to
obtain a powder, which was placed in a desiccator at room
temperature until use.
Animals and treatments
Wild-type C57BL/6 male mice (aged 7–9 weeks,
weighing 20–25 g) were obtained from Charles River Laboratories,
Inc. (Wilmington, MA, USA). All animals were maintained on food and
water ad libitum and housed in microisolation cages. All
experiments with animals were approved by and performed according
to the guidelines of the China Medical University Ethics Committee
(Shenyang, China). The mice were fasted overnight prior to
administration of acetaminophen (300 mg/kg, intraperitoneal) or
phosphate-buffered saline (PBS) control. A number of the mice with
acute liver failure were then treated with Schisandra
chinensis 3 h after the acetaminophen treatment (50 mg/kg,
intraperitoneal).
Hepatotoxicity verification
Blood samples were collected by cardiac puncture 12
h after the end of treatment. The samples were analyzed for serum
alanine transaminase (ALT) and aspartate transaminase (AST)
(Beijing Gersion Bio-Technology Co., Ltd., Beijing, China).
Briefly, the values of the serum ALT and AST activities were
derived according to the ‘absorptivity micromolar extinction
coefficient’ of NADH at 340 nm and were expressed in terms of unit
per liter. Pyruvate is reduced to lactate by lactate dehydrogenase
with the simultaneous oxidation of NADH to NAD, which was monitored
by measuring the rate of decrease in absorbance at 340 nm.
Histopathological analysis
Liver tissue samples were collected following the
blood collection. The samples were fixed with 10% formaldehyde in
PBS for 24 h, dehydrated in a graded ethanol series, embedded in
paraffin and sliced at a thickness of 5 μm. The paraffin sections
were stained with hematoxylin and eosin for histopathological
analysis.
Measurement of hepatic glutathione
levels
Samples of liver (50 mg) were minced in ice-cold 5%
metaphosphoric acid (1:10), homogenized and then centrifuged at
3,000 × g for 10 min at 4°C. The supernatants were filtered through
a 0.2-μm syringe filter, and the reduced glutathione (GSH) and
oxidized glutathione disulfide (GSSG) were quantified using the
respective colorimetric assay kits (Beyotime Institute of
Biotechnology, Beijing, China).
Measurement of hepatic Cyp activity
To prepare the microsomes, liver samples (1 g) were
homogenized at 4°C in two volumes (w/v) 10 mM Tris-base (pH 7.4)
containing 1.5% KCl using a Teflon-glass homogenizer (DuPont,
Wheaton, NJ, USA). The homogenates were centrifuged at 1,000 × g
(10 min, 4°C), and then the supernatants were collected and
centrifuged at 12,000 × g (20 min, 4°C) to remove cellular debris,
followed by centrifugation at 100,000 × g (1.5 h, 4°C). The
microsomes were resuspended in homogenization buffer containing 0.5
mM phenylmethanesulfonylfluoride and centrifuged at 100,000 × g (90
min, 4°C). The pellets were resuspended in 0.25 M sucrose
containing 10 mM Tris-base (pH 7.4) and stored at −80°C. The levels
of Cyp2e1, Cyp1a2 and Cyp3a activity were measured according to the
methods of Gardner et al (17). To assess Cyp isoform specificity,
enzyme activity levels were measured following the addition of
either 1 μM of the Cyp1a2 inhibitor, rutaecarpine, or of the Cyp3a
inhibitor, ketoconazole.
Mice hepatocytes
According to the methods of Qian et al
(18), hepatocytes were isolated
from overnight-fasted wild-type C57BL/6 male mice by collagenase
digestion and plated on type 1 collagen-coated 24-well microtiter
plates, 6-cm culture dishes or glass bottom Petri dishes in
Waymouth’s medium MB-752/1 (HiMedia Laboratories, Mumbai, India)
supplemented with 2 mM L-glutamine, 10% fetal calf serum, 100 nM
insulin, 100 nM dexamethasone, 100 U/ml penicillin and 100 μg/ml
streptomycin. Cell viability was identified as >90% by trypan
blue exclusion, according to the manufacturer’s instructions
(Beyotime Institute of Biotechnology). After 4 h, the hepatocytes
were placed in hormonally defined medium consisting of RPMI-1640
supplemented with 240 nM insulin, 2 mM L-glutamine, 1 μg/ml
transferrin, 0.3 nM selenium, 1.5 μM free fatty acids, 100 U/ml
penicillin and 100 μg/ml streptomycin.
