Introduction
The emergence of engineered cartilage tissue has
provided novel approaches for the repair of cartilage defects in
plastic and reconstructive surgery. Seeding chondrocytes onto
biodegradable scaffolds to construct three dimensional cartilage
tissue for implantation may be a highly promising strategy for the
repair of cartilage defects (1,2).
Scaffolds represent one of the three essential factors necessary
for tissue engineering and have a significant role in cartilage
regeneration. Various biodegradable polymers have been explored for
use as scaffolds for cartilage tissue engineering, including
calcium alginate gel and polyglycolic acid (3–6).
Among these, the novel polyhydroxyalkanoates (PHAs) have been shown
to be biocompatible, biodegradable and thermoplastic polyesters,
which, due to their enhanced biomechanical properties, may be ideal
for use as biomedical materials. The PHA that has attracted the
most interest is poly(3-hydroxybutyrate) (PHB). Numerous strains of
bacteria have been identified to be capable of producing PHB in
high yields. Poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV), a
copolymer containing hydroxyvalerate and PHB in varying ratios, is
a particularly useful material due to its less crystalline and more
flexible structure, and greater ease of processability than PHB
(7).
Previous studies have used PHBV as a biomaterial for
cartilage tissue engineering. In one such study, Kose et al
(8) investigated the use of
macroporous PHBV scaffolds in the repair of full-thickness
cartilage defects (side length, 4.5 mm; depth, 4 mm) in rabbits
in vivo. At 8 and 20 weeks after seeding, minimal foreign
body reaction was observed and the chondrocytes seeded onto the
PHBV matrices showed early cartilage formation. Furthermore, the
newly formed cartilage had the appearance of normal articular
cartilage (8). Lu et al
(9) confirmed the feasibility of
engineering an entire meniscal structure in a total meniscectomy
rabbit model using biodegradable PHBV scaffolds and cultured
allogeneic meniscal cells. However, PHBV is a hydrophobic
polyester. In a previous study, we revealed that the poor
hydrophilicity and mechanical strength associated with PHBV
resulted in a low percentage of cell adherence and the formation of
thin cartilage layers with poor biomechanical properties (10). Therefore, the present study aimed
to investigate the improvement of the hydrophilic characteristics
and mechanical strength of PHBV scaffolds.
Bioglass® (BG) is a bioactive inorganic
material composed of specific proportions of SiO2,
Na2O, CaO and P2O5, and its
incorporation into PHBV has previously been reported to be capable
of improving the hydrophilicity and mechanical strength of the
composites (7,11). However, few reports have shown
whether the incorporation of BG into PHBV scaffolds has potential
in cartilage tissue engineering. Therefore, in the present study it
was hypothesized that the incorporation of BG into PHBV would
generate composite scaffolds with enhanced properties for cartilage
engineering compared with scaffolds solely composed of PHBV.
To investigate this hypothesis, PHBV scaffolds and
PHBV scaffolds containing 10% BG (w/w) (PHBV/10% BG) were prepared.
Chondrocytes were seeded onto the scaffolds and cultured in
vitro for 24 h, prior to 10 weeks of in vivo
implantation to observe the formation of engineered cartilage
tissue on the different scaffolds. The extracellular matrix (ECM)
production, size, structure and biomechanical properties of the
neocartilage were analyzed to compare the structure and function of
the engineered cartilage tissue produced by the different
scaffolds.
Materials and methods
Ethics statement
All experimental procedures performed in this study
were approved by the Ethics Committee of the Shanghai Jiao Tong
University School of Medicine (Shanghai, China).
Preparation of PHBV and PHBV/10% BG
scaffolds
PHBV, (molecular weight, 300,000 Da) containing 3
mol% 3-hydroxyvalerate was obtained from Tianan Biologic Material
Co. Ltd. (Ningbo, China). A solvent casting/particulate leaching
method was used in the preparation of the PHBV and composite
PHBV10% BG scaffolds as described in a previous study (12). PHBV powder (1 g) was dissolved in
10 ml chloroform to generate a solution with a concentration of 10%
(w/v). To produce the PHBV/10% BG composite scaffolds, the solution
was supplemented with 0.11 g BG powder and stirred continuously for
2 h to ensure uniform dispersal of the powder. NaCl particles were
subsequently added to the solution as pore-generating additives.
The solution was then cast in a 60-mm diameter, 3-mm long
Teflon® mold and air-dried in a fume hood for 24 h to
allow solvent evaporation. Any remaining solvent was eliminated
through vacuum drying at 60°C for 48 h. The pore-generating
additives were then leached from the dried samples by immersion in
deionized water. Samples were subsequently subjected to further
vacuum drying to produce porous scaffolds, which were referred to
as PHBV and PHBV/10% BG scaffolds, respectively. Scaffolds were cut
into identical rectangular prisms that measured 4 mm in length and
were 3-mm thick. Additional PHBV and PHBV/10% BG films were
prepared using an identical method; however, the porogen addition
and particulate leaching processes were eliminated so that the
water contact angles could be determined. AgNO3
titration was performed to ensure complete NaCl leaching from the
scaffolds (12).
