Introduction
The root of Salvia miltiorrhiza Bunge (S.
Bunge), a perennial plant in the Salvia genus, is highly valued in
traditional Chinese Medicine. The most effective compounds for
medical treatment extracted from the root of S. Bunge are several
tanshinone derivatives, including tanshinones I, IIA and IIB,
dihydrotanshinone and cryptotanshinone (1). Previous studies mainly focused on the
pharmacological effects of tanshinones I and IIA; however, rarely
on cryptotanshinone. Tanshinones I and IIA are diterpene quinones
exerting anti-inflammatory, anti-oxidative, cytotoxic,
apoptosis-inducing and growth-inhibitory effects on numerous types
of cancer cells, including human gastric (2), hepatocellular (3), breast (4), and cervical cancer cells (5). Given that cryptotanshinone is
structurally homologous to tanshinones I and IIA, their
pharmacological effects may be similar. In the present study the
anticancer effect of cryptotanshinone on human lung cancer, was
assessed in vitro and in vivo, investigating the
pharmacological effects and potential clinical use of
cryptotanshinone.
Materials and methods
Drug treatments and assessment of
cytotoxic effects
Cryptotanshinone was purchased from LKT
Laboratories, Inc. (St. Paul, MN, USA). The human A549 lung
adenocarcinoma epithelial cell line was propagated at 37°C with 5%
CO2 in Roswell Park Memorial Institute (RPMI)-1640 cell
culture medium (GIBCO-BRL, Carlsbad, CA, USA) supplemented with
L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10%
(v/v) fetal bovine serum (FBS; GIBCO, USA). A549 cells
(5×103 cells/well) were seeded in a 96-well plate
(Corning, Acton, MA, USA). Cells were treated with cryptotanshinone
dissolved in 0.1% DMSO at final concentrations of 1, 5, 20 and 50
μM for 24, 36 and 48 h, respectively. The control group was treated
with 0.1% DMSO. The MTT assay was performed to investigate cell
growth inhibition and cytotoxicity. The colony-forming assay was
performed to assess cell survival following treatment with
cryptotanshinone. A549 cells (n=250 were seeded in a 35 mm tissue
culture dish (Corning) and allowed to attach overnight prior to
treatment with 1, 5, 20 and 50 μM of cryptotanshinone dissolved in
DMSO or 0.1% DMSO (control), incubated for 10 days. The colonies
were stained with 0.5% crystal violet in methanol/acetic acid (3:1)
and those composed of >50 cells were counted. Experiments were
performed three times in duplicate.
Cell cycle and analysis of associated
genes
According to previous studies, a final
cryptotanshinone concentration of 20 μM is the ideal concentration
for in vitro experiments. A549 cells were seeded in a 6-well
plate (Corning) at a density of 1×105/ml and treated at
a final cryptotanshinone concentration of 20 μM for 24 h. Cells
were trypsinized, centrifuged (4°C, 800 g/min, 10 min), washed with
phosphate-buffered saline (PBS) and fixed with cold 70% ethanol/30%
PBS at 4°C overnight. Cells were digested by 1,000 units RNase A
(Invitrogen, Carlsbad, CA, USA), and then stained with 1% propidium
iodide (Sigma, St. Louis, MO, USA) at 37°C for 30 min. The DNA
profiles were determined within 4 h of staining by flow cytometry
(FCM; BD FACSArray™, BD Biosciences, Franklin Lakes, NJ, USA). The
expression of cell cycle and apoptosis-associated genes was tested
using Cell Cycle PCR Array (PAHS-020Z, SABiosciences, Quiagen,
Hilden, Germany). The value of the control group was set as 1 while
the relative values for the cryptotanshinone group were calculated
in correlation with the control group. Significant changes (>2-
or <0.5-fold) in the levels of G2/M cell
cycle-associated genes were shown.
Western blot analysis of cell cycle and
apoptosis
Total protein extracted (40 μg) from A549 cells
treated with cryptotanshinone at a final concentration of 20 μM for
48 h was boiled with 4X loading buffer at 100°C for 5 min prior to
injection in a 12.5% SDS-PAGE. Following electrophoresis and
transfer, the polyvinylidene fluoride (PVDF) membrane (Invitrogen,
USA) was blocked in 5% non-fat milk for 1 h, incubated overnight at
4°C with B-cell lymphoma 2 (Bcl2) rabbit polyconal immunoglobulin G
(IgG) (1:1000; Signalway Antibody LLD, College Park, MD, USA),
Bcl2-associated X (Bax) rabbit polyconal IgG (1:1000; Signalway
Antibody), p53 rabbit polyconal IgG (1:1200; AnboBio, San
Francisco, CA, USA), cyclin-dependent kinase inhibitor 1A
[p21(CIP1/WAF1)], cyclin B1 and cell division cycle 25C (Cdc25C)
rabbit polyconal IgG (1:800; Cell Signaling Technology, Inc.,
Danvers, MA, USA), or cyclin D kinase 1 (CDK1) rabbit polyconal
IgG, (1:1000; Merck Millipore, Billerica, MA, USA). Cells were then
washed with 1X Tris-PBS, incubated with the secondary antibody
[goat anti-rabbit IgG-horseradish peroxidase (HRP), Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA] for 1 h at room
temperature, washed and exposed. β-actin was used as the native
control. Images were captured on Kodak film (Kodak, Rochester, NY,
USA) for analysis. The value (target protein/β-actin) in the
control group was set as 1 while the relative values for the
cryptotanshinone group were calculated in correlation with the
control group.