Cell growth inhibition assays
The cells were plated in 96-well plates (1,500
cells/well) and allowed to attach overnight. Subsequently,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (Sigma-Aldrich, Carlsbad, CA, USA; final concentration,
0.5 mg/ml) was added to the wells and the cells were incubated for
4 h. Absorbance was measured at 550–560 nm by using a microplate
reader (Bio-Rad, Hercules, CA, USA).
Annexin V-fluroescein isothiocynate
(FITC) and propidium iodide (PI) double staining
Following the manufacturer’s instructions (Apoptosis
Detection kit; KeyGen Biotech Co., Ltd., Nanjing, China), the cells
were washed and resuspended in binding buffer prior to incubation
in FITC-labeled Annexin V and PI for 10 min. The suspensions were
immediately analyzed by a FACSCalibur machine (BD Biosciences,
Baltimore, MD, USA).
Cell cycle analysis
Cells were collected and centrifuged at 1,500 × g
for 5 min, and the pellet was resuspended in 100 μl PBS at a
density of 1×106 cells/ml. Cold ethanol (900 μl, 70%)
was added to the mixture for 1 h on ice. The cells were collected
by centrifugation at 1,500 × g for 5 min. The pellet was then
resuspended in 100 μl PBS containing RNase A (0.2 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) and maintained at room
temperature for 30 min. The cells were recovered by centrifugation
and the pellets were resuspended in 350 μl PBS containing PI (50
μg/ml; KeyGen Biotech Co., Ltd.) and analyzed by flow cytometry
using the FACSCalibur machine.
Determination of mitochondrial membrane
potential (MMP)
MMP was analyzed using the fluorescent dye
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) according to the manufacturer’s instructions (KeyGen
Biotech Co. Ltd.). Briefly, cells were plated on a six-well culture
plate. Following treatment for 24 h, the cells were washed twice
with PBS, harvested and incubated with 20 nM JC-1 for 30 min in the
dark. MMP was then analyzed using the FACSCalibur machine.
Quantification of cellular ROS
Cells (5×105) were cultured in 12-well
tissue culture plates overnight, and then cotreated with drugs and
2′,7′-dichlorofluorescin diacetate (DCF-DA), an ROS-sensitive dye.
Following treatment, the cells were harvested and suspended in PBS.
The relative fluorescence intensities of the cells were quantified
using the FACSCalibur machine.
26S proteasome activity assay
The 26S proteasome function was assayed as described
previously (19). The assay was
based on the detection of the fluorophore 7-amino-4-methylcoumarin
(AMC) following cleavage from the labeled substrate Suc-LLVY-AMC
(Boston Biochem Inc., Cambridge, MA, USA). This fluorogenic
proteasome substrate was added to the cell lysate at a final
concentration of 80 μM in 1% dimethylsulfoxide. Adenosine
triphosphate-dependent cleavage activity was monitored continuously
by detection of free AMC using a microplate reader (Bio-Rad) at
380/460 nm at 37°C.
Western blot analysis
Cell extracts were resolved on SDS-PAGE and then
transferred to nitrocellulose membranes. These membranes were
developed and visualized with electrochemiluminescence (Pierce,
Waltham, MA, USA). The primary antibodies used are listed in
Table I.
 | Table IAntibodies used in the western blot
analysis. |
Table I
Antibodies used in the western blot
analysis.
| Antibody | Santa Cruz (sc)
catalog number | Dilution |
|---|
| anti-JNK | sc-7345 | 1:200 |
| anti-p-JNK | sc-293136 | 1:200 |
| anti-Bax | sc-7480 | 1:200 |
| anti-Bcl-xL | sc-8392 | 1:200 |
| anti-Bcl-2 | sc-783 | 1:200 |
| anti-P-Bcl-2 | sc-16323 | 1:200 |
| anti-β-actin | sc-47778 | 1:1000 |
Statistical analysis
All values are presented as the mean ± the standard
error of the mean. Student’s paired t-test was used to identify
statistically significant differences. Kaplan-Meier survival plots
were generated and comparisons were made with log-rank statistics.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using GraphPad
Prism 4 software (GraphPad Software Inc., San Diego, CA, USA).