Characterization of the PHBV and
PHBV/10%BG scaffolds
Scaffold porosity was determined by measuring the
mass and dimensions of the scaffolds as described previously
(13). In order to test the
compressive strength, PHBV and PHBV/10% BG scaffolds that measured
6 mm in diameter and were 3-mm thick were prepared. The compressive
strength of the scaffolds was determined using an AG-1 Shimadzu
mechanical tester (Shimadzu Co., Kyoto, Japan), with a crosshead
speed of 0.5 mm/min.
The water absorptivity of the scaffolds was
determined as described previously (14). Dry scaffolds were weighed to obtain
the dry weight (Wdry), prior to being placed in deionized water at
room temperature for 4 h. This ensured that water absorption was
equilibrated. The hydrated scaffolds were then extracted from the
water, filter paper was utilized to remove the free surface water
and the scaffolds were weighed to obtain the wet weight (Wwet). The
water absorption ratio was calculated as follows: Water absorption
ratio (%)=(Wwet−Wdry)/Wdry × 100.
Determination of hydrophilicity
Scaffold hydrophilicity was analyzed by measuring
the water contact angles of the nonporous composite cuboids.
Contact angles were measured at 25°C using a contact angle
goniometer (model SZ10-JC2000A; Maikailun Co., Xiamen, China). The
sessile drop method was used to obtain the measurements, and five
different locations were analyzed for each specimen. A total of 0.5
μl deionized water was deposited onto the surface of the specimen
at each location. The degree of reproducibility for the different
specimens was within ±4.0°. Three specimens were tested for each
sample.
Cell isolation and construction of
engineered cartilage
Auricular cartilage was harvested from the ears of
New Zealand white rabbits (SLAC Lab. Animal Ltd., Shanghai, China),
aged between three and five days, prior to being cut into small
pieces as previously described (15). The cartilage slices were digested
using 0.2% (w/v) collagenase II to release the chondrocytes, which
were then cultured in Dulbecco’s Modified Eagle Medium (DMEM)
supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100
μg/ml streptomycin. The chondrocytes were incubated at 37°C in 5%
CO2 and the medium was changed every three days.
Following two subcultures, 30 μl chondrocyte suspension (5 ×
107 cells/ml) at passage 2 was deposited onto each
scaffold, prior to the addition of 5 ml DMEM after 4 h. After 24 h,
the constructs were harvested and subcutaneously implanted into
nude mice (SLAC Lab. Animal Ltd.). Ten weeks after implantation,
the specimens were harvested.
Cell adhesion
The PHBV and composite PHBV/10% BG substrates were
soaked in 75% ethanol for 48 h prior to overnight sterilization
using ultraviolet radiation and washing with sterile
phosphate-buffered saline (PBS, pH 7.4). Chondrocytes were then
seeded onto the sterilized substrates in a 48-well plate (density,
70 cells/mm2) and maintained in a CO2
incubator for 3 h, prior to the addition of 1 ml fresh medium to
each well. Cell viability was determined using an MTT-based
colorimetric assay and the percentage of adhered cells was
calculated in accordance with a method described previously
(16).
Gross observation of in vivo engineered
tissue
The constructs were harvested 10 weeks after
implantation and images were captured so that the side length,
volume and thickness of the constructs could be measured. At each
time-point, the volume was determined using a volumenometer, and
the side length and thickness were measured using a vernier
caliper.
Quantitative analysis of in vivo
cartilage formation
After 10 weeks in vivo culture, the Wwet,
glycosaminoglycan (GAG) (17) and
total collagen (18) content, and
mechanical strength (19) were
determined using previously described methods.
Histology evaluation
Ten weeks after implantation, representative
cartilaginous tissue formed on the PHBV and PHBV/10% BG scaffolds
was fixed in neutral buffered formalin, prior to being embedded in
paraffin and sectioned into 5-μm thick specimens. The
cross-sections were stained using hematoxylin and eosin and
Safranin-O. Immunostaining of the 10-μm cryosections was performed
with an anti-type II collagen antibody. Non-specific binding sites
were blocked by immersing the samples overnight in PBS containing
1% goat serum at 4°C. The sections were then incubated for 4 h at
25°C in PBS containing 1% bovine serum albumin (BSA) and an
anti-type II collagen antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at a dilution of 1:100. Following three washes
with PBS, samples were incubated in PBS containing 3% BSA and then
in PBS containing 1% BSA and a horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G antibody (Santa Cruz Biotechnology,
Inc.) at a dilution of 1:150 at 25°C for 4 h. Color development was
performed using diaminobenzidine tetrahydrochloride (Santa Cruz
Biotechnology, Inc.) (2).