Generation and measurement of tumor
xenograft mice
Human lung tumor tissue (patient consent was
obtained from the patient’s family, provided by Wuwei Tumor
Hospital) was cut into 3×3×3 mm3 sections in RPMI-1640
(Gibco) and then subcutaneously injected into the right lower limbs
of 24 male nude mice (50 days old; weight, ~18 g, provided by the
Animal Research Center, Lanzhou University, China) kept under
specific pathogen-free conditions at 24±2°C and 55±15% humidity.
Seven days post-xenotransplantation, the mice with tumor sizes
>150 mm3 were used. Half of these nude mice were
subcutaneously injected with 100 μg/g cryptotanshinone per day
around the xenotransplantation area for a total of 20 days,
according to our previously established method (6). The body weight and tumor size of the
nude mice were measured every two days following treatment with
cryptotanshinone. Mice with weight loss of >40% or tumor sizes
of >2,000 mm3 were sacrificed according to the animal
welfare requirements. Tumor sizes were calculated as V=π/6 × a ×
b2 where a is the major and b, the minor axis. Mice were
sacrificed by CO2 inhalation for 3 min. The present
study was approved by the Medical Ethics Committee of Wuwei Tumor
Hospital, Gansu, China.
Hematoxylin and eosin (H&E) staining,
terminal dioxynucleotidyl transferase deoxyuridine triphosphate
nick end-labeling (TUNEL) and immunohistochemistry (IHC)
detection
Lung tumors transplanted into nude mice were
collected on the 20th day and subjected to pathological detection
and assessment of apoptosis. Briefly, 4 μm tumor sections were
deparaffinized by treatment with fresh xylene as well as 100, 95
and 70% alcohol for 3 min, which was repeated once. The sections
were stained in Harris hematoxylin solution for 5 min,
differentiated in 1% acid alcohol for 30 sec, blued in 0.2% ammonia
water for 30 sec, stained with eosin-phloxine solution for 1 min
and then dehydrated with 95 and 100% alcohol as well as xylene for
3 min, which was repeated once. TUNEL detection for apoptosis
analysis of 4 μm paraffin sections was performed using the QIA33
FragEL™ DNA Fragmentation Detection kit (Merck KGaA, Darmstadt,
Germany) according to the manufacturer’s instructions. Paraffin
sections (4 μm) were prepared and stained with Bcl2 rabbit
polyclonal IgG (1:200; Signalway Antibody), Bax rabbit polyconal
IgG (1:200), p53 rabbit polyconal IgG (1:300; Anbobio) and caspase
3 rabbit polyconal IgG (1:200; Cell Signaling Technology) to assess
apoptosis in lung tumor tissue following the protocol (Nikon
TE2000; Nikon, Tokyo, Japan).
Data analysis
In the present study, all experiments were performed
at least three times, both in vitro and in vivo. Data
were presented as the mean ± standard deviation. Calculation and
analysis were performed using SPSS 13.0 (USA). These results were
evaluated by the Student’s t-test (two groups) or one-way ANOVA
(multiple groups). A significant difference was defined as
P<0.05 and P<0.01.
Results
Cell growth and colonogenic survival
inhibition
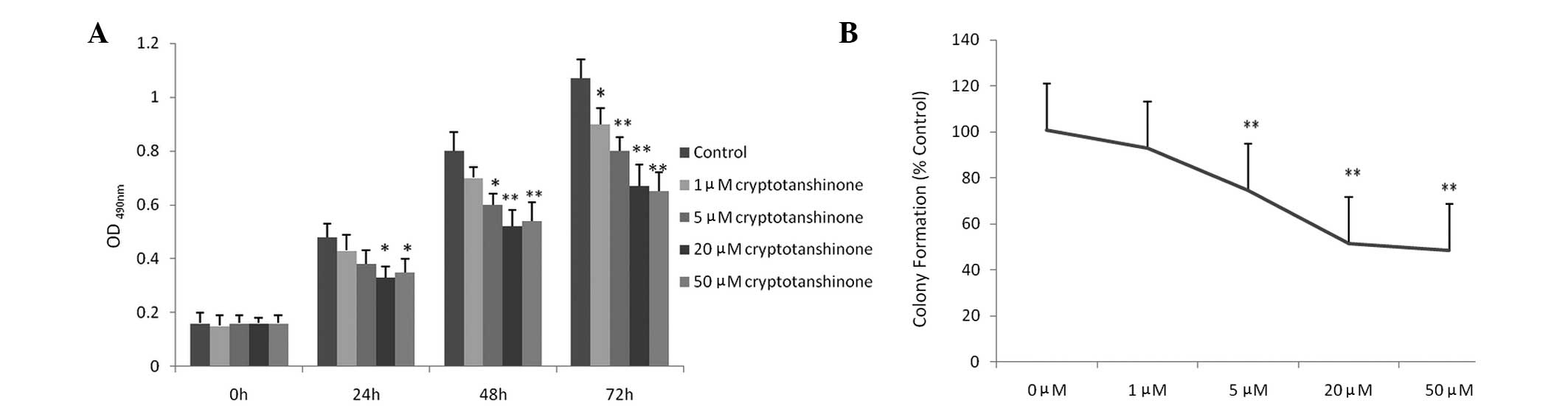
As shown in Fig.