Results
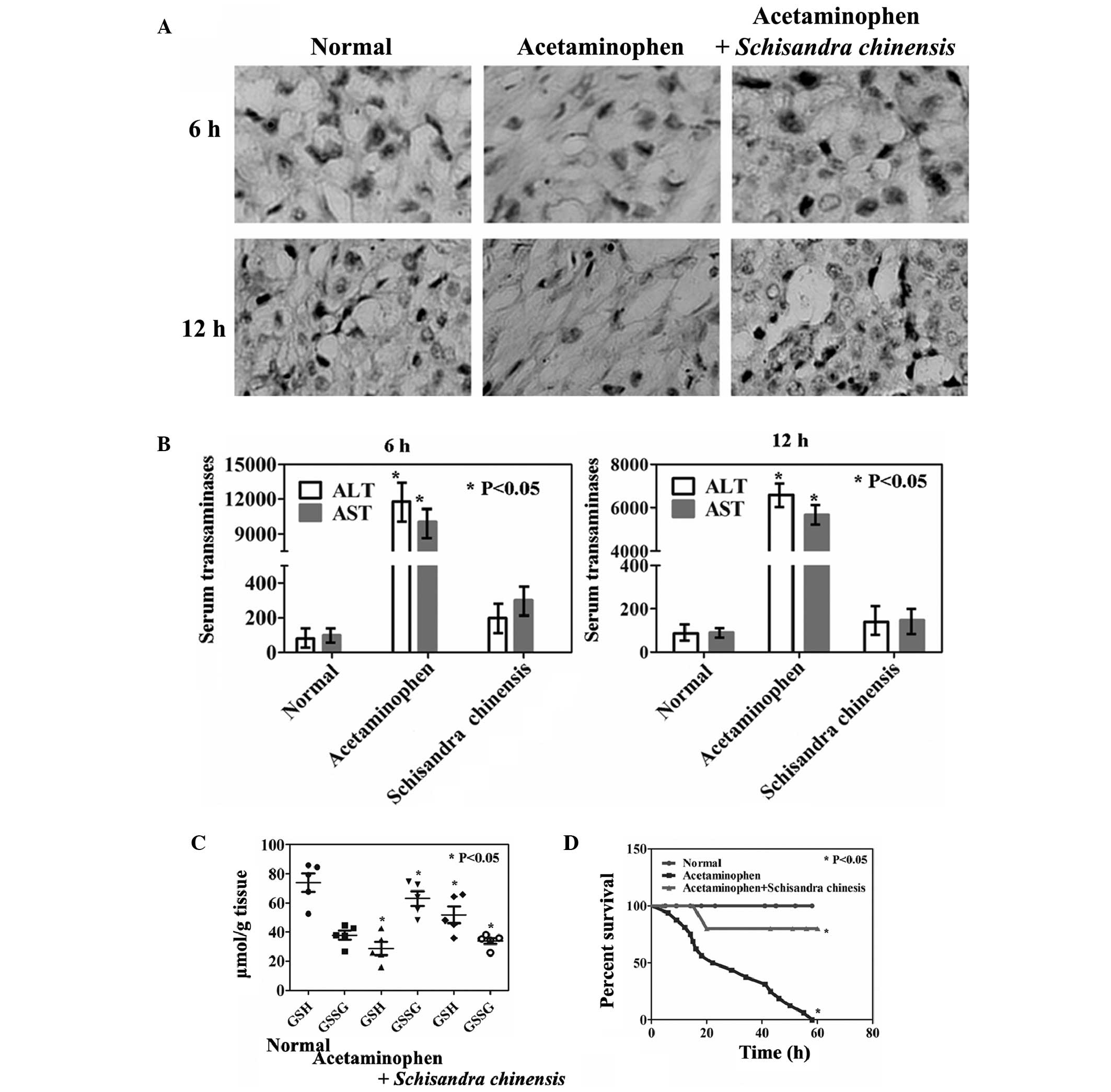
Effects of Schisandra chinensis on
acetaminophen-induced hepatotoxicity in vivo
The effects of a diet containing Schisandra
chinensis on mice with acetaminophen-induced liver injury were
analyzed. Hepatocellular cytoplasmic degeneration, bridging
necrosis and severe congestion were observed in mice treated with
acetaminophen (Fig. 1A). Compared
with those of the untreated mice, the mice administered
acetaminophen exhibited a rapid induction of hepatotoxicity with
significant elevations in the levels of serum ALT and AST (Fig. 1B, P<0.05). Treatment with
Schisandra chinensis appeared to inhibit
acetaminophen-induced hepatotoxicity, as reduced levels of serum
transaminases and marginal structural alterations in the liver were
observed (Fig. 1A and B).