Statistical analysis
All data are presented as the mean ± standard
deviation for n=6. Differences between the PHBV and PHBV/10% BG
scaffolds were analyzed using the Student’s t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
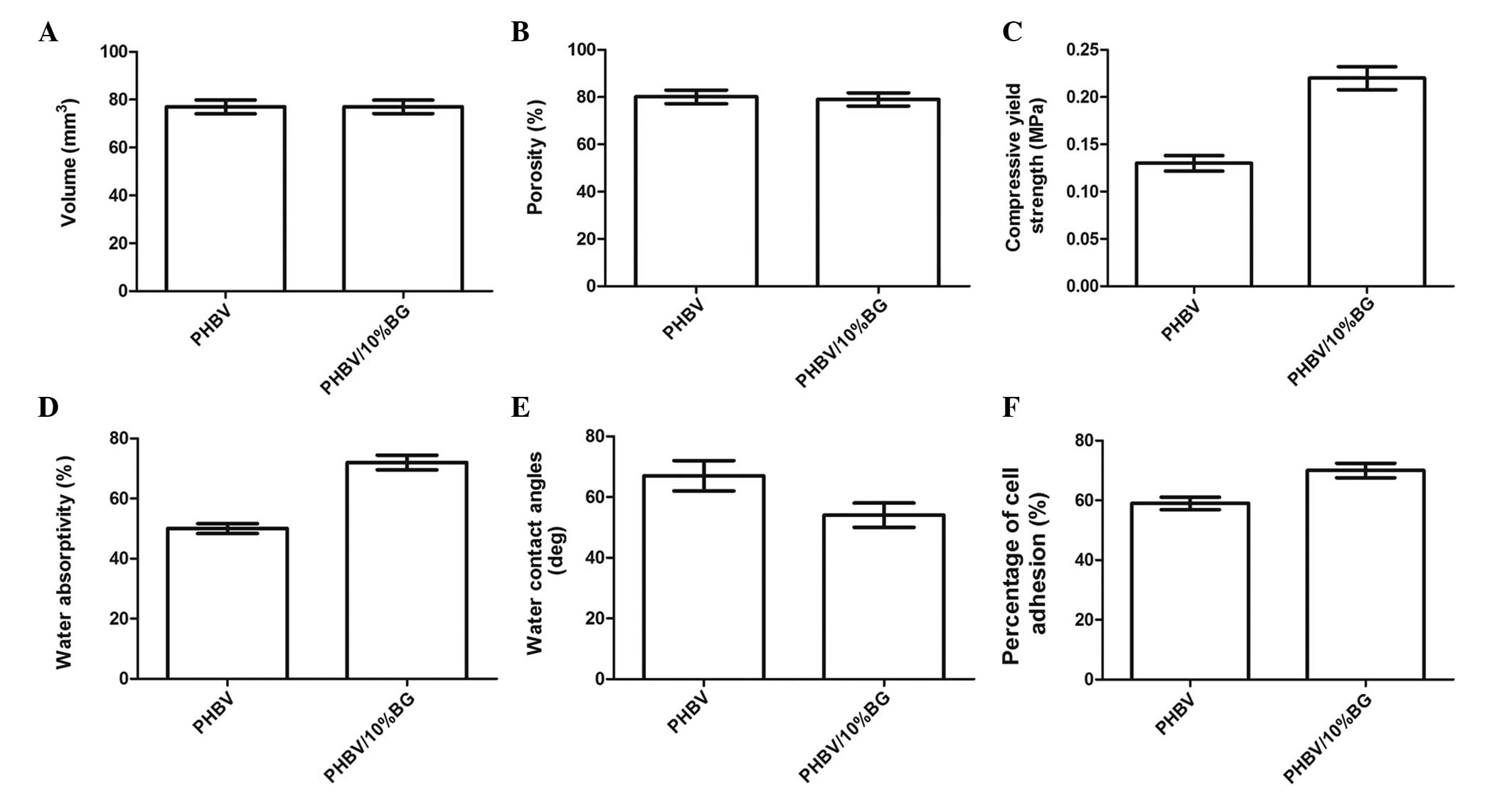
Scaffold characterization
The PHBV and PHBV/10% BG scaffolds were cut into
rectangular prisms with no significant differences in size or
volume (P>0.05) (Fig. 1A).
Furthermore, no significant difference was observed in the porosity
of the scaffolds between the PHBV and PHBV/10% BG groups
(P>0.05) (Fig. 1B). However,
with the addition of 10% BG (w/w) to the PHBV scaffolds,
compressive strength and water absorptivity were observed to
increase from 0.13±0.02 to 0.22±0.05 MPa and from 50±3 to 72±5%,
respectively (P<0.05) (Fig. 1C and
D).
Hydrophilicity and cell adhesion of
different scaffolds
As shown in Fig.
1E, the water contact angles of the PHBV/10% BG composites
(54±1.5°) were observed to be significantly lower than those of the
PHBV specimens (66±2°) (P<0.01), indicating that the addition of
BG to PHBV enhanced the surface hydrophilicity. Furthermore, the
percentage of adhered cells increased from 59±5 to 70±7% with the
addition of 10% BG (w/w) (Fig.
1F).