1A, cryptotanshinone exerted a dose-dependent cytotoxic effect
on A549 cells. Compared with the control group, 1 μM
cryptotanshinone showed no obvious growth inhibitory effect within
48 h; however, an effect was observed within 72 h. The growth and
viability of A549 cells treated with cryptotanshinone for 24–72 h
were significantly inhibited at concentrations >5 μM. No obvious
improvement of the inhibitory effect was observed at >20 μM.
These results indicate that cryptotanshinone is cytotoxic to A549
cells and thus inhibits cancer cell growth. In the presence of
cryptotanshinone at concentrations >5 μM, colony formation of
A549 cells was significantly inhibited, while 1 μM cryptotanshinone
showed no obvious inhibition effect in the clonogenic assay
(Fig. 1B). Both the cell growth
and clonogenic survival inhibition study indicated that
cryptotanshinone was able to inhibit the growth of A549 cells and
20 μM served as an ideal concentration for subsequent
experiments.
Cell cycle and analysis of associated
genes
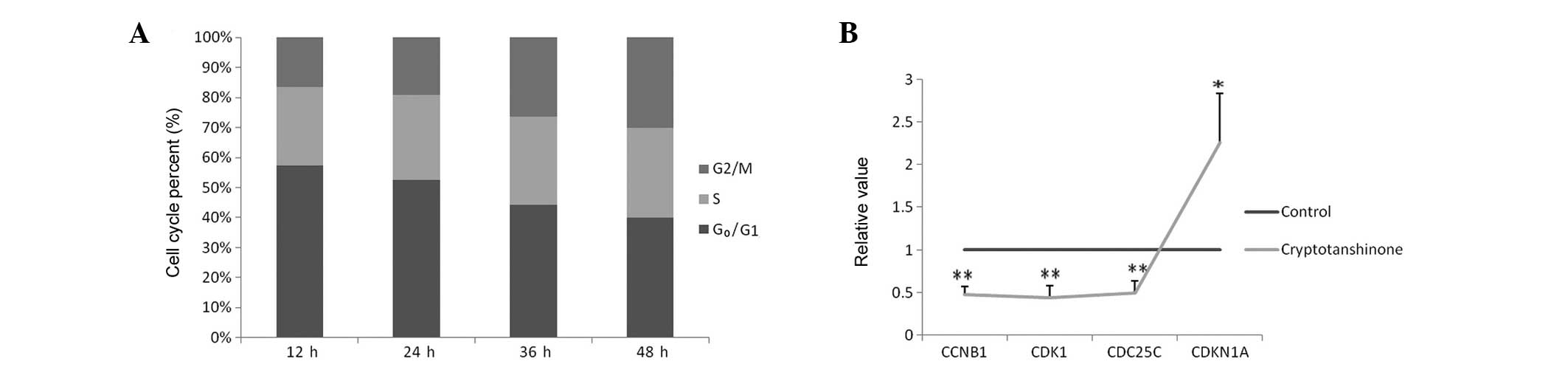
As shown in Fig.
2A, compared with the control group, the percentage of cells in
G0/G1 phase was decreased following treatment
with 20 μM cryptotanshinone, while the percentage in the
G2/M phases increased, indicating that cryptotanshinone
was able to cause growth arrest in A549 cells at the
G2/M phase. Based on this knowledge, G2/M
phase regulatory genes were assessed. Cyclin B1, CDK1 and Cdc25C
were downregulated (<0.5-fold), while the expression of
p21(CIP1/WAF1) was upregulated (>2-fold) according to
quantitative polymerase chain reaction (qPCR). For other
G2/M phase regulatory genes, no obvious changes were
detected. Data are presented in Fig.
2B.
Western blot analysis
As shown in Fig.
3A, the expression of the G2/M phase-associated
proteins cyclin B1, CDK1 and CDC25C was downregulated, while
p21(CIP1/WAF1) was upregulated following treatment with 20 μM
cryptotanshinone, which was in accordance with the results of the
qPCR analysis. This result indicated that cryptotanshinone was able
to cause cell cycle arrest at the G2/M phase of A549
cells both at the gene and the protein level. Concerning
apoptosis-associated proteins, the expression of Bcl-2 was
downregulated, while p53 and Bax were upregulated following
treatment with 20 μM cryptotanshinone. This result indicated that
cryptotanshinone was able to induce and promote the apoptosis of
A549 cells (Fig. 3B).