Acetaminophen treatment alone resulted in a rapid reduction in the
levels of GSH and an increase in the levels of GSSG. However, the
levels of GSH in the acetaminophen and Schisandra chinensis
combined treatment group showed a restored trend compared with
those of the untreated group (Fig.
1C, P<0.05). No significant differences were identified in
the levels of microsomal Cyp2e1 and Cyp3a activity among the mice
in the different groups. However, the levels of Cyp1a2 were
significantly suppressed by Schisandra chinensis
administration compared with those in the acetaminophen-treated
mice (Table II, P<0.05).
Furthermore, a significantly different survival rate between the
untreated group and the acetaminophen-treated group, as well as
between the acetaminophen-treated group and the acetaminophen- and
Schisandra chinensis-treated group in the Cox model was
identified (Fig. 1D,
P<0.05).
 | Table IIEffects of Schisandra
chinensis on the levels of Cyp activity in mouse liver
microsomes. |
Table II
Effects of Schisandra
chinensis on the levels of Cyp activity in mouse liver
microsomes.
| Treatment
group |
|---|
|
|
|---|
| Enzyme | Normal | Acetaminophen | Acetaminophen +
Schisandra chinensis |
|---|
| Cyp2e1 | 1.07±0.04 | 1.04±0.03 | 1.12±0.06 |
| Cyp1a2 | 33.8±1.5 | 145.2±27.8 | 42.7±2.2a |
| Cyp1a2 +
rutaecarpine | 1.5±0.1 | 5.6±0.3 | 1.8±0.2a |
| Cyp3a | 5.9±0.8 | 8.3±0.5 | 6.4±0.7 |