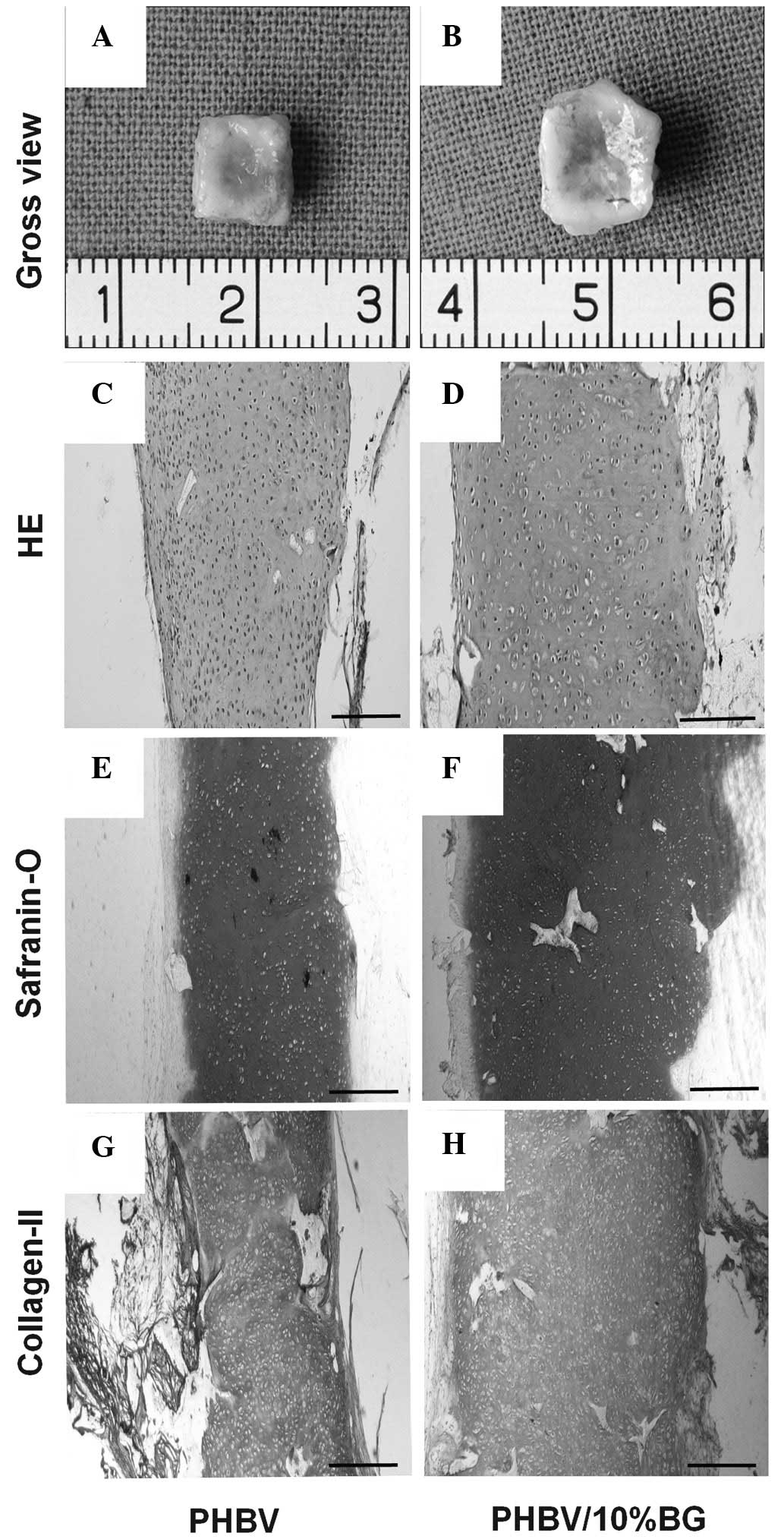
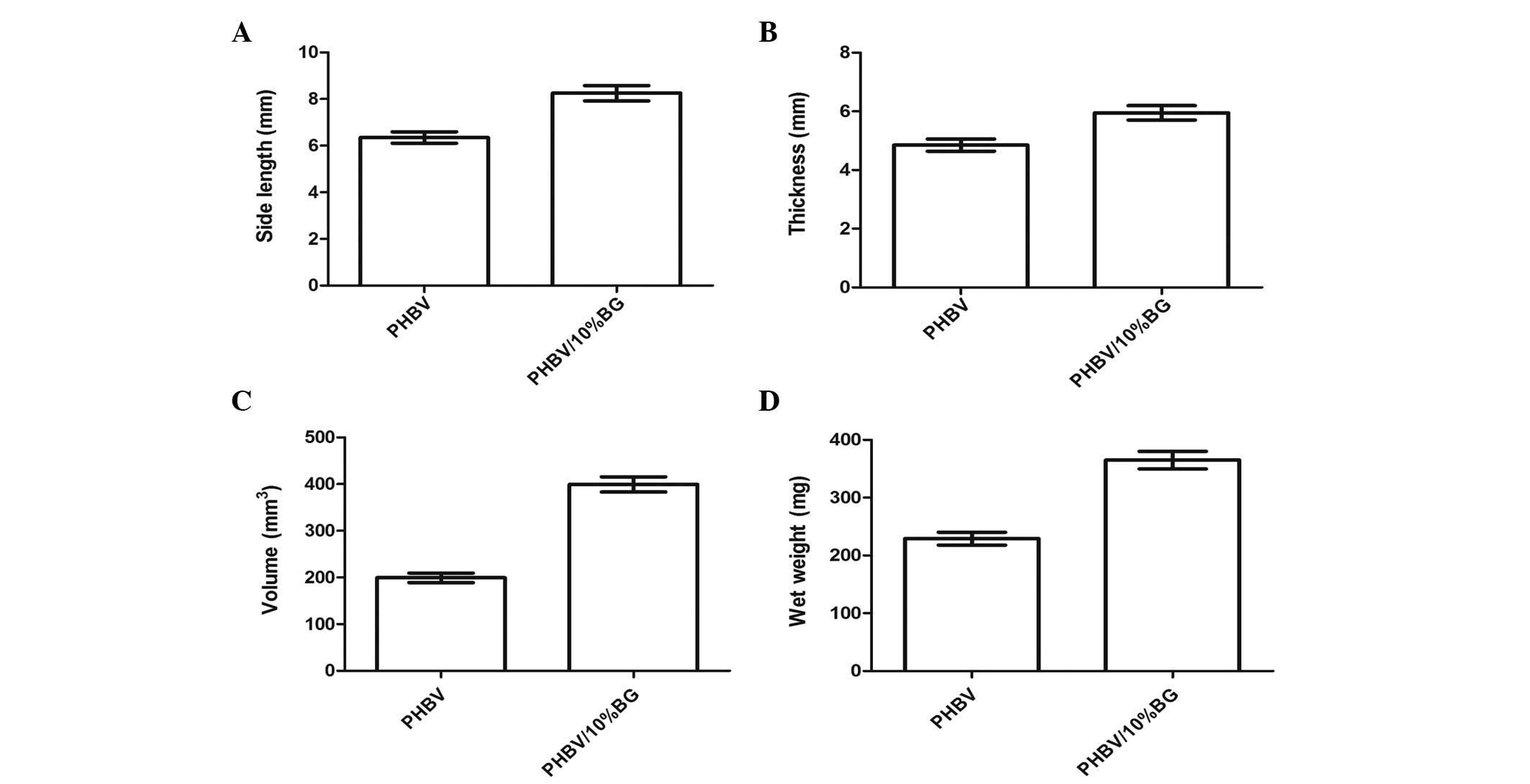
Gross evaluation of the in vivo
engineered constructs
Variations in the gross shape and size of the in
vivo engineered constructs were recorded to assess the impact
of the two different scaffolds on three dimensional cartilage
tissue formation. Following in vivo implantation, the
cell-scaffold constructs were observed to maintain their original
size in the two groups, and form an ivory-white cartilage-like
tissue (Fig. 2A and B).
