Introduction
Melanogenesis is the process of melanin synthesis
and delivery through various enzymes (1). In particular, tyrosinase,
tyrosinase-related protein 1 (TRP1), and tyrosinase related protein
2 (TRP2) are key enzymes in melanin synthesis (2,3). The
melanin synthesizing enzymes transfer L-tyrosine to melanin though
a multistep transformation (4).
Microphthalmia-associated transcription factor (MITF)
transcriptionally regulates these key enzymes in melanocytes
(5). Therefore, the regulation of
MITF is central for hyper- or hypo-pigmentation diseases and for
skin whitening for cosmetic purposes (6,7).
Centella asiatica is a medicinal plant widely
used in South Asia. C. asiatica extracts have therapeutic
applications, particularly in neuroprotection and wound healing
(8–11). In addition, its extracts are widely
used for the treatment of inflammatory skin disorders, including
leprosy, lupus, varicose ulcers, eczema, atopic dermatitis and
psoriasis (12). The C.
asiatica extract is mainly administered as a titrated extract.
Titrated extract of C. asiatica (TECA) contains asiatic
acid, asiaticoside and madecassic acid (12,13).
In dermatology, these components are used to achieve preventive and
therapeutic effects. Asiaticoside prevents ultraviolet A-dependent
photoaging by suppressing ultraviolet A-induced reactive oxygen
species production (14). In
addition, asiatic acid, madecassic acid and asiaticoside induce
collagen I synthesis (15).
Recently, Saraf et al (16) indicated that a cosmetic formulation
containing C. asiatica may possess de-melanogenic potential
(16). However, the mechanism
behind the activity of C. asiatica in melanocytes has yet to
be elucidated. Therefore, a detailed understanding of the changes
in melanogenesis is important for elucidating the mechanism of
asiaticoside-dependent hypopigmentation. The present study aimed to
determine how C. asiatica, particularly asiaticoside,
affects melanogenesis.
Materials and methods
Materials and cell culture
TECA was obtained from Bayer (Leverkusen, Germany).
Asiatic acid, madecassic acid, asiaticoside and
1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione (IBMX) were
purchased from Sigma Aldrich (St. Louis, MO, USA). B16F10 cells
were purchased from Korea Cell Line Bank (Seoul, Korea) and were
cultured in Dulbecco’s modified Eagle’s medium (Sigma Aldrich)
supplemented with 10% fetal bovine serum (Sigma Aldrich) and 1%
penicillin-streptomycin (Gibco®, Life Technologies,
Carlsbad, CA, USA). Cultures were maintained at 37°C with 5%
CO2.
Viability assays
The B16F10 cells were seeded in 96-well plates at a
density of 4×103 cells per well. The cells were cultured
with TECA, asiatic acid, madecassic acid or asiaticoside. After 24
or 48 h, the cells were incubated with 0.5 mg/ml MTT for 2 h.
Formazan crystals were dissolved in dimethyl sulfoxide. The mean
absorbance at 405 nm was assessed using an EL800 Absorbance
Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Assessment of melanin content
The melanin content was assessed using the method
previously described by Hosoi et al with several
modifications (17). The B16F10
cells were seeded at a density of 2×105 cells in 60-mm
culture dishes. The cells were cultured with TECA, asiatic acid,
madecassic acid or asiaticoside. Following incubation, the
melanocytes were washed in phosphate-buffered saline and lysed in 1
N NaOH. The mean absorbance at 405 nm was detected using an EL800
Absorbance Microplate Reader (Molecular Devices).
Western blot analysis
The B16F10 cells were cultured with TECA, asiatic
acid, madecassic acid or asiaticoside. Following incubation, the
cells were harvested and lysed in radioimmunoprecipitation assay
buffer [50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5%
deoxycholic acid and 0.1% sodium dodecyl sulfate]. The total
protein concentration of the cell lysates was determined using a
protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal
amounts of protein were separated with 12 or 10% SDS-PAGE and
transferred to nitrocellulose membranes (Whatman, Dassel, Germany).
The membrane was blocked in 5% skimmed milk. Anti-tyrosinase goat
polyclonal IgG, anti-TRP1 goat polyclonal IgG, anti-MITF rabbit
monoclonal IgG and anti-β-actin mouse monoclonal IgG antibodies
were used to detect specific proteins and were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The membranes were
visualized using SuperSignal West Pico (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and Las-4000 (Fujifilm, Minato-ku, Japan).
Quantitative polymerase chain reaction
(qPCR)
Total RNA from the B16F10 cells was isolated using
RiboEX (Geneall, Seoul, Korea) according to the manufacturer’s
instructions. cDNA was synthesized using reverse transcriptase
(Bioneer Corp., Daejeon, Korea) and used as template for the PCR.