| Cyp3a +
ketoconazole | 1.08±0.08 | 1.04±0.05 | 0.98±0.06 |
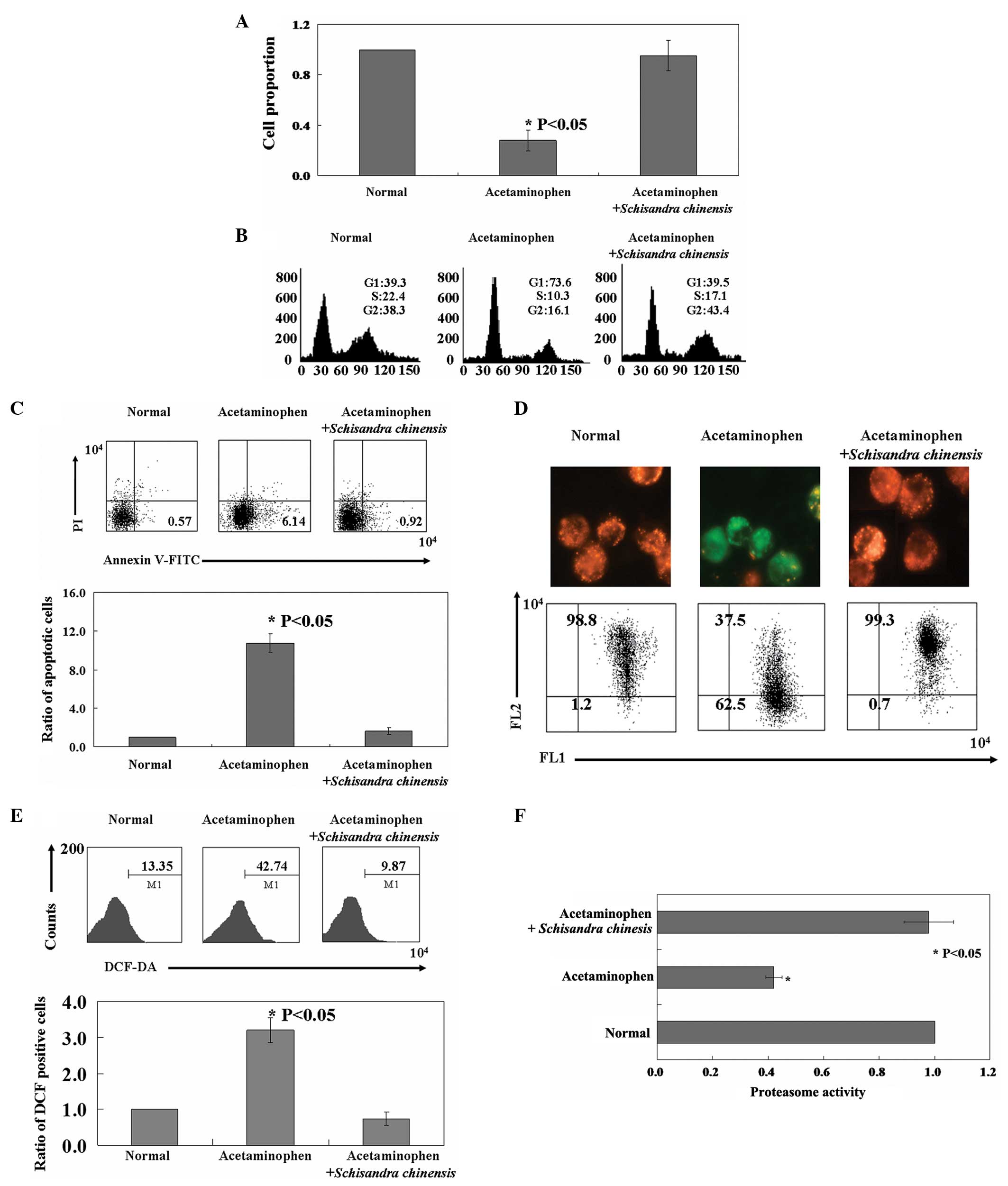
Effects of Schisandra chinensis on
acetaminophen-induced hepatotoxicity in vitro
The proliferation of hepatocytes was inhibited by
acetaminophen as revealed using the MTT assay (Fig. 2A, P<0.05). PI staining of cells
revealed that acetaminophen-treated cells were arrested in the
G1 phase (Fig. 2B).
Annexin V-FITC and PI double staining was performed to detect
apoptotic cells. In the cells with acetaminophen treatment, the
apoptotic ratio was 11–12-fold higher than that of the untreated
cells (Fig. 2C, P<0.05). As
shown in Fig. 2D, the red/green
ratio, used to measure the MMP, in the normal cells (1.2% green,
98.8% red) was reversed following acetaminophen treatment (62.5%
green, 37.5% red). The fluorescent dye DCF-DA was used to measure
the ROS content in cells following acetaminophen treatment. As
shown in Fig. 2E, acetaminophen
treatment directly induced an increase in the fluorescence
intensity of the cells (42.7%) when compared with that of the
normal cells (13.4%, P<0.05). Furthermore, acetaminophen was
found to inhibit proteasome activity in hepatocytes (Fig. 2F, P<0.05). Consistent with the
results obtained in vivo, Schisandra chinensis
protected hepatocytes against acetaminophen-induced apoptosis, ROS
release and injury to mitochondria and proteasomes (Fig. 2).