Quantitative analysis revealed that in the PHBV/10% BG group, the
thickness, side length, volume and Wwet were significantly higher
than those in the PHBV group after 10 weeks in vivo
transplantation (P<0.05) (Fig.
3).
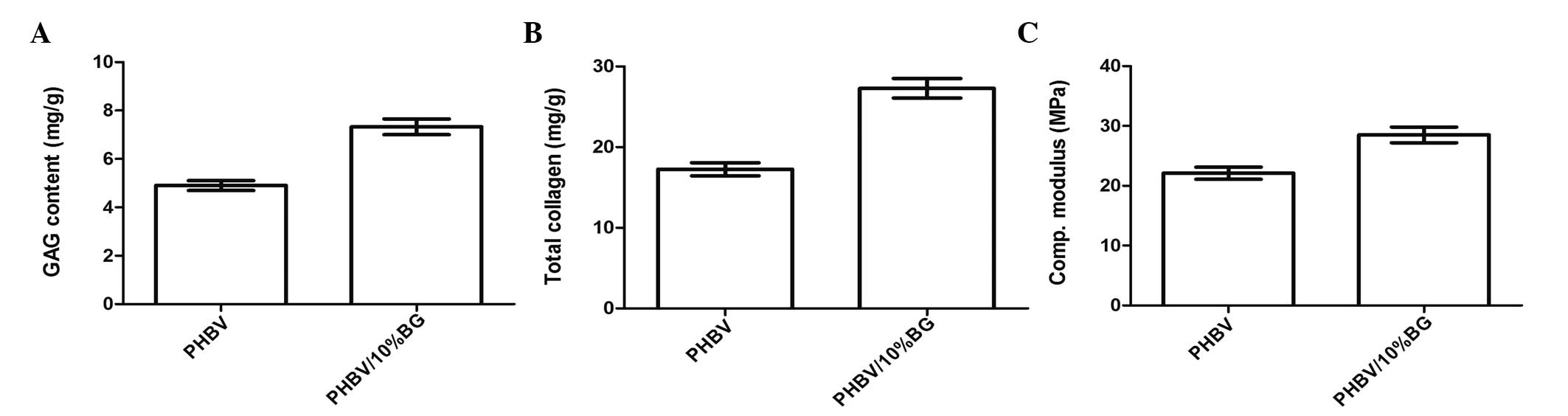
Collagen and GAG content and compressive
modulus
Quantitative analysis further revealed that there
were significant differences in the ECM and mechanical properties
between the samples of regenerated cartilage in the PHBV and
PHBV/10% BG groups (P<0.05) (Fig.
4A and B).