The PCR mixtures contained template, 0.5 μM primers and HOT FIREPol
Eva Green® qPCR Mix Plus (Solis Biodyne, Tartu, Estonia)
from a SYBR green I-based qPCR kit. Reactions were run in LinegeneK
(BioER, Hangzhou, China) using the following program: 5 min
denaturation at 95°C, followed by 40 cycles of 15 sec denaturation
at 95°C, 15 sec annealing at 58°C and 15 sec polymerization at
72°C. The sequences of the primers used were as follows: Tyrosinase
forward, 5′-ACACACTGGAAGGATTTGCC-3′ and reverse,
5′-GAGCGGTATGAAAGGAACCA-3′; and β-actin forward,
5′-CGACAGGATGCAGAAGGAG-3′ and reverse, 5′-ACATCTGCTGGAAGGTGGA-3′.
The relative quantity of all samples was calculated using a serial
dilution standard curve.
Chromatin immunoprecipitation (ChIP)
Following treatment with TECA and TECA components,
the cells were washed in ice-cold phosphate-buffered saline and
cross-linked for 15 min at 25°C with 1% formaldehyde. The
cross-linked cells were harvested and resuspended in ChIP lysis
buffer [50 mM HEPES (pH 7.5), 140 mM NaCl, 1% Triton ×100]. The
cells were sonicated and immunoprecipitated with the anti-MITF
antibody (Santa Cruz Biotechnology, Inc.). Following
immunoprecipitation, cross-linked proteins were degraded using
protease K, and DNA was collected using the PCR purification kit
(Geneall). The tyrosinase promoter site was detected using PCR. The
following primer sequences for the tyrosinase promoter were used:
Forward, 5′-AGTCATGTGCTTTGCAGAAGAT-3′ and reverse,
5′-CAGCCAAGAACATTTTCTCCTT-3′.
Results
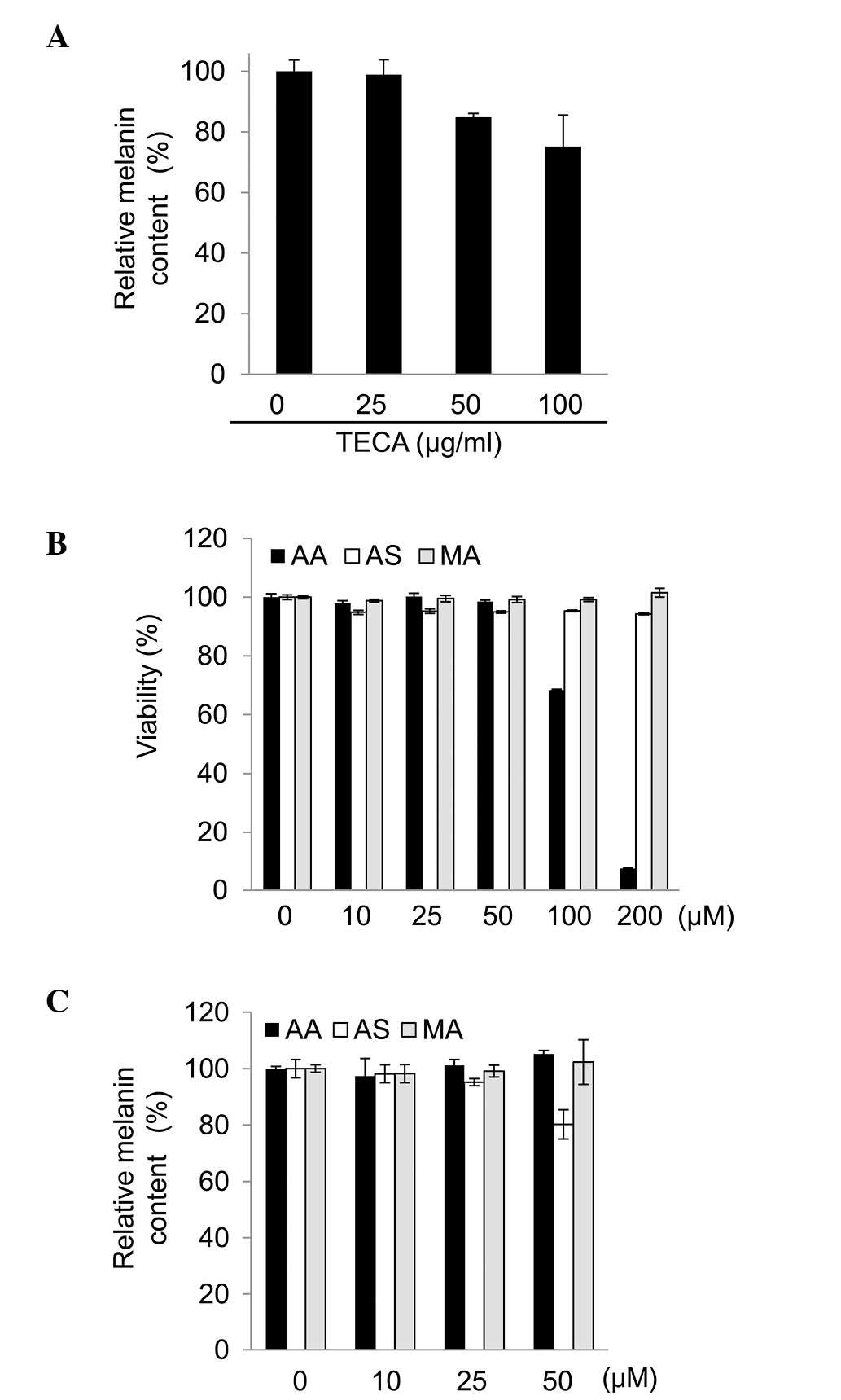
TECA represses melanogenesis in B16F10
cells
To examine whether TECA possesses cytotoxic
activity, the B16F10 cells were treated with TECA at various
concentrations (0, 10, 25, 50, 100 and 200 μg/ml) for 48 h. After
48 h, no significant changes were observed in B16F10 cell viability
with 10 to 100 μg/ml TECA (data not shown). Therefore, <100
μg/ml TECA was used in the following experiments. As shown in
Fig. 1A, TECA decreased the
melanin content in the B16F10 cells in a concentration-dependent
manner.