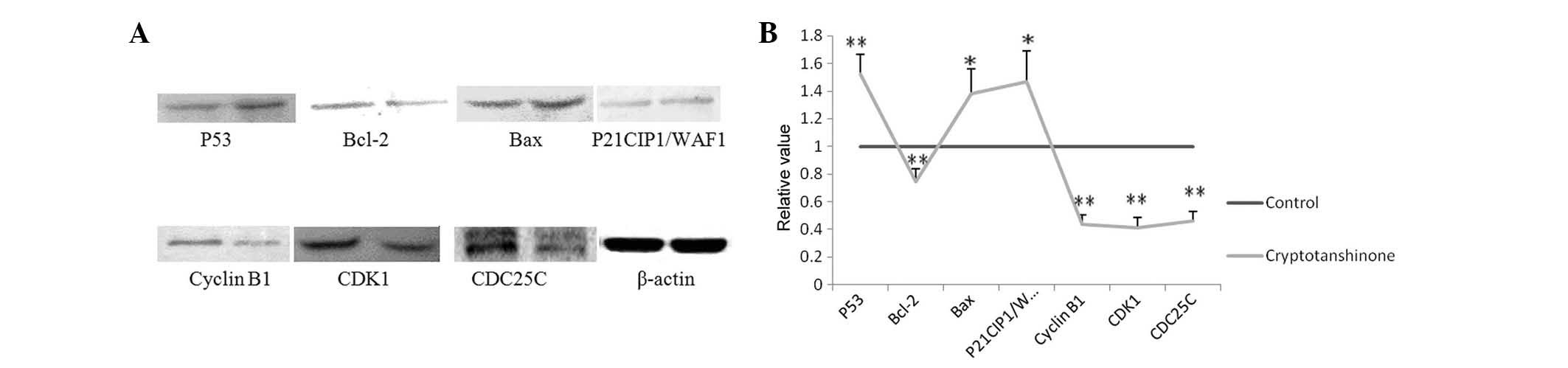 | Figure 3(A) Expression of G2/M
phase-associated proteins cyclin B1, CDK1 and CDC25C were
downregulated, while p21CIP1/WAF1 was upregulated following
treatment with 20 μM cryptotanshinone, indicating that
cryptotanshinone was able to cause G2/M-phase arrest.
The expression of Bcl-2 was downregulated while p53 and Bax were
up-regulated following treatment with 20 μM cryptotanshinone,
indicating cryptotanshinone was able to induce and promote
apoptosis of A549 cells (left, control group; right,
cryptotanshinone group). (B) Data analysis for the relative value
of protein expression. A significant difference was defined as
*P<0.05 and **P<0.01. Bcl-2, B-cell
lymphoma 2; Bax; Bcl-2 associated X; p21CIP1/WAF1, cyclin-dependent
kinase inhibitor 1A; CDK1; cyclin D kinase 1; Cdc25C, cell division
cycle 25C; G2/M, growth phase 2/mitosis phase. |
Analysis of body weight and tumor size of
xenografts
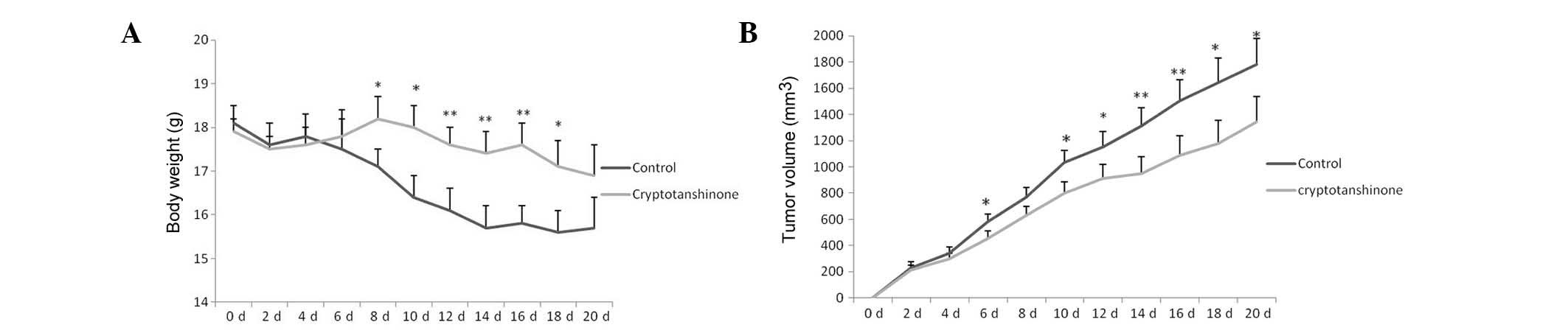
As shown in Fig.
4A, the tumor in the control group exhibited rapid growth.