Histology and immunohistochemistry
The engineered tissue was subjected to histological
and immunohistochemical analysis to assess the formation of
neocartilage. Cartilage-like tissue was observed to have formed in
the cell-scaffold constructs in the PHBV and PHBV/10% BG groups;
this tissue exhibited a notable cartilage-like lacunar structure
with strong expression of type II collagen (Fig. 4). However, the full-thickness
histological and immunohistochemical staining revealed that the
cartilage-like tissue layers generated by the cell-scaffold
constructs in the PHBV/10% BG group were thicker than those
generated in the pure PHBV group (Fig.
2).
Discussion
Engineered cartilage tissue is used to repair
cartilage defects and has been suggested to be an ideal therapy for
the clinic. The basic method underlying tissue engineering is to
seed cells directly onto a biodegradable scaffold material and then
implant the cell-scaffold complex subcutaneously to construct the
required tissue (6,20). Therefore, scaffolds have a
significant role in cartilage tissue engineering. The following
properties are required for ideal scaffolds: (i) The mechanical
strength necessary for the creation of a macroporous scaffold that
retains its structure following implantation, particularly in the
reconstruction of hard, load-bearing tissues; (ii) the ability to
biodegrade at a controllable rate that approximates the rate of
tissue regeneration under the culture conditions of interest; and
(iii) histocompatibility that promotes cell-biomaterial
interactions, cell adhesion and ECM deposition (21,22).
On the scaffolds, chondrocytes should maintain their chondrogenic
phenotype and produce ECM components to eventually replace the
scaffolds (23,24). The chemistry and physicochemical
properties of the scaffolds determine whether the seeded cells are
able to grow and maintain their morphology and phenotype (25,26).
Such properties include: (i) External geometry, including macro-
and micro-structure and interconnectivity; (ii) surface properties,
including surface energy, chemistry, charge and surface area; (iii)
porosity and pore size; (iv) interface adherence and
biocompatibility; (v) degradation characteristics, including
biodegradation; and (vi) mechanical competence, including
compressive and tensile strength (22,25–27).
PHBV has demonstrated potential as a chondrocyte carrier for
cartilage engineering (7).
However, the cartilage tissue grown using PHBV matrices is not, at
present, suitable for clinical application due to the poor
hydrophilicity and mechanical strength associated with PHBV, which
results in the engineered cartilage tissue exhibiting poor
biomechanical properties. The hydrophilicity of a material has been
reported to significantly influence cell adhesion, growth and
proliferation. Improving the surface hydrophilicity of a material
may enhance its ability to interact with cells and elicit
controlled cellular adhesion, while maintaining a stable
differentiated phenotype (28,29).
Certain techniques have been reported to enhance the hydrophilicity
of PHBV, including combining PHBV with the polymer
poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide) (30), oxygen and nitrogen plasma treatment
(31) and covalently immobilizing
a water-soluble chitosan/chondroitin-6-sulfate polyelectrolyte
complex onto the surface of PHBV membranes using ozone-induced
oxidation and polyacrylic acid graft polymerization (32). Li et al (16) demonstrated that incorporating
hydrophilic inorganic materials into hydrophobic polymers may be a
feasible approach to improve the hydrophilicity of these
composites.
Previous studies have indicated that the
incorporation of bioactive glass into PHBV is capable of improving
hydrophilicity and mechanical properties (11). BG is an inorganic material not
normally present in bone or cartilage tissue; however, bioactive
glass has been utilized in tissue engineering, and studies
(33,34) have shown that this material is
capable of promoting the growth and proliferation of osteoblasts.
Bal et al (35) reported
that bioactive glass was superior to bone allografts with respect
to integrating into the adjacent host bone, regenerating
hyaline-like tissue at the graft surface and expressing type II
collagen in the articular cartilage. Therefore, in the present
study, 10% BG was incorporated into PHBV in order to generate
porous composite scaffolds for in vitro and in vivo
investigations. Hydrophilicity was observed to be significantly
enhanced in the PHBV/10% BG scaffolds compared with that of the
pure PHBV scaffolds, with the water contact angle decreasing from
66 to 54°with the addition of 10% BG (w/w). The enhanced
hydrophilicity was associated with an increase in cell adhesion
from 59 to 70%. Histological and immunohistochemical analyses of
the in vivo engineered cartilage confirmed these findings.
The cartilage-like tissue layers generated using the PHBV/10% BG
scaffolds were thicker than those formed using the pure PHBV
scaffolds, due to the higher percentage of cell adhesion in the
PHBV/10% BG group.
Ideal scaffolds require appropriate mechanical
properties; therefore, various attempts have been made to improve
the mechanical properties of PHBV scaffolds. Wang et al
(36) reported that the addition
of 5% (w/w) acetylated chitin nanocrystals to PHBV scaffolds
improved the tensile strength and Young’s modulus by 44 and 67%,
respectively, compared with improvements of 24 and 43% in
PHBV/chitin nanocrystal composites. Furthermore, it has been
reported that combining PHBV with Ecoflex may improve the
mechanical properties of PHBV and thereby promote its application
in tissue engineering (37). The
incorporation of bioactive inorganic materials into PHBV has also
been reported to improve the mechanical strength of the scaffold
(16). In the present study, the
addition of BG into PHBV scaffolds was observed to generate
scaffolds with enhanced mechanical properties and an increased
capacity for cartilage formation. In addition, the composite
scaffolds produced from the incorporation of 10% (w/w) BG into PHBV
exhibited an increase in compressive yield strength, from 0.13 to
0.22 MPa. This suggests that BG may have a strengthening effect on
PHBV scaffolds; however, the mechanism by which this is achieved is
yet to be elucidated.