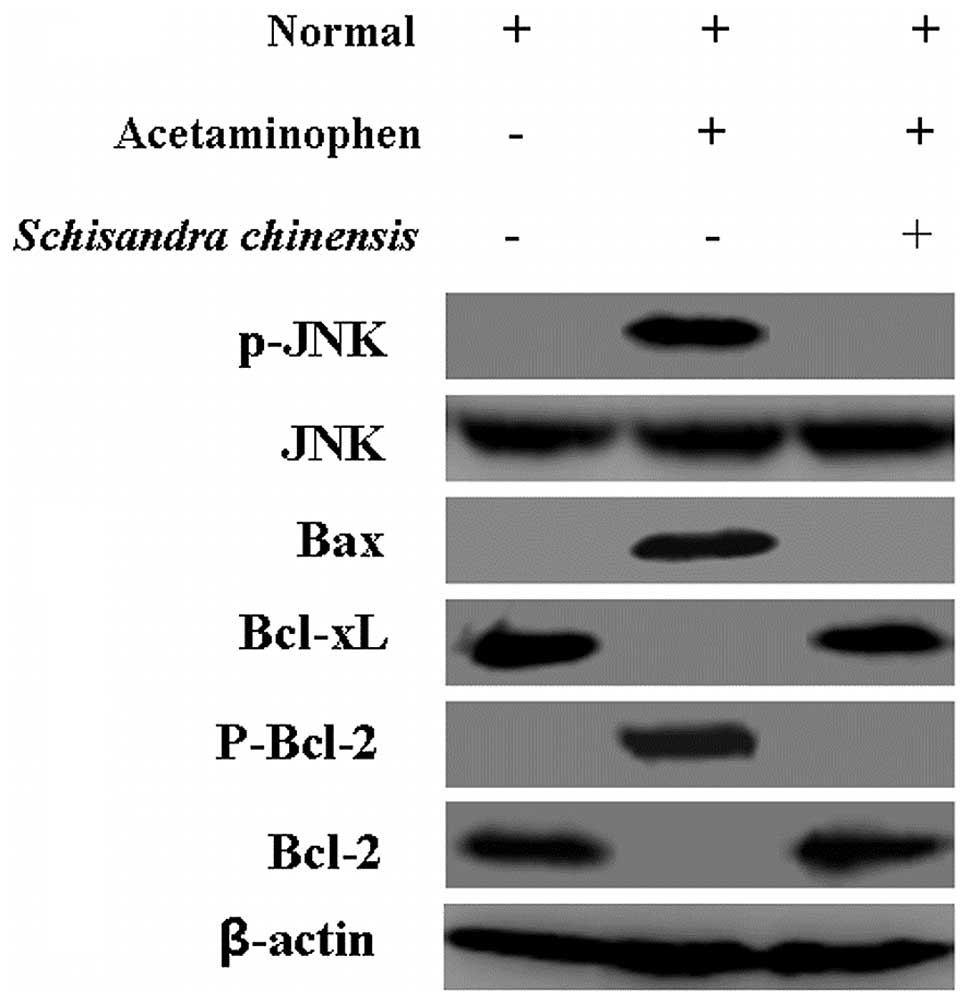
Mechanism(s) of Schisandra
chinensis-mitigated acetaminophen-induced hepatotoxicity in
hepatocytes
The aforementioned results revealed the changes of
mitochondria in hepatocytes. Due to these results, the changes were
further analyzed; expression of Bax, Bcl-xL, Bcl-2 and p-Bcl-2 was
detected using western blot analysis. As shown in Fig. 3 (lanes 1 and 2), a reduction in the
Bcl-2 and Bcl-xl expression levels and an increase in Bax and
P-Bcl-2 expression levels were identified in hepatocytes with
acetaminophen treatment compared with those in the normal cells.
The levels of these proteins were markedly reversed following
Schisandra chinensis treatment (Fig. 3, lanes 2 and 3). The levels of
phosphor-c-Jun N-terminal kinase (p-JNK) were upregulated by
acetaminophen treatment but the levels of total JNK did not change
compared with those in the normal cells (Fig. 3, lanes 1 and 2). However, p-JNK was
inhibited following Schisandra chinensis treatment (Fig. 3, lane 3). β-actin served as an
internal control for all western blotting.
Discussion
Acetaminophen is a commonly used analgesic drug that
in overdose results in hepatic necrosis and liver failure (1). In the present study, acute liver
injury in mice was successfully established using acetaminophen, as
detemined by the levels of the serum transaminases and histological
changes. Elevations in ALT and AST levels are characteristic of
acute acetaminophen overdose (20,21).
The levels of ALT and AST were confirmed to be elevated in the mice
in the present study following acetaminophen treatment compared
with those in the untreated mice. GSH is key in scavenging ROS
(22). Consistent with the results
of previous studies (23,24), acetaminophen intoxication resulted
in reduced GSH levels in mice compared with those in the control
group.