Tumors with uncontrolled growth require large amounts of nutrition,
and thus, weaken the animals’ bodies. However, the tumor size in
the xenografts decreased gradually in the cryptotanshinone group
compared with the control group. As shown in Fig. 4B, due to the rapid growth of the
tumors and their use of nutrition, the body weight of the animals
decreased rapidly in the control group. These animals appeared to
be weak due to bearing large tumors. However, the body weight in
the cryptotanshinone group decreased less rapidly compared with the
control group due to the inhibition of tumor growth. The physical
and mental condition of the animals in the cryptotanshinone group
appeared to be improved as compared to the control group. The
present study results indicate that cryptotanshinone was not only
able to inhibit the growth of human lung cancer in vivo, but
also improve the physical and mental status of tumor-bearing
mice.
Assessment of apoptosis in xenograft
tumors
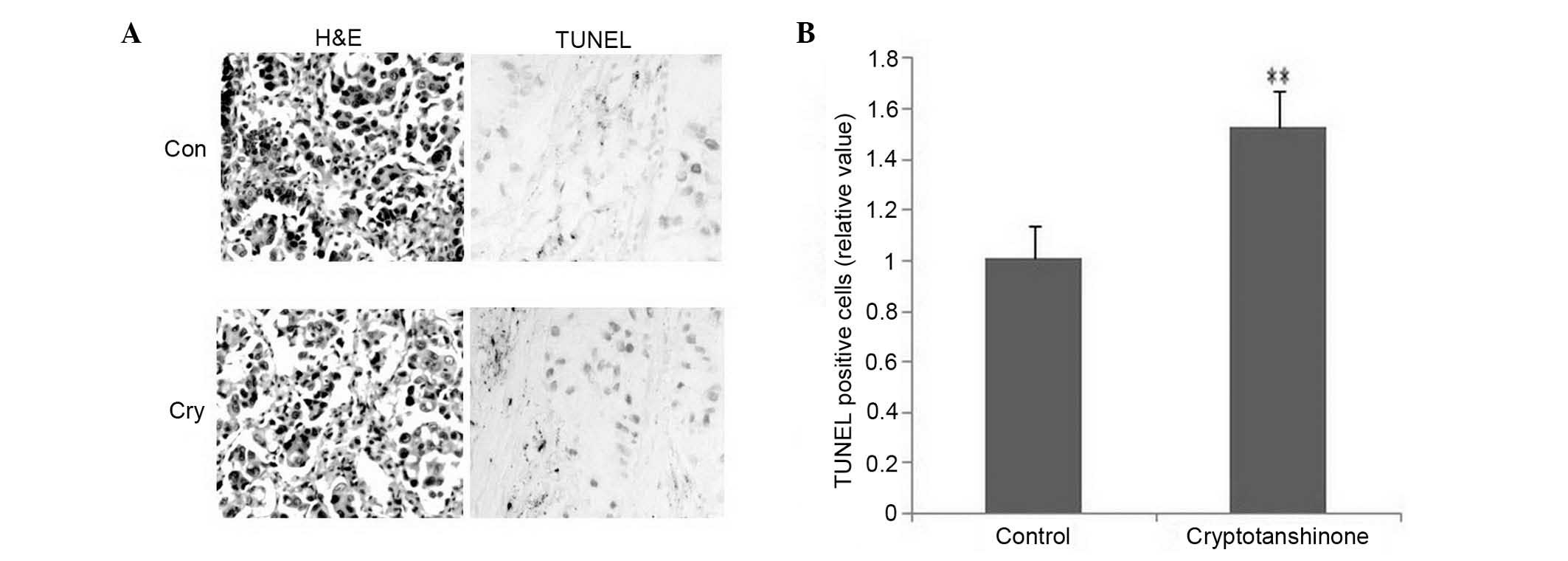
The inhibition of tumor growth was caused and
promoted by apoptosis. As shown in Fig. 5A (left), compared with the
cryptotanshinone group, a larger number of abnormal hyperplasia
with abundant cytoplasm, irregularities with pleomorphic nuclei and
crude chromatin granules were detected for epithelial cells in the
control group, accompanied by infiltration of inflammatory cells
using H&E staining for pathological detection. As shown in
Fig. 5A (right), an increased
number of apoptotic cells was detected in the cryptotanshinone
group using a TUNEL kit. These increased positive cells indicated
enhanced apoptosis following treatment with cryptotanshinone. Data
were analyzed in Fig. 5B for TUNEL
detection. IHC was used to detect apoptotic key factors in
situ. As shown in Fig. 6, p53
(nuclear staining, indicated by arrow) was increased, Bcl-2
(cytoplasmic staining, indicated by arrow) was decreased, Bax
(cytoplasmic staining, indicated by arrow) was increased, caspase 3
(nuclear staining, indicated by arrow) was increased in the
cryptotanshinone group compared with the control. The present study
indicated that cryptotanshinone was able to promote apoptosis in
human lung cancer xenografts and inhibit the growth of tumors in
vivo. The key factors involved in this process are p53,
Bax/Bcl2 and caspase 3. This result was in accordance with the
western blot analysis and supported the conclusion thereof.
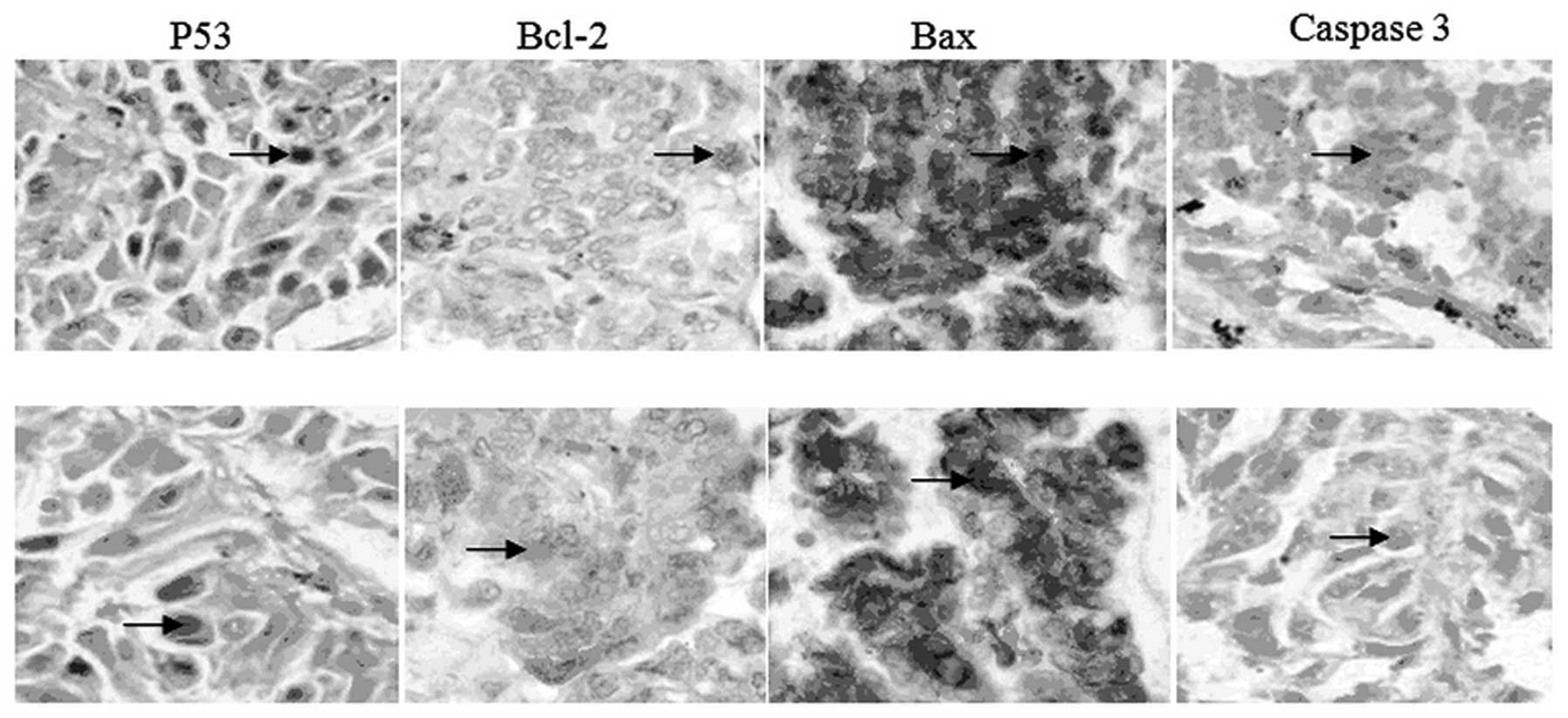 | Figure 6IHC detection for key factors of
apoptosis. P53 (nuclear staining, indicated by arrow), Bax
(cytoplasm staining, indicated by arrow) and caspase 3 (nuclear
staining, indicated by arrow) were increased, while Bcl-2
(cytoplasm staining, indicated by arrow) was decreased in the
cryptotanshinone group (Cry, lower panel) compared with the control
group (Con, upper panel). H&E, TUNEL, magnification, ×100; IHC,
magnification, ×400). IHC, immunohistochemistry; H&E,
hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase
deoxyuridine triphosphate nick end-labeling. Bcl-2, B-cell lymphoma
2; Bax; Bcl-2 associated X. |
Discussion
Lung cancer is a leading cause of cancer mortality
in China. An increasing number of people, particularly adult men,
die from lung cancer more than from any other type of cancer. Lung
cancer is caused by uncontrolled cell growth in the lung, then
spreads to nearby tissue in metastasis, and eventually, to other
parts of the body. Lung cancer includes small-cell lung carcinoma
(SCLC) and non-small-cell lung carcinoma (NSCLC). The
identification of new anti-lung cancer drugs is highly important.