The compressive modulus of the cell-scaffold
constructs in the PHBV/10% BG group in the present study was
observed to be significantly higher than that in the pure PHBV
group. This may be a consequence of the enhanced mechanical
strength associated with the PHBV/10% BG scaffolds compared with
that in the PHBV scaffolds, or the thicker cartilage-like tissue
formed with the PHBV/10% BG scaffolds and the fact that the
improved mechanical properties of the neocartilage tissue are
determined by the content of the ECM. Studies have revealed that
improvements in mechanical strength may be partially elicited by
the homogeneous structure and the ECM content, specifically with
regard to the GAG and total collagen content (38–40).
This is consistent with the findings from the analysis of the GAG
and total collagen content in the present study. It was observed in
the present study that the GAG and total collagen content was
significantly higher in the PHBV/10% BG group than that in the PHBV
group, which contributed to the improved mechanical properties of
the neocartilage tissue.
In conclusion, the present study has demonstrated
that composite PHBV/10% BG scaffolds exhibit improved
hydrophilicity and mechanical properties and also form neocartilage
with enhanced biochemical and biomechanical properties. Although
the specific mechanism by which this is achieved is yet to be
elucidated, the incorporation of BG into PHBV may be beneficial for
the generation of composite scaffolds with enhanced properties for
cartilage engineering compared with pure PHBV scaffolds.
Acknowledgements
The authors would like to thank Professor Li Huang
(Department of Plastic and Reconstructive Surgery, Tongji Hospital
Affiliated to Tongji Medical College of Huazhong University of
Science and Technology, China) for the help and support provided.
This research was supported by the National Natural Science
Foundation of China (81272129).
References
|
1
|
Vacanti CA, Langer R, Schloo B and Vacanti
JP: Synthetic polymers seeded with chondrocytes provide a template
for new cartilage formation. Plast Reconstr Surg. 88:753–759. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xue K, Zhu Y, Zhang Y, Chiang C, Zhou G
and Liu K: Xenogeneic chondrocytes promote stable subcutaneous
chondrogenesis of bone marrow-derived stromal cells. Int J Mol Med.
29:146–152. 2012.PubMed/NCBI
|
|
3
|
Zhu S, Zhang T, Sun C, Yu A, Qi B and
Cheng H: Bone marrow mesenchymal stem cells combined with calcium
alginate gel modified by hTGF-β1 for the construction of
tissue-engineered cartilage in three-dimensional conditions. Exp
Ther Med. 5:95–101. 2013.PubMed/NCBI
|
|
4
|
Gu Y, Zhu W, Hao Y, Lu L, Chen Y and Wang
Y: Repair of meniscal defect using an induced myoblast-loaded
polyglycolic acid mesh in a canine model. Exp Ther Med. 3:293–298.
2012.PubMed/NCBI
|
|
5
|
Kuhne M, John T, El-Sayed K, et al:
Characterization of auricular chondrocytes and auricular/articular
chondrocyte co-cultures in terms of an application in articular
cartilage repair. Int J Mol Med. 25:701–708. 2010.PubMed/NCBI
|
|
6
|
Schlegel W, Nürnberger S, Hombauer M,
Albrecht C, Vécsei V and Marlovits S: Scaffold-dependent
differentiation of human articular chondrocytes. Int J Mol Med.
22:691–699. 2008.PubMed/NCBI
|
|
7
|
Sun J, Wu J, Li H and Chang J: Macroporous
poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for cartilage
tissue engineering. Eur Polym J. 41:2443–2449. 2005. View Article : Google Scholar
|
|
8
|
Köse GT, Korkusuz F, Ozkul A, et al:
Tissue engineered cartilage on collagen and PHBV matrices.
Biomaterials. 26:5187–5197. 2005.PubMed/NCBI
|
|
9
|
Lu HD, Cai DZ, Wu G, Wang K and Shi DH:
Whole meniscus regeneration using polymer scaffolds loaded with
fibrochondrocytes. Chin J Traumatol. 14:195–204. 2011.PubMed/NCBI
|
|
10
|
Wu J, Xue K, Li H, Sun J and Liu K:
Improvement of PHBV scaffolds with Bioglass for cartilage tissue
engineering. PLoS One. 8:e715632013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Du R and Chang J: Fabrication,
characterization, and in vitro degradation of composite scaffolds
based on PHBV and bioactive glass. J Biomater Appl. 20:137–155.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H and Chang J: Fabrication and
characterization of bioactive wollastonite/PHBV composite
scaffolds. Biomaterials. 25:5473–5480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Q, Grijpma DW and Feijen J: Porous
polymeric structures for tissue engineering prepared by a
coagulation, compression moulding and salt leaching technique.
Biomaterials. 24:1937–1947. 2003. View Article : Google Scholar
|
|
14
|
Zheng X, Yang F, Wang S, et al:
Fabrication and cell affinity of biomimetic structured
PLGA/articular cartilage ECM composite scaffold. J Mater Sci Mater
Med. 22:693–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitney GA, Mera H, Weidenbecher M,
Awadallah A, Mansour JM and Dennis JE: Methods for producing
scaffold-free engineered cartilage sheets from auricular and
articular chondrocyte cell sources and attachment to porous
tantalum. Biores Open Access. 1:157–165. 2012.PubMed/NCBI
|
|
16
|
Li H, Zhai W and Chang J: In vitro
biocompatibility assessment of PHBV/Wollastonite composites. J
Mater Sci Mater Med. 19:67–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enobakhare BO, Bader DL and Lee DA:
Quantification of sulfated glycosaminoglycans in
chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene
blue. Anal Biochem. 243:189–191. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reddy GK and Enwemeka CS: A simplified
method for the analysis of hydroxyproline in biological tissues.
Clin Biochem. 29:225–229. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carey J, Small CF and Pichora DR: In situ
compressive properties of the glenoid labrum. J Biomed Mater Res.
51:711–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Leong KF, Du Z and Chua CK: The
design of scaffolds for use in tissue engineering. Part I
Traditional factors. Tissue Eng. 7:679–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dhandayuthapani B, Yoshida Y, Maekawa T
and Kumar DS: Polymeric scaffolds in tissue engineering
application: A review. Int J of Polym Sci. 2011:1–19. 2011.
View Article : Google Scholar
|
|
23
|
Surrao DC, Khan AA, McGregor AJ, Amsden BG
and Waldman SD: Can microcarrier-expanded chondrocytes synthesize
cartilaginous tissue in vitro? Tissue Eng Part A. 17:1959–1967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schuh E, Kramer J, Rohwedel J, et al:
Effect of matrix elasticity on the maintenance of the chondrogenic
phenotype. Tissue Eng Part A. 16:1281–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hambleton J, Schwartz Z, Khare A, et al:
Culture surfaces coated with various implant materials affect
chondrocyte growth and metabolism. J Orthop Res. 12:542–552. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyan BD, Hummert TW, Dean DD and Schwartz
Z: Role of material surfaces in regulating bone and cartilage cell
response. Biomaterials. 17:137–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gugala Z and Gogolewski S:
Differentiation, growth and activity of rat bone marrow stromal
cells on resorbable poly(L/DL-lactide) membranes. Biomaterials.
25:2299–2307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lydon MJ, Minett TW and Tighe BJ: Cellular
interactions with synthetic polymer surfaces in culture.
Biomaterials. 6:396–402. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Wachem PB, Beugeling T, Feijen J,
Bantjes A, Detmers JP and van Aken WG: Interaction of cultured
human endothelial cells with polymeric surfaces of different
wettabilities. Biomaterials. 6:403–408. 1985.PubMed/NCBI
|
|
30
|
Li X, Liu KL, Wang M, et al: Improving
hydrophilicity, mechanical properties and biocompatibility of
poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through
blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide).
Acta Biomater. 5:2002–2012. 2009.
|
|
31
|
Wang Y, Lu L, Zheng Y and Chen X:
Improvement in hydrophilicity of PHBV films by plasma treatment. J
Biomed Mater Res A. 76:589–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu DG, Lin WC, Lin CH and Yang MC:
Cytocompatibility and antibacterial activity of a PHBV membrane
with surface-immobilized water-soluble chitosan and
chondroitin-6-sulfate. Macromol Biosci. 6:348–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Köse GT, Korkusuz F, Korkusuz P, Purali N,
Ozkul A and Hasirci V: Bone generation on PHBV matrices: an in
vitro study. Biomaterials. 24:4999–5007. 2003.PubMed/NCBI
|
|
34
|
Köse GT, Korkusuz F, Korkusuz P and
Hasirci V: In vivo tissue engineering of bone using
poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) and collagen
scaffolds. Tissue Eng. 10:1234–1250. 2004.PubMed/NCBI
|
|
35
|
Bal BS, Rahaman MN, Jayabalan P, et al: In
vivo outcomes of tissue-engineered osteochondral grafts. J Biomed
Mater Res B Appl Biomater. 93:164–174. 2010.PubMed/NCBI
|
|
36
|
Wang B, Li J, Zhang J, et al:
Thermo-mechanical properties of the composite made of poly
(3-hydroxybutyrate-co-3-hydroxyvalerate) and acetylated chitin
nanocrystals. Carbohydr Polym. 95:100–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang A, Gan Y, Yu H, et al: Improvement of
the cytocompatibility of electrospun
poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] mats by
Ecoflex. J Biomed Mater Res A. 100:1505–1511. 2012.PubMed/NCBI
|
|
38
|
Rieppo J, Töyräs J, Nieminen MT, et al:
Structure-function relationships in enzymatically modified
articular cartilage. Cells Tissues Organs. 175:121–132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mikic B, Isenstein AL and Chhabra A:
Mechanical modulation of cartilage structure and function during
embryogenesis in the chick. Ann Biomed Eng. 32:18–25. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bastiaansen-Jenniskens YM, Koevoet W, de
Bart AC, et al: Contribution of collagen network features to
functional properties of engineered cartilage. Osteoarthritis
Cartilage. 16:359–366. 2008. View Article : Google Scholar : PubMed/NCBI
|