Schisandra chinensis has been used in China,
Korea and Japan to regulate various pathophysiological conditions,
including hepatitis and cancer (6). In the present study, Schisandra
chinensis was confirmed, with histological evidence, to inhibit
acetaminophen-induced acute liver injury. For example, the levels
of ALT and AST were reduced in mice following Schisandra
chinensis treatment compared with those in the mice which only
received acetaminophen. Hu et al (25) found that Schisandra
chinensis inhibited the reduction in the levels of GSH and
reduced the levels of GSSG, and similar effects were identified in
the present study. Acetaminophen-induced hepatotoxicity involves
oxidative stress, which is generated as a consequence of
Cyp-mediated NAPQI formation and inflammatory cell production of
ROS (26,27). In the present study, Schisandra
chinensis inhibited the elevated levels of ROS induced by
acetaminophen. Furthermore, the normal levels of hepatic Cyp1a2
activity were restored in mice following Schisandra
chinensis treatment.
In the in vitro experiments,
acetaminophen-induced apoptosis in hepatocytes was associated with
changes in the MMP. Changes in the levels of Bcl-2 and Bax in
hepatocytes were also identified following acetaminophen treatment
compared with those in the normal cells. However, Schisandra
chinensis may be able to treat acute liver injury though
protection of the mitochondria. Disruption of lysosomes in
hepatocytes following acetaminophen treatment has been observed in
previous studies (13,15). Notably, Schisandra chinensis
was also found to protect lysosomes in the present study.
Activation of hepatic JNK is recognized as a key
event in the progression and exacerbation of acetaminophen
toxicity, and inhibition of p-JNK has been shown to protect mice
against acetaminophen-induced hepatotoxicity (28,29).
In the present study, Schisandra chinensis was found to
inhibit p-JNK expression levels compared with those in the
acetaminophen-treated hepatocytes
In conclusion, the present study provides evidence
that Schisandra chinensis positively inhibited
acetaminophen-induced hepatotoxicity in vivo and in
vitro. The collective results showed that Schisandra
chinensis may protect mitochondria and lysosomes. Furthermore,
Schisandra chinensis inhibited the p-JNK signaling pathway.
These findings provide further support for the clinical application
of Schisandra chinensis in the treatment and prevention of
acetaminophen-induced hepatotoxicity.
Acknowledgements
The authors thank Dr Ming Fan for carefully
proofreading the manuscript and providing valuable comments.
References
|
1
|
Fontana RJ: Acute liver failure including
acetaminophen overdose. Med Clin North Am. 92:761–794. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moyer AM, Fridley BL, Jenkins GD, et al:
Acetaminophen-NAPQI hepatotoxicity: a cell line model system
genome-wide association study. Toxicol Sci. 120:33–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daly FF, Fountain JS, Murray L, Graudins A
and Buckley NA; Panel of Australian and New Zealand clinical
toxicologists. Guidelines for the management of paracetamol
poisoning in Australia and New Zealand - explanation and
elaboration. A consensus statement from clinical toxicologists
consulting to the Australasian poisons information centres. Med J
Aust. 188:296–301. 2008.
|
|
4
|
James LP, Gill P and Simpson P: Predicting
risk in patients with acetaminophen overdose. Expert Rev
Gastroenterol Hepatol. 7:509–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: an overview of Russian research
and uses in medicine. J Ethnopharmacol. 118:183–212. 2008.
|
|
6
|
Hancke JL, Burgos RA and Ahumada F:
Schisandra chinensis (Turcz.) Baill. Fitoterapia.
70:451–471. 1999. View Article : Google Scholar
|
|
7
|
Chen DF, Zhang SX, Xie L, et al: Anti-AIDS
agents-XXVI. Structure-activity correlations of gomisin-G-related
anti-HIV lignans from Kadsura interior and of related synthetic
analogues. Bioorg Med Chem. 5:1715–1723. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu PY, Mak DH, Poon MK and Ko KM: In
vivo antioxidant action of a lignan-enriched extract of
Schisandra fruit and an anthraquinone-containing extract of
Polygonum root in comparison with schisandrin B and emodin. Planta
Med. 68:951–956. 2002.PubMed/NCBI
|
|
9
|
Ram VJ: Herbal preparations as a source of
hepatoprotective agents. Drug News Perspect. 14:353–363.
2001.PubMed/NCBI
|
|
10
|
Turk B and Turk V: Lysosomes as ‘suicide
bags’ in cell death: myth or reality? J Biol Chem. 284:21783–21787.
2009.
|
|
11
|
Szopa A and Ekiert H: In vitro cultures of
Schisandra chinensis (Turcz.) Baill (Chinese magnolia vine)
- a potential biotechnological rich source of therapeutically
important phenolic acids. Appl Biochem Biotechnol. 166:1941–1948.