Cryptotanshinone, together with tanshinones I, IIA, IIB and
dihydrotanshinone, are the most abundant constituents of the root
of Salvia miltiorrhiza, a perennial plant of the Salvia
genus, which is highly valued for its roots in traditional Chinese
Medicine. Most studies focused on the antioxidant and
anti-inflammatory effects (7) of
tanshinone I and IIA, which exert an anti-cancer effect (8). However, to the best of our knowledge,
cryptotanshinone has rarely been studied.
The growth inhibition of human lung cancer cells by
cryptotanshinone was investigated using the MTT assay and the
clonogenic survival inhibition was assessed. The results indicated
that cryptotanshinone exerted a dose-dependent cytotoxic effect on
A549 cells. Concentrations of cryptotanshinone >5 μM showed the
significant inhibition of cell growth and clonogenic survival;
however, no obvious improvement was observed >20 μM. Cell growth
and clonogenic survival are regulated by the cell cycle. The cell
cycle is a series of events leading to cell division and
duplication, which consists of four distinct phases: G1
phase, S phase (synthesis), G2 phase (interphase) and M
phase (mitosis). The cell cycle is controlled by checkpoints that
ensure the fidelity of cell divisions in eukaryotic cells (9). The first checkpoint is situated at
the end of the G1 phase and the second checkpoint at the
end of the G2 phase (10). The cell cycle analysis performed in
the present study using FCM showed that cryptotanshinone was able
to cause G2 phase arrest in A549 cells, indicated by an
increased percentage of cells in G2/M and decreased
percentage of cells in G0/G1. This triggers
the start of the M phase (mitotic phase), thus inhibiting cancer
cell growth. The cell cycle is driven by cyclin-dependent kinases
(CDK) associated with cyclins. It was then attempted to identify
the regulators of the observed G2 phase inhibition. The
expression of G2/M-associated cyclin B/CDK1 and Cdc25C
was observed, together with cyclin-dependent kinase inhibitor (CKI)
p21(CIP1/WAF1) (11). The results
indicated that following treatment with cryptotanshinone, the
expression of cyclin B1, CDK1 and Cdc25C was downregulated at the
gene and the protein level, while p21 was upregulated. Accumulation
of cyclin B increases the activity of CDK1 prior to entering
mitosis, which has a key role in the regulation of cell division.
The Cdc25 phosphatase family regulates the dephosphorylation of
cyclin B/CDK1 and triggers entry into mitosis, together with the
suppression of p53-induced growth inhibition (12). p21(CIP1/WAF1) acts as a regulator
of cell cycle progression, controlled by the tumor suppressor
protein p53. Growth arrest by p21 is able to promote cell
differentiation and death, preventing cell proliferation (13).
Xenografts are effective models for assessing the
effect of compounds on the formation and growth of tumors, and are
widely used in pre-clinical studies. The tumor size and body weight
are the most important data. In the present study, the body weight
of the nude mice was decreased in the control group due to the
rapid growth of the tumor. These animals appeared to be weak as a
portion of their nutrition was used by the tumor. Following
treatment with cryptotanshinone, the body weight of the nude mice
was decreased less rapidly than in the control, together with an
improved physical and mental condition. Without any treatment, the
tumor size increased rapidly. The uncontrolled growth of the tumor
used up the nutrition and emaciated the mice. The tumor size
decreased less rapidly in the cryptotanshinone group. The present
study indicated that cryptotanshinone was able to inhibit the
growth of human lung tumors in an in vivo model. Tumor
growth occurs due to numerous reasons, of which an important one is
the imbalance of cell proliferation and cell death pathways.
Over-expression of anti-apoptotic factors, and under-expression of
pro-apoptotic factors may lead to increased proliferation and lack
of cell death, resulting in cancer. In the present study, an
increased number of abnormal hyperplasia with abundant cytoplasm,
irregularities and pleomorphism of the nucleus, crude chromatin
granules were detected for epithelial cells in the control group,
accompanied by infiltration of inflammatory cells to a greater
extent than in the cryptotanshinone-treated group using H&E
staining for pathological detection. The present study indicated
that cryptotanshinone arrested tumor growth. Apoptosis, also known
as programmed cell death (PCD), is a series of biochemical events
leading to morphological changes, which include blebbing, cell
shrinkage, nuclear fragmentation, chromatin condensation,
chromosomal DNA fragmentation and finally cell death (14). Apoptosis of cancer cells,
controlled by a diverse range of cell signals, is considered as an
efficient method for cancer treatment (15). Numerous key regulators are involved
in apoptosis, among which p53, Bcl-2 and members of the caspase
family are most important. Bcl-2 family members share one or more
of the four characteristic domains (BH1, BH2, BH3 and BH4), and
thus form hetero- or homodimers, acting as anti-[Bcl-2, b-cell
lymphoma extra large (Bcl-xl) and Mcl1] or pro-apoptotic (Bax)
regulators (16). Following
induction of apoptosis, Bax may be involved in p53-mediated
apoptosis, and induce the opening of the mitochondrial
voltage-dependent anion channel, releasing cytochrome c and other
pro-apoptotic factors from the mitochondria (17,18),
leading to the activation of caspases, among which caspase 3 is the
key factor (19). The expression
of Bax was upregulated by the tumor suppressor protein p53. Bcl-2
had the opposite role during this process (20). The present study revealed that
cryptotanshinone enhanced the expression of p53 and Bax and
downregulated the expression of Bcl-2 both in vitro and
in vivo, which suggested that cryptotanshinone induced
apoptosis in cancer cells. Cryptotanshinone activated caspase 3
in vivo, indicating that this apoptosis may be
caspase-mediated.
In conclusion, the present study has demonstrated
that cryptotanshinone causes growth-inhibition, cell cycle arrest,
apoptosis and inhibition of tumor formation in human lung cancer
both in vitro and in vivo. These results suggest that
cryptotanshinone is a potential compound for the treatment and
prevention of human lung cancer. Future studies on the anti-cancer
effect of cryptotanshinone should be conducted.
Acknowledgements
The present study was supported by the research
program of of Wuwei Tumor Hospital Research Program and Jiangsu
Provincial Natural Science Foundation (BK20130219).
References
|
1
|
Wang JW and Wu JY: Tanshinone biosynthesis
in Salvia miltiorrhiza and production in plant tissue
cultures. Appl Microbiol Biotechnol. 88:437–449. 2010.
|
|
2
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuxian X, Feng T, Ren L and Zhengcai L:
Tanshinone II-A inhibits invasion and metastasis of human
hepatocellular carcinoma cells in vitro and in vivo. Tumori.
95:789–795. 2009.PubMed/NCBI
|
|
4
|
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ,
Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim HJ: Tanshinone I
effectively induces apoptosis in estrogen receptor-positive (MCF-7)
and estrogen receptor-negative (MDA-MB-231) breast cancer cells.
Int J Oncol. 33:485–491. 2008.PubMed/NCBI
|
|
5
|
Pan TL, Hung YC, Wang PW, Chen ST, Hsu TK,
Sintupisut N, Cheng CS and Lyu PC: Functional proteomic and
structural insights into molecular targets related to the growth
inhibitory effect of tanshinone IIA on HeLa cells. Proteomics.
10:914–929. 2010.PubMed/NCBI
|
|
6
|
Zhang Y, Yao Y, Wang H, Guo Y, Zhang H and
Chen L: Effects of salidroside on glioma formation and growth
inhibition together with improvement of tumor microenvironment.
Chin J Cancer Res. 25:520–526. 2013.PubMed/NCBI
|
|
7
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma H, Fan Q, Yu J, Xin J and Zhang C:
Novel microemulsion of tanshinone IIA, isolated from Salvia
miltiorrhiza Bunge, exerts anticancer activity through inducing
apoptosis in hepatoma cells. Am J Chin Med. 41:197–210.
2013.PubMed/NCBI
|
|
9
|
Heber-Katz E, Zhang Y, Bedelbaeva K, Song
F, Chen X and Stocum DL: Cell cycle regulation and regeneration.
Curr Top Microbiol Immunol. 367:253–276. 2013.PubMed/NCBI
|
|
10
|
Vleugel M, Hoogendoorn E, Snel B and Kops
GJ: Evolution and function of the mitotic checkpoint. Dev Cell.
23:239–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallorini M, Cataldi A and di Giacomo V:
Cyclin-dependent kinase modulators and cancer therapy. BioDrugs.
26:377–391. 2012.PubMed/NCBI
|
|
12
|
Miyazaki T and Arai S: Two distinct
controls of mitotic cdk1/cyclin B1 activity requisite for cell
growth prior to cell division. Cell Cycle. 6:1419–1425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 years after. Curr Opin Oncol. 25:52–58.
2013.PubMed/NCBI
|
|
14
|
Wlodkowic D, Skommer J and Darzynkiewicz
Z: Cytometry of apoptosis. Historical perspective and new advances.
Exp Oncol. 34:255–262. 2012.PubMed/NCBI
|
|
15
|
Rebucci M and Michiels C: Molecular
aspects of cancer cell resistance to chemotherapy. Biochem
Pharmacol. 85:1219–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barillé-Nion S, Bah N, Véquaud E and Juin
P: Regulation of cancer cell survival by BCL2 family members upon
prolonged mitotic arrest: opportunities for anticancer therapy.
Anticancer Res. 32:4225–4233. 2012.PubMed/NCBI
|
|
17
|
Kubli DA and Gustafsson ÅB: Mitochondria
and mitophagy: the yin and yang of cell death control. Circ Res.
111:1208–1221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monian P and Jiang X: Clearing the final
hurdles to mitochondrial apoptosis: regulation post cytochrome C
release. Exp Oncol. 34:185–191. 2012.PubMed/NCBI
|
|
19
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cakir E, Yilmaz A, Demirag F, Oguztuzun S,
Sahin S, Yazici UE and Aydin M: Prognostic significance of
micropapillary pattern in lung adenocarcinoma and expression of
apoptosis-related markers: caspase-3, bcl-2, and p53. APMIS.
119:574–580. 2011. View Article : Google Scholar : PubMed/NCBI
|