Introduction
Hepatitis B virus (HBV) infection is a serious
public health problem affecting more than 400 million individuals
worldwide (1). During HBV
infection, the host immune responses, particularly the cellular
immune response, mediate clearance of HBV infection and the
pathogenesis of liver injury is dependent on the balance between
HBV replication and the viral-specific cytotoxic T-lymphocyte (CTL)
response (2). In patients with an
acute self-limiting HBV infection, the CD4+ and
CD8+ T-cell response with T helper 1 type cytokine
profile is crucial for the control of the infection (3,4).
However, patients with chronic hepatitis B (CHB) often exhibit
impairment of HBV-specific T-cell activity, which is characterized
by a weak immune response to HBV and lack of the vigorous specific
CD4+ and CD8+ T-cell response (4,5).
The precise mechanisms responsible for this impaired
T-cell response to the chronicity of HBV infection are not fully
understood. One scenario is the potential role of host-mediated
immunosuppressive mechanisms that may be activated following
persistent antigenic exposure (6).
Previously, regulatory T cells (Tregs) were focused to have an
indispensable role in maintaining immunological unresponsiveness to
antigens (7,8), as they have a prominent role in
immunoregulation and tolerance. During the acute-resolved HBV
infection, circulating Treg frequency is low in the acute phase,
but is significantly increased during the convalescent phase and
then returns to a normal level along with disease resolution
(9). In patients with CHB, the
increased Treg frequencies in the peripheral blood and liver may
significantly suppress HBV-specific CTL responses; however, they
are found to be decreased upon hepatitis B e antigen (HBeAg)
seroconversion (10,11). Furthermore, the decreased frequency
of hepatitis B c antigen (HBcAg)-specific Tregs accompanied by
increased HBcAg peptide-specific CTLs partially account for the
acute exacerbation in patients with CHB (12).
It has been suggested that Treg can be induced
through a repetitive stimulation of T cells by high concentrations
of antigen for longer periods of time (13). The high viral load present in
peripheral blood of HBV patients may possibly provide such a
stimulus. For CHB, two major types of antiviral drugs are widely
used: Nucleotide analogs (including lamivudine, defovir, entecavir,
tenofovir and telbivudine) and interferon (conventional and
pegylated interferon alfa) (14,15).
During the course of pegylated interferon treatment, the decline of
circulating Tregs together with a partial recovery of the immune
responses was able to predict favorable responses (16,17).
The frequency of HBV-specific CTL in the peripheral blood of
responders was significantly higher compared with that of
non-responders following lamivudine treatment (18–20).
Previous studies have also reported that adefovir-induced viral
load reduction results in a decline of circulating Treg together
with a partial recovery of the immune response, as indicated by a
decrease in percentages of Treg and an increased HBV-specific
proliferation (21). Concomitantly
with a quantitative reduction in viral replication, the frequency
of CD4+ T cells and the CD4+/CD8+
ratio increased during effective telbivudine therapy (22). These findings indicated that
effective antiviral treatment can sustain the inhibition of viral
replication and antigen production, which may potentially result in
a decrease of Treg induction, leading to restoration of the immune
response. However, this hypothesis was challenged by a recent
report in which a greater increase in HBcAg-specific Tregs was
correlated with a higher rate of sustained responsiveness to
antiviral therapy in patients with CHB (23).
Telbivudine is a novel orally bioavailable antiviral
drug with high potency and selectivity against HBV (24). Multinational studies have
demonstrated the superiority of telbivudine over lamivudine for the
treatment of CHB, particularly in terms of viral load reduction,
serum alanine aminotransferase (ALT) normalization, HBeAg loss and
reduced viral resistance (25–28).
Certain studies indicated that the rapid inhibition of the HBV load
resulting from continued telbivudine treatment may cause a
restoration of the impaired T cell response in patients with CHB
(22).
In the present study, it was hypothesized that
patients with CHB have a higher proportion of Treg compared with
healthy controls and that the inhibition of viral replication by
continued telbivudine treatment can reduce proportions of Treg in
the peripheral blood and enhance the antiviral immune response. To
evaluate the effects of telbivudine and its correlation with the
HBV DNA load in patients with CHB, the proportions of peripheral
blood CD4+CD25+CD127low and
CD8+CD25+ T cells, and the associated mRNA
levels of Forkhead/winged helix transcription factor (FoxP3, it is
a transcription factor specifically expressed by
CD4+CD25+ Treg cells), were examined and
detected in 22 patients with CHB undergoing telbivudine
treatment.
Patients and methods
Study participants
A total of 22 unrelated patients (16 males and 6
females; age, 36±6.5 years) who were diagnosed with chronic
hepatitis B and received antiviral treatment with telbivudine (600
mg orally per day) between October 2009 and December 2010 at
Southwest Hospital (Chongqing, China) were recruited for the
present study. Nineteen age- and gender-matched healthy blood
donors were selected as the control group. The diagnostic criteria
of CHB were based on the related literature recommended by the
Chinese Medical Association (29).
All the patients were selected according to the following criteria:
(i) All hepatitis B surface antigen (HBsAg) carriers were positive
for both HBsAg and antibody to HBV core antigen of the
immunoglobulin G (IgG) type for at least 12 months; (ii)
HBeAg-positive; (iii) age between 18 and 60 years; (iv) HBV
DNA≥6log10 copies/ml; (v) ALT levels ≥2 fold the upper
limit of that of normal patients and (vi) no antiviral, immune
suppressive or immunomodulatory treatment during the last six
months. Patients who were pregnant and co-infected with the human
immunodeficiency virus, hepatitis A, C or D and patients with other
types of hepatitis were excluded from the present study.
For the patients with CHB, clinical, biochemical,
virological and immunological parameters were assessed in the study
at three fixed time-points (baseline, three and six months). The
clinical characteristics at baseline of the 22 patients are shown
in Table I. Peripheral blood
mononuclear cells (PBMCs) were obtained at baseline and subsequent
to three and six months of telbivudine treatment. All the subjects
provided informed consent to participate in the study (Trial
Registration: Chinese Clinical Trial Registry ChiCTR-TRC-12001987),
as approved by the ethical committee of the Southwest Hospital
(Chongqing, China).
 | Table IBasic characteristics of the
participants. |
Table I
Basic characteristics of the
participants.
| Characteristic | Chronic hepatitis B
patients, n=22 | Healthy blood
donors, n=19 |
|---|
| Gender,
male/female | 16/6 | 14/5 |
| Age, years | 36±6.5 | 38.6±12.1 |
| HBsAg | 22 | 0 |
| Anti-HBs IgG | 0 | 19 |
| HBeAg positive | 22 | 0 |
| Serum HBV DNA
load | >6
log10 copies/ml | Not detectable |
| TBil, μmol/l | 29.6
(17.4–52.8) | 8.4 (3.9–16.5) |
| ALT IU/l | 143.2
(95.1–224.3) | 17.5
(11.0–27.3) |
| AST IU/l | 132.1
(103.2–198.5) | 14.2
(9.5–23.2) |
Biochemical and virological
assessment
Routine liver function tests included ALT, aspartate
aminotransferase and total bilirubin. These assays were performed
with routine automated techniques (upper limit of normal: 50 U/l,
40 U/l and 21.0 μmol/l, respectively; HITACHI L-7600; Hitachi
Medical Corp., Hitachi, Japan). HBV markers (HBsAg, anti-HBs,
HBeAg, anti-HBe and anti-HBc), anti-HAV, anti-hepatitis C virus
(HCV), anti-hepatitis D virus (HDV) and anti-human immunodeficiency
virus (HIV) were determined by commercial enzyme immunoassay kits
using the Abbott IMX system (Abbott Laboratories, North Chicago,
IL, USA). Serum HBV DNA levels were quantified using the Roche
Amplicor Monitor assay (the limit threshold of the assay was 400
copies/ml) according to the manufacturer’s instructions (Roche,
Branchburg, NJ, USA).
Isolation of the PBMCs and flow
cytometric analysis
PBMCs from patients with CHB and healthy donors were
isolated from heparinized whole blood by density gradient
centrifugation using Ficoll-Histopaque (Sigma, St. Louis, MO, USA).
The cells were washed twice with RPMI 1640 (Bio Whittaker,
Verviers, Belgium) and were frozen in RPMI 1640 containing 20%
fetal calf serum (Hyclone, Logan, UT, USA) and 10% dimethyl
sulfoxide. PBMCs from different time-points were stored at −135°C
for further analysis.
When the flow cytometric analysis was performed, the
frozen PBMCs were washed once in phosphate-buffered saline (PBS)
containing 0.3% bovine serum albumin and stained with
fluorescently-labeled antibodies for the surface markers
CD4-fluorescein isothiocyanate, CD25-phycoerythrin (PE),
CD127-Alexa Fluor 647 and CD8- APC-Alexa Flour 750 (BD Biosciences
PharMingen, San Diego, CA, USA) for 20 min at 4°C. The cells were
then washed twice with PBS containing 1% fetal calf serum,
immediately analyzed using a FACScan flow cytometer
(Becton-Dickinson, Franklin Lanes, NJ, USA) and analyzed using Cell
Quest software (FACScalibur™, CELLQuest Pro™
software, Beckton-Dickinson).
Forkhead/winged helix transcription
factor (FoxP3) mRNA quantification
RNA was isolated from the PBMC samples of all
patients and controls using TRIzol® solution (Roche).
The FoxP3 mRNA levels were quantified using a BioRad IQTM5
multicolor quantitative polymerase chain reaction (qPCR) detection
system (BioRad, Hercules, CA, USA) with SYBR® Green I
probes (Toyobo, Osaka, Japan), using β-actin as an internal
control. Primers used for qPCR of FoxP3 and β-actin mRNAs were as
follows: FoxP3 forward, 5′-AAGGAAAGGAGGATGGACG-3′ and reverse,
3′-CAGGCAAGACAGTGGAAACC-5′; β-actin forward,
5′-CGTGGACATCCGCAAAGAC-3′ and reverse, 3′-CTCGCTCCAACCGACTGCT-5′.
Data from qPCR using SYBR Green I probes were analyzed by the
standard curve method as described previously (30).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 9.0; SPSS Inc., Chicago, IL, USA). Continuous
data are presented as the mean ± standard deviation, unless
specified otherwise, and the significance was analyzed with the
t-test. Flow cytometry and FoxP3 data were analyzed using the
Mann-Whitney U test. All flow cytometry and functional data were
compared to the levels at baseline using a Wilcoxon matched pairs
signed rank sum test. P>0.05 was considered to indicate a
statistically significant difference.
Results
The proportion of circulating Treg is
increased in CHB patients and can be decreased during telbivudine
treatment
To demonstrate the importance of Treg in chronic HBV
infection, the proportion of Treg present in the peripheral blood
of patients with CHB (n=22) and healthy individuals (n=19) was
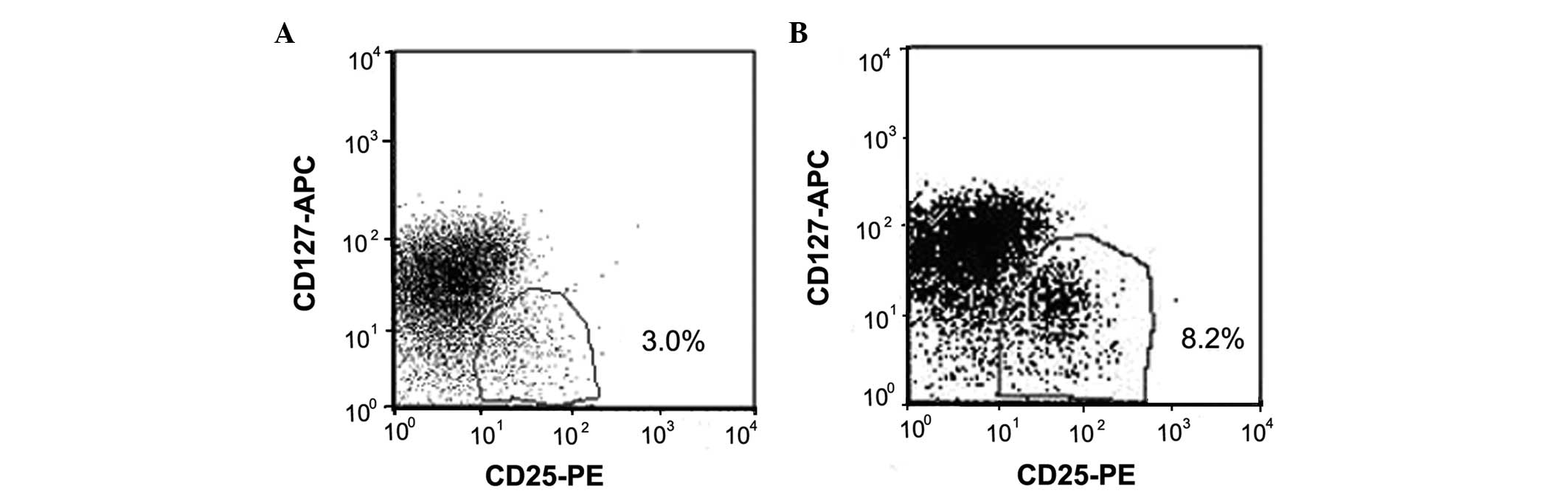
first detected and compared. Figure
1 shows the typical dot plots of the proportion of peripheral
blood CD4+CD25+CD127low T cells
among CD4+ T cells for PBMCs from a representative
patient with CHB (Fig. 1A) and a
healthy individual (Fig. 1B).
Typical dot plots of the proportion of the peripheral blood
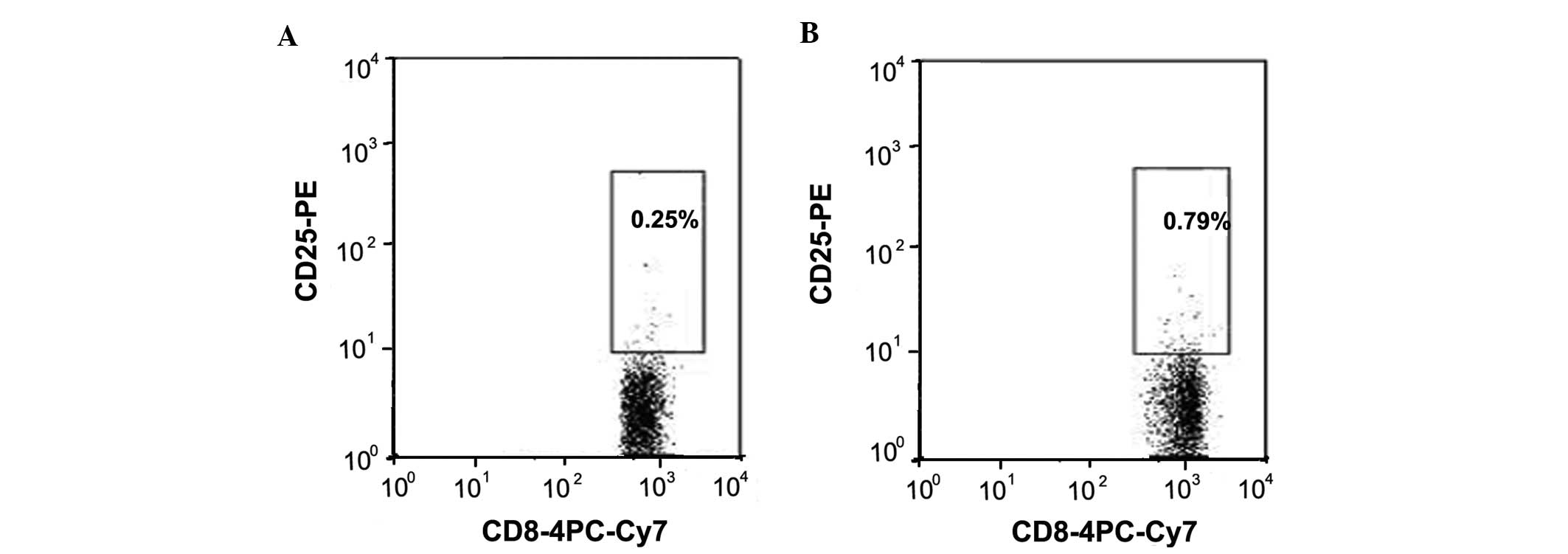
CD8+CD25+ T cells among CD8+ T
cells from a representative patient with CHB (Fig. 2A) and a healthy individual
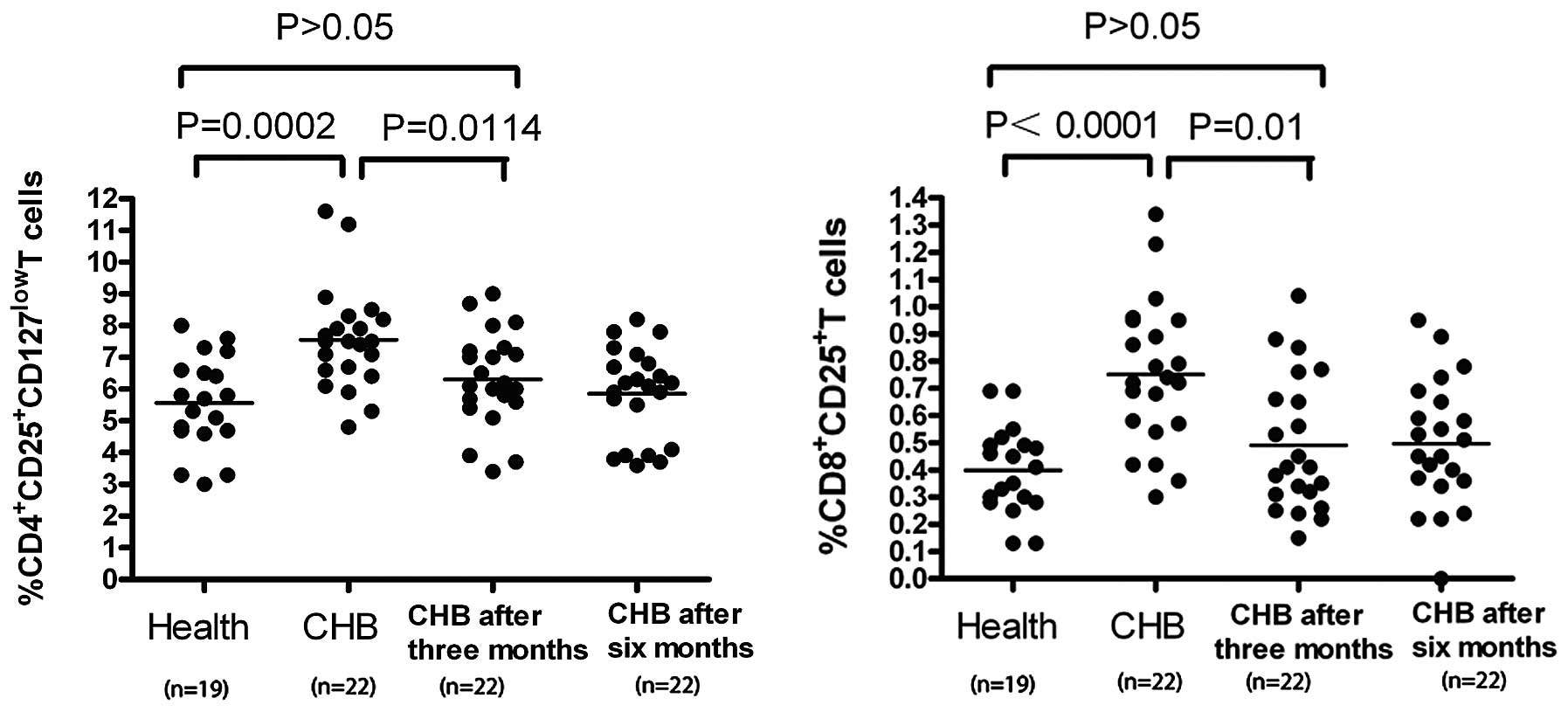
(Fig. 2B) are shown in Figure 2. As Figure 3 shows, patients with CHB
demonstrated a significantly higher percentage of
CD4+CD25+CD127low T cells within
their population of CD4+ T cells (7.55±1.61%) compared
with healthy controls in the peripheral blood (5.6±0.62%)
(P=0.002). Patients with CHB also showed a significantly higher
percentage of CD8+CD25+ T cells within their
population of CD8+ T cells (0.75±0.27%) compared with
healthy controls (0.39±0.09%) (P<0.0001).
To assess the effect of treatment with telbivudine
on the immune response, the proportion of Treg from patients’ PBMCs
were then compared at different time-points prior to and during
antiviral treatment. Subsequent to three and six months of therapy,
a significant decrease in the percentage of
CD4+CD25+CD127low Treg (baseline,
7.55±1.61% vs. three months, 6.31±1.50% vs. six months, 5.86±1.44%,
P=0.00114) was observed. Similarly, after three and six months of
therapy, a significant decrease in the percentage of
CD8+CD25+ Treg (baseline, 0.75±0.27% vs.
three months, 0.49±0.25% vs. six months, 0.50±0.23%; P=0.01;
Fig. 3) were also identified.
Subsequent to six months of telbivudine treatment, the proportion
of peripheral blood
CD4+CD25+CD127low and
CD8+CD25+ T cells in patients with CHB was
comparable to the proportion of the healthy controls
(P>0.05).
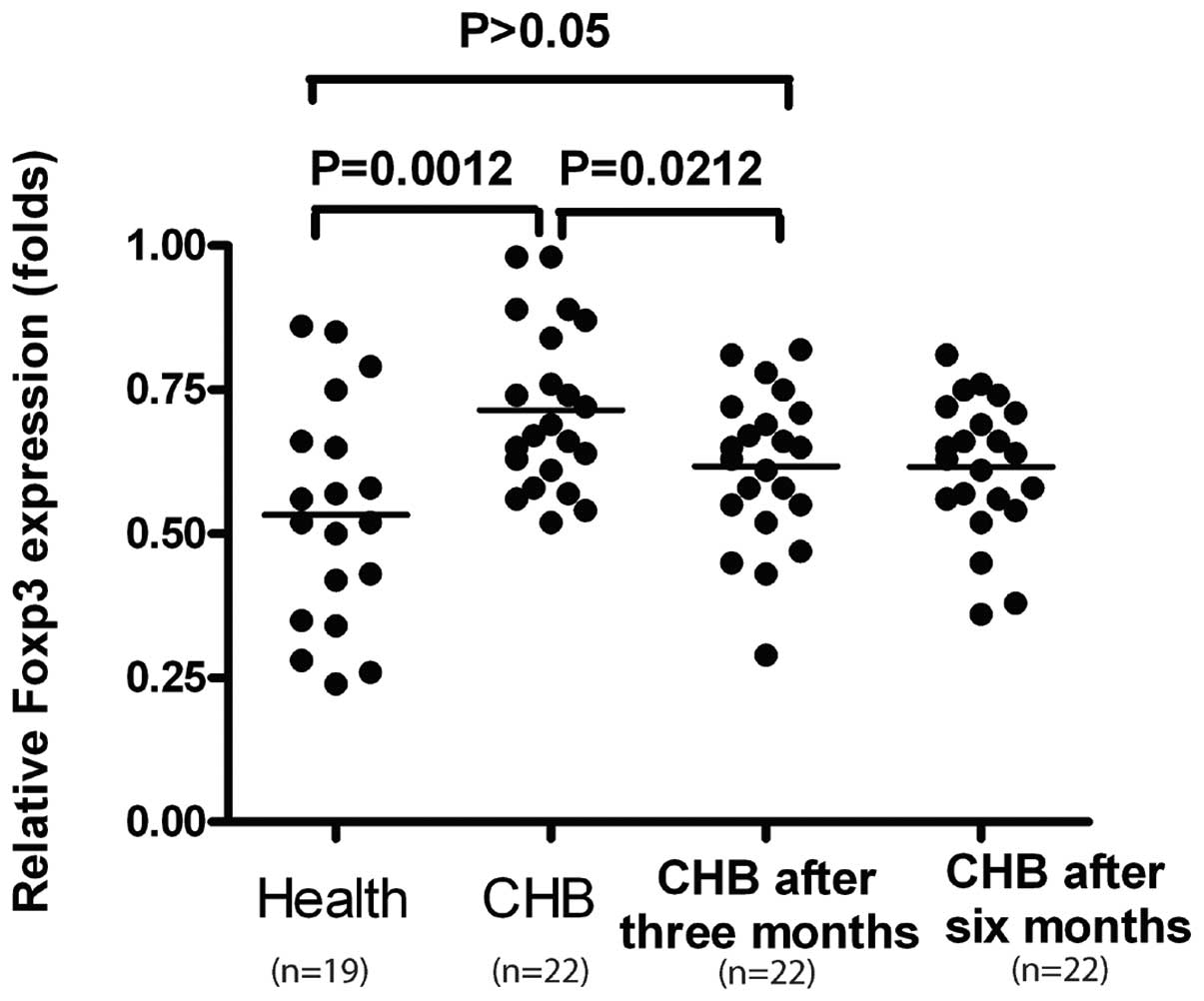
FoxP3 RNA expression is increased in
patients CHB and can be decreased during telbivudine treatment
Since FoxP3 is considered to be the most specific
marker for Treg, the relative FoxP3 mRNA levels of the peripheral
blood samples from all the patients with CHB (one patient with
three samples: At baseline, subsequent to three and six months of
telbivudine treatment) and healthy controls were determined by
qPCR. The FoxP3 mRNA levels in PBMCs in patients with chronic HBV
infection were significantly higher compared with those in the
healthy controls (0.72±0.12 vs. 0.51±0.15; P=0.0012). Subsequent to
three and six months of telbivudine treatment, the relative levels
of Foxp3 mRNA in PBMCs from patients with CHB were significantly
decreased from 0.72±0.12 at baseline to 0.62±0.13 (P=0.0212) and
0.61±0.12 after three and six months treatment, respectively
(P=0.0231) (Fig. 4). Following
telbivudine treatment, the relative levels of Foxp3 mRNA in PBMCs
in patients with CHB were comparable to the levels in the healthy
controls (P>0.05).
Correlation between Treg and clinical
parameters
All 22 chronic patients with HBV in the present
study were HBeAg-positive with ≥6log10 copies/ml HBV DNA
and ≥2 times the upper limit of the normal serum ALT levels prior
to telbivudine treatment. Subsequent to three months of telbivudine
treatment, the HBV DNA levels of all 22 patients with CHB had
dropped to 105 copies/ml or less, and those of 15
patients (15/22, 68.2%) dropped to 103 copies/ml or
less; the serum ALT levels of 17 patients (17/22, 77.3%) dropped to
40 U/l or less, and two (9.1%) patients identified as
HBeAg-negative. Subsequent to six months of telbivudine treatment,
the ALT levels of all 22 patients with CHB had dropped to normal
levels (40 U/l or less); the HBV DNA levels of all 19 patients with
CHB (19/22, 86.4%) had dropped to 103 copies/ml or less,
and four patients (18.2%) identified as HBeAg-negative. During
treatment, no HBsAg loss was observed in the present study.
Following telbivudine treatment, no correlation was observed
between hepatic inflammation and the percentage of peripheral blood
Treg, since the ALT levels of patients whose proportions of Treg
were reduced to normal levels showed significant differences.