2012.
|
|
12
|
Kurz T, Terman A and Brunk UT: Autophagy,
ageing and apoptosis: the role of oxidative stress and lysosomal
iron. Arch Biochem Biophys. 462:220–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchiyama A, Kim JS, Kon K, Jaeschke H,
Ikejima K, Watanabe S and Lemasters JJ: Translocation of iron from
lysosomes into mitochondria is a key event during oxidative
stress-induced hepatocellular injury. Hepatology. 48:1644–1654.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boya P and Kroemer G: Lysosomal membrane
permeabilization in cell death. Oncogene. 27:6434–6451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kon K, Kim JS, Uchiyama A, Jaeschke H and
Lemasters JJ: Lysosomal iron mobilization and induction of the
mitochondrial permeability transition in acetaminophen-induced
toxicity to mouse hepatocytes. Toxicol Sci. 117:101–108. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaeschke H and Bajt ML: Intracellular
signaling mechanisms of acetaminophen-induced liver cell death.
Toxicol Sci. 89:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gardner CR, Hankey P, Mishin V, Francis M,
Yu S, Laskin JD and Laskin DL: Regulation of alternative macrophage
activation in the liver following acetaminophen intoxication by
stem cell-derived tyrosine kinase. Toxicol Appl Pharmacol.
262:139–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian T, Nieminen AL, Herman B and
Lemasters JJ: Mitochondrial permeability transition in pH-dependent
reperfusion injury to rat hepatocytes. Am J Physiol.
273:C1783–C1792. 1997.PubMed/NCBI
|
|
19
|
Fekete MR, McBride WH and Pajonk F:
Anthracyclines, proteasome activity and multi-drug-resistance. BMC
Cancer. 5:1142005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larson AM, Polson J, Fontana RJ, et al:
Acetaminophen-induced acute liver failure: results of a United
States multicenter, prospective study. Hepatology. 42:1364–1372.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ostapowicz G, Fontana RJ, Schiødt FV, et
al; U.S. Acute Liver Failure Study Group. Results of a prospective
study of acute liver failure at 17 tertiary care centers in the
United States. Ann Intern Med. 137:947–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaeschke H: Glutathione disulfide
formation and oxidant stress during acetaminophen-induced
hepatotoxicity in mice in vivo: the protective effect of
allopurinol. J Pharmacol Exp Ther. 255:935–941. 1990.
|
|
23
|
Chiu H, Gardner CR, Dambach DM,
Brittingham JA, Durham SK, Laskin JD and Laskin DL: Role of p55
tumor necrosis factor receptor 1 in acetaminophen-induced
antioxidant defense. Am J Physiol Gastrointest Liver Physiol.
285:G959–G966. 2003.PubMed/NCBI
|
|
24
|
Gardner CR, Gray JP, Joseph LB, et al:
Potential role of caveolin-1 in acetaminophen-induced
hepatotoxicity. Toxicol Appl Pharmacol. 245:36–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu D, Cao Y, He R, Han N, Liu Z, Miao L
and Yin J: Schizandrin, an antioxidant lignan from Schisandra
chinensis, ameliorates Aβ1–42-induced memory impairment in
mice. Oxid Med Cell Longev. 2012:7217212012.PubMed/NCBI
|
|
26
|
Das J, Ghosh J, Manna P and Sil PC:
Acetaminophen induced acute liver failure via oxidative stress and
JNK activation: protective role of taurine by the suppression of
cytochrome P450 2E1. Free Radic Res. 44:340–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez FJ: Role of cytochromes P450 in
chemical toxicity and oxidative stress: studies with CYP2E1. Mutat
Res. 569:101–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gunawan BK, Liu ZX, Han D, Hanawa N,
Gaarde WA and Kaplowitz N: c-Jun N-terminal kinase plays a major
role in murine acetaminophen hepatotoxicity. Gastroenterology.
131:165–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanawa N, Shinohara M, Saberi B, Gaarde
WA, Han D and Kaplowitz N: Role of JNK translocation to
mitochondria leading to inhibition of mitochondria bioenergetics in
acetaminophen-induced liver injury. J Biol Chem. 283:13565–13577.
2008. View Article : Google Scholar : PubMed/NCBI
|