Asiaticoside, the major component of
TECA, represses melanogenesis in B16F10 cells
As melanogenesis was repressed by TECA, the present
study also investigated which of its components contributed to the
repression of this process. The cytotoxic effects of the major
components of TECA (asiaticoside, asiatic acid and madecassic acid)
were assessed in the B16F10 cells using MTT assays. The
dose-response curves to the TECA components in the B16F10 cells are
shown in Fig. 1. Asiaticoside and
madecassic acid (0–200 μM) had no effect on cell viability
(Fig. 1B). However, asiatic acid
induced cytotoxicity at concentrations of >100 μM in the B16F10
cells. Therefore, the melanin content was assessed following
incubation with 50 μM asiatic acid. Under non-toxic conditions, the
melanin content was decreased by asiaticoside, but not by asiatic
acid or madecassic acid (Fig. 1C).
As tyrosinase has a significant role in melanogenesis, the effects
of asiaticoside on tyrosinase protein and mRNA expression in the
B16F10 cells were assessed. Fig. 2A
and B shows that asiaticoside suppressed tyrosinase mRNA and
protein expression in the B16F10 cells.
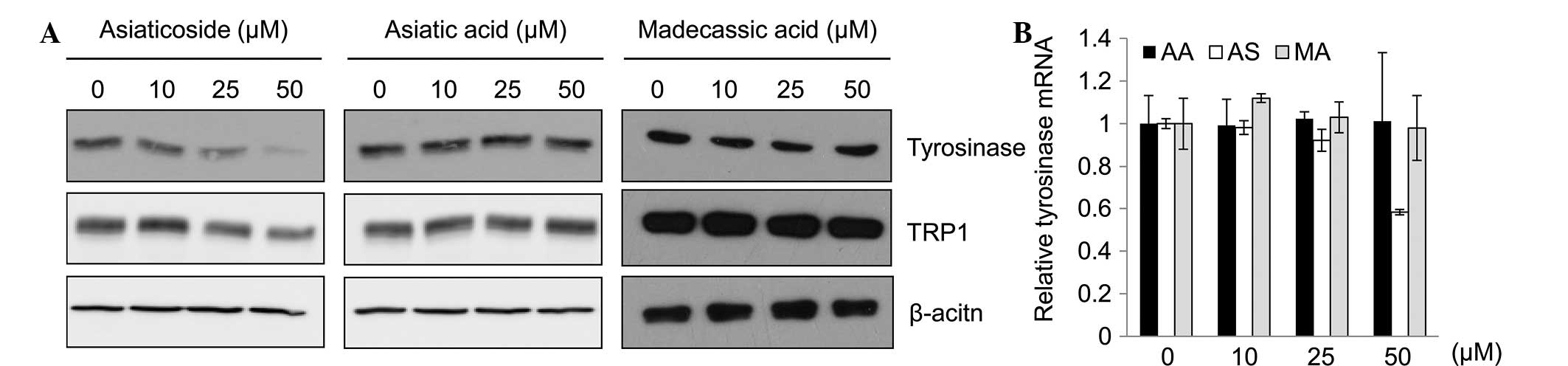 | Figure 2Effect of asiaticoside on tyrosinase
expression. B16F10 cells were incubated with the indicated
concentrations of TECA or TECA components (asiatic acid,
asiaticoside and madecassic acid). (A) Following treatment with
asiatic acid, asiaticoside and madecassic acid, the expression
levels of tyrosinase, TRP1, and TRP2 were analyzed using SYBR Green
I-based quantitative polymerase chain reaction (qPCR) assays. (B)
The protein levels of tyrosinase were analyzed by western blot
analysis. β-actin was used as a loading control. TECA, titrated
extract of Centella asiatica; AA, asiatic acid; AS,
asiaticoside; MA, madecassic acid; TRP, tyrosinase-related
protein. |