Subsequent to six months of telbivudine treatment, the percentages
of Treg of four HBeAg-negative patients were significantly
decreased to normal levels. A weak correlation was observed between
the percentage decrease in HBV DNA and that in the proportion of
Treg after three (r=0.2812 and P<0.05) and six (r=0.5475 and
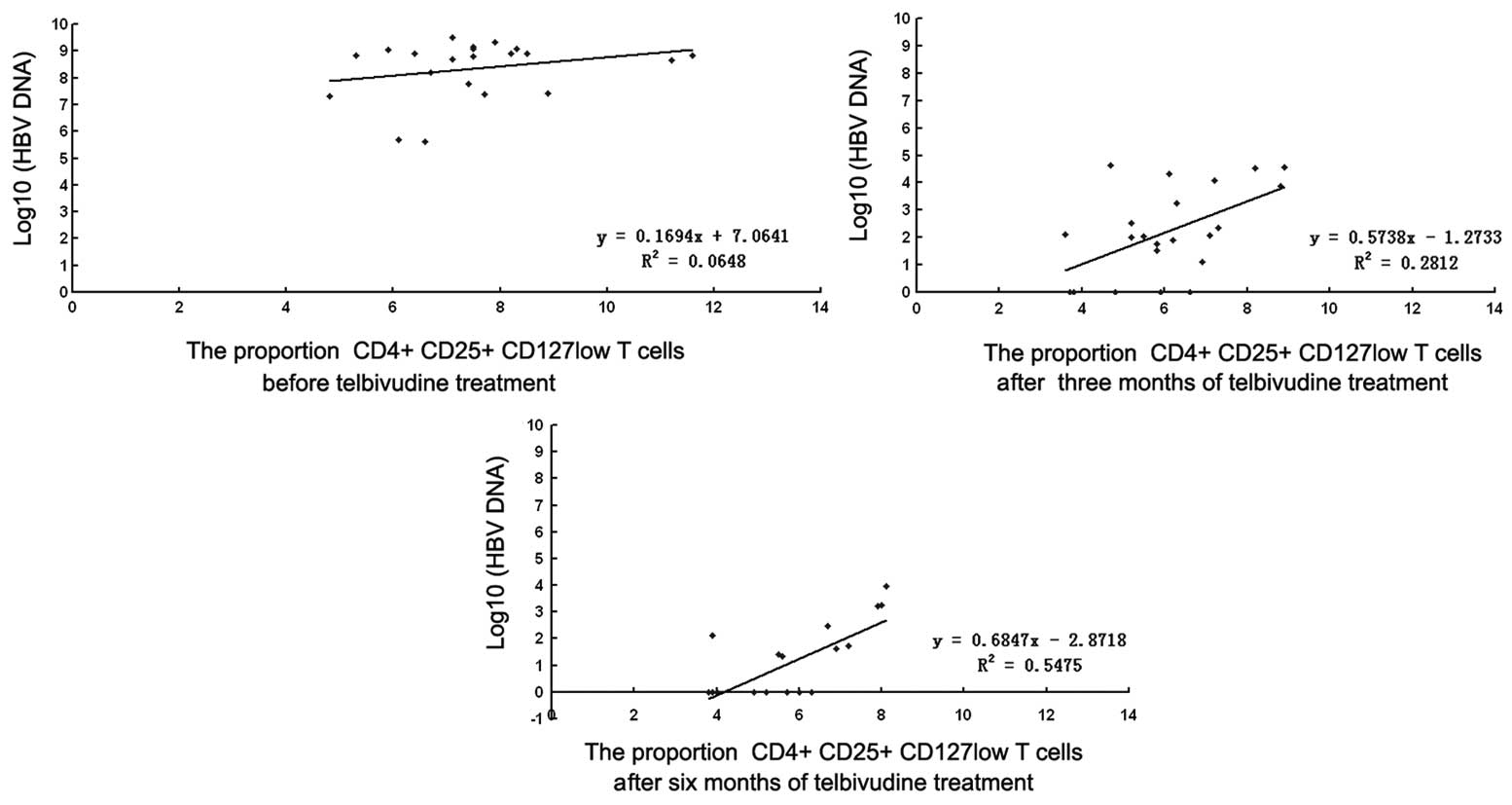
P<0.05) months of telbivudine treatment (Fig. 5).
The proportions of peripheral blood
CD8+CD25+ T cells among CD8+ T
cells in patients with CHB were low (0.1–1.3% at baseline). No
correlation was observed between the viral load or hepatic
inflammation (ALT) and the percentage of the peripheral blood
CD8+CD25+ T cells. However, the percentages
of CD8+CD25+ T cells in four patients
exhibiting HBeAg loss were also significantly decreased in the
present study.
Discussion
Consistent with the results of other previous
studies (24–28), the results of the present study
demonstrated telbivudine’s marked potency to reduce HBV DNA levels.
In the present study, continued telbivudine treatment was
identified to be capable of rapidly reducing HBV DNA levels
together with continuing improvement of biochemical and virological
parameters through six months of treatment in patients with
CHB.
Furthermore, the present study demonstrated the
importance of Tregs in CHB. The frequency and functional properties
of Tregs are significant due to increased numbers of Tregs, which
may favor the development of chronic viral infections and influence
the course of the disease and effectiveness of antiviral treatment.
However, it had remained controversial whether circulating
CD4+CD25+ Treg is frequency increased in
patients with CHB and whether the frequency is correlated with HBV
replication (31,32). The results of the present study
indicated that patients with CHB infection displayed higher levels
of Treg in their PBMCs compared with healthy controls. In addition,
the relative FoxP3 mRNA levels were higher in PBMCs of patients
with CHB. FoxP3 is a transcription factor specifically expressed by
CD4+CD25+ Treg cells (33–35).
A previous study showed that FoxP3 mRNA expression is relatively
unique to the CD4+CD25+ cell population of
PBMCs and measuring FoxP3 mRNA expression in CD4+ cell
populations or even total PBMCs is more practical compared with
isolating the CD4+CD25+ cell population to
evaluate CD4+CD25+ Treg activity and predict
a clinical outcome. The higher relative levels of FoxP3 mRNA in
patients with CHB in the present study indicate that patients with
CHB have a higher percentage of Treg in the peripheral blood
compared with healthy controls. This is in agreement with a
previous study, which also identified a higher percentage of
CD4+CD25+FoxP3+ Treg in patients
with CHB compared with healthy controls (36). However, an early study did not find
the diversity of the Treg distribution among the asymptomatic
carriers, patients with CHB and healthy individuals (11). The possible reason for this
difference between different studies may be the different detection
methods of Treg used in these studies, among various other specific
circumstances. Certain studies have found that the virus-specific
induction of Treg may result in two different consequences: It may
have key roles in the process to prevent excessive
immunopathological injury and it may also cause the establishment
of persistent viral infection (37–41).
Furthermore, antiviral treatment with telbivudine
was observed to be capable of downregulating the proportion of Treg
in PBMCs and enhancing the antiviral immune response of patients
with CHB. An abundance of experimental data has confirmed that
CD4+CD25+ Tregs can suppress effective
antiviral immune responses, in particular, in the chronic
infections caused by the HIV (42)
and HCV (43). A number of studies
have shown that during the course of nucleotide analogs and
interferon treatment, the decline of circulating Tregs together
with a partial recovery of the immune responses can predict
favorable responses (16,20–22).
However, this standpoint was oppugned by a recent study in which
greater increases of Tregs were correlated with a higher rate of
sustained responsiveness to antiviral therapy (23). In the present study, the proportion
of peripheral blood
CD4+CD25+CD127low and
CD8+CD25+ T cells in patients with CHB was
observed to be decreased over three or six months of telbivudine
treatment to a level comparable to that of the healthy controls.
Concomitantly, the levels of Foxp3 mRNA in PBMCs from patients with
CHB also decreased over three or six months of telbivudine
treatment to a level comparable to that prior to treatment. The
results indicate that the inhibition of viral replication reduced
regulatory T cells and enhanced the antiviral immune response in
chronic hepatitis B. It is hypothesized that patients effectively
treated with telbivudine not only showed an enhanced reconstitution
of the CD4 response, but also exhibited a significant enhancement
in stimulation of HBV-specific CTL activity and reduced HBV serum
titers, efficiently resulting in a significant increase in the
frequency of CTL and a greater magnitude of cytokine production,
which was partly proved previously (21,22,44).
Last and most importantly, the results of the
present study indicated that the proportions of peripheral blood
CD4+CD25+CD127low T cells were
paralleled with its HBV DNA inhibition upon telbivudine treatment.
The present study revealed a positive correlation between the
CD4+CD25+CD127low Treg frequency
and the serum HBV DNA levels, indicating that the upregulation of
Tregs may be associated with an increase in HBV replication. This
result is identical to those of previous studies (36,45).
However, two earlier studies did not find any significant
association between circulating Treg frequency and HBV DNA titer in
patients with CHB (10,11). In addition, certain studies support
an association between increased CD4+CD25+
Treg and HBeAg, as well as impaired viral clearance (36), while others did not find this
correlation (10). By contrast,
the present study did not identify any association of the HBeAg
status with either
CD4+CD25+CD127low or
CD8+CD25+ T cell frequency. Differences in
the methods, reagents and samples used in these studies may account
for the discrepancies. Following telbivudine treatment, no
correlation was observed between ALT and the percentage of the
peripheral blood Treg in the present study, which was in agreement
with a previous study (10).
However, another study reported that Treg accumulated and expanded
locally at the site of infection (46). Alltogether, the preliminary data of
the current study indicated that the elevation in the number of
circulating Tregs in patients with CHB decreased following
antiviral treatment and the antigen-specific T-cell response to
HBsAg was more significantly suppressed by Tregs. These results
support the hypothesis that CHB infection leads to the induction of
suppressive Tregs which inhibit antiviral immune responses.
In conclusion, the findings of the present study
indicate that telbivudine treatment not only reduces serum HBV DNA
levels rapidly, but is also beneficial to restore the antiviral
immune response. Effective telbivudine treatment indirectly affects
the immune system by downregulating the proportion of the
peripheral blood CD4+CD25+CD127low
and CD8+CD25+ T cells markedly, which may be
predictive of the responsiveness to telbivudine therapy.
Acknowledgements
The present study was supported in part by the State
Key Project Specialized for Infectious Diseases (no.
2012ZX10002-004).
References
|
1
|
Lai CL, Ratziu V, Yuen MF and Poynard T:
Viral hepatitis B. Lancet. 362:2089–2094. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guidotti LG and Chisari FV: Immunobiology
and pathogenesis of viral hepatitis. Annu Rev Pathol. 1:23–61.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rehermann B, Fowler P, Sidney J, et al:
The cytotoxic T lymphocyte response to multiple hepatitis B virus
polymerase epitopes during and after acute viral hepatitis. J Exp
Med. 181:1047–1058. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertoletti A and Naoumov NV: Translation
of immunological knowledge into better treatments of chronic
hepatitis B. J Hepatol. 39:115–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertoletti A, D’Elios MM, Boni C, et al:
Different cytokine profiles of intraphepatic T cells in chronic
hepatitis B and hepatitis C virus infections. Gastroenterology.
112:193–199. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Zhang JY, Wang LF and Wang FS:
Immunopathogenesis and prognostic immune markers of chronic
hepatitis B virus infection. J Gastroenterol Hepatol. 27:223–230.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar
|
|
9
|
Xu D, Fu J, Jin L, et al: Circulating and
liver resident CD4+CD25+ regulatory T cells actively influence the
antiviral immune response and disease progression in patients with
hepatitis B. J Immunol. 177:739–747. 2006.
|
|
10
|
Stoop JN, van der Molen RG, Baan CC, et
al: Regulatory T cells contribute to the impaired immune response
in patients with chronic hepatitis B virus infection. Hepatology.
41:771–778. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franzese O, Kennedy PT, Gehring AJ, et al:
Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T
cells in patients with hepatitis B virus infection. J Virol.
79:3322–3328. 2005.
|
|
12
|
Feng IC, Koay LB, Sheu MJ, et al:
HBcAg-specific CD4+CD25+ regulatory T cells modulate immune
tolerance and acute exacerbation on the natural history of chronic
hepatitis B virus infection. J Biomed Sci. 14:43–57. 2007.
|
|
13
|
Taams LS, Vukmanovic-Stejic M, Smith J, et
al: Antigen-specific T cell suppression by human CD4+CD25+
regulatory T cells. Eur J Immunol. 32:1621–1630. 2002.
|
|
14
|
Liaw YF and Chu CM: Hepatitis B virus
infection. Lancet. 373:582–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dienstag JL: Hepatitis B virus infection.
N Engl J Med. 359:1486–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sprengers D, Stoop JN, Binda RS, et al:
Induction of regulatory T-cells and interleukin-10-producing cells
in non-responders to pegylated interferon-alpha therapy for chronic
hepatitis B. Antivir Ther. 12:1087–1096. 2007.PubMed/NCBI
|
|
17
|
Rico MA, Quiroga JA, Subirá D, et al:
Hepatitis B virus-specific T-cell proliferation and cytokine
secretion in chronic hepatitis B e antibody-positive patients
treated with ribavirin and interferon alpha. Hepatology.
33:295–300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maini MK, Reignat S, Boni C, et al: T cell
receptor usage of virus-specific CD8 cells and recognition of viral
mutations during acute and persistent hepatitis B virus infection.
Eur J Immunol. 30:3067–3078. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boni C, Penna A, Bertoletti A, et al:
Transient restoration of anti-viral T cell responses induced by
lamivudine therapy in chronic hepatitis B. J Hepatol. 39:595–605.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai SL, Sheen IS, Chien RN, et al:
Activation of Th1 immunity is a common immune mechanism for the
successful treatment of hepatitis B and C: tetramer assay and
therapeutic implications. J Biomed Sci. 10:120–135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stoop JN, van der Molen RG, Kuipers EJ,
Kusters JG and Janssen HL: Inhibition of viral replication reduces
regulatory T cells and enhances the antiviral immune response in
chronic hepatitis B. Virology. 361:141–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Li X, Ye B, et al: Effect of
telbivudine therapy on the cellular immune response in chronic
hepatitis B. Antiviral Res. 91:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koay LB, Feng IC, Sheu MJ, et al:
Hepatitis B virus (HBV) core antigen-specific regulatory T cells
confer sustained remission to anti-HBV therapy in chronic hepatitis
B with acute exacerbation. Hum Immunol. 72:687–698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nash K: Telbivudine in the treatment of
chronic hepatitis B. Adv Ther. 26:155–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai CL, Gane E, Liaw YF, et al:
Telbivudine versus lamivudine in patients with chronic hepatitis B.
N Engl J Med. 357:2576–2588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai CL, Leung N, Teo EK, et al: A 1-year
trial of telbivudine, lamivudine, and the combination in patients
with hepatitis B e antigen-positive chronic hepatitis B.
Gastroenterology. 129:528–536. 2005.PubMed/NCBI
|
|
27
|
Lai CL, Gane E, Hsu CW, et al: Two-year
results from the GLOBE trial in patients with hepatitis B: greater
clinical and antiviral efficacy for telbivudine (LdT) vs.
lamivudine Hepatology. 44(Suppl 1): 222A2006.
|
|
28
|
Liaw YF, Gane E, Leung N, et al: 2-Year
GLOBE trial results: telbivudine Is superior to lamivudine in
patients with chronic hepatitis B. Gastroenterology. 136:486–495.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinese Society of Hepatology, Chinese
Medical Association; Chinese Society for Infectious Diseases,
Chinese Medical Association. Guideline on prevention and treatment
of chronic hepatitis B in China (2005). Chin Med J (Engl).
120:2159–2173. 2007.PubMed/NCBI
|
|
30
|
Shaik GM, Dráberová L and Dráber P,
Boubelík M and Dráber P: Tetraalkylammonium derivatives as
real-time PCR enhancers and stabilizers of the qPCR mixtures
containing SYBR Green I. Nucleic Acids Res. 36:e932008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lan RY, Cheng C, Lian ZX, et al:
Liver-targeted and peripheral blood alterations of regulatory T
cells in primary biliary cirrhosis. Hepatology. 43:729–737. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Gowans EJ, Chougnet C, Plebanski M
and Dittmer U: Natural regulatory T cells and persistent viral
infection. J Virol. 82:21–30. 2008. View Article : Google Scholar
|
|
33
|
Fontenot JD and Rudensky AY: A well
adapted regulatory contrivance: regulatory T cell development and
the forkhead family transcription factor Foxp3. Nat Immunol.
6:331–337. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of CD4+CD25+ regulatory
T cells. Nat Immunol. 4:330–336. 2003.
|
|
35
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang G, Liu A, Xie Q, et al: Association
of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and
viral clearance in patients with hepatitis B. Int Immunol.
19:133–140. 2007.
|
|
37
|
Belkaid Y: Regulatory T cells and
infection: a dangerous necessity. Nat Rev Immunol. 7:875–888. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Belkaid Y and Rouse BT: Natural regulatory
T cells in infectious disease. Nat Immunol. 6:353–360. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alatrakchi N and Koziel M: Regulatory T
cells and viral liver disease. J Viral Hepat. 16:223–229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rushbrook SM, Hoare M and Alexander GJ:
T-regulatory lymphocytes and chronic viral hepatitis. Expert Opin
Biol Ther. 7:1689–1703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Billerbeck E, Bottler T and Thimme R:
Regulatory T cells in viral hepatitis. World J Gastroenterol.
13:4858–4864. 2007.PubMed/NCBI
|
|
42
|
Aandahl EM, Michaëlsson J, Moretto WJ,
Hecht FM and Nixon DF: Human CD4+ CD25+ regulatory T cells control
T-cell responses to human immunodeficiency virus and
cytomegalovirus antigens. J Virol. 78:2454–2459. 2004.
|
|
43
|
Cabrera R, Tu Z, Xu Y, et al: An
immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in
hepatitis C virus infection. Hepatology. 40:1062–1071. 2004.
|
|
44
|
Lau GK, Cooksley H, Ribeiro RM, et al:
Impact of early viral kinetics on T-cell reactivity during
antiviral therapy in chronic hepatitis B. Antivir Ther. 12:705–718.
2007.PubMed/NCBI
|
|
45
|
Peng G, Li S, Wu W, Sun Z, Chen Y and Chen
Z: Circulating CD4+ CD25+ regulatory T cells correlate with chronic
hepatitis B infection. Immunology. 123:57–65. 2008.
|
|
46
|
Cao D, Malmström V, Baecher-Allan C,
Hafler D, Klareskog L and Trollmo C: Isolation and functional
characterization of regulatory CD25brightCD4+ T cells from the
target organ of patients with rheumatoid arthritis. Eur J Immunol.
33:215–223. 2003.PubMed/NCBI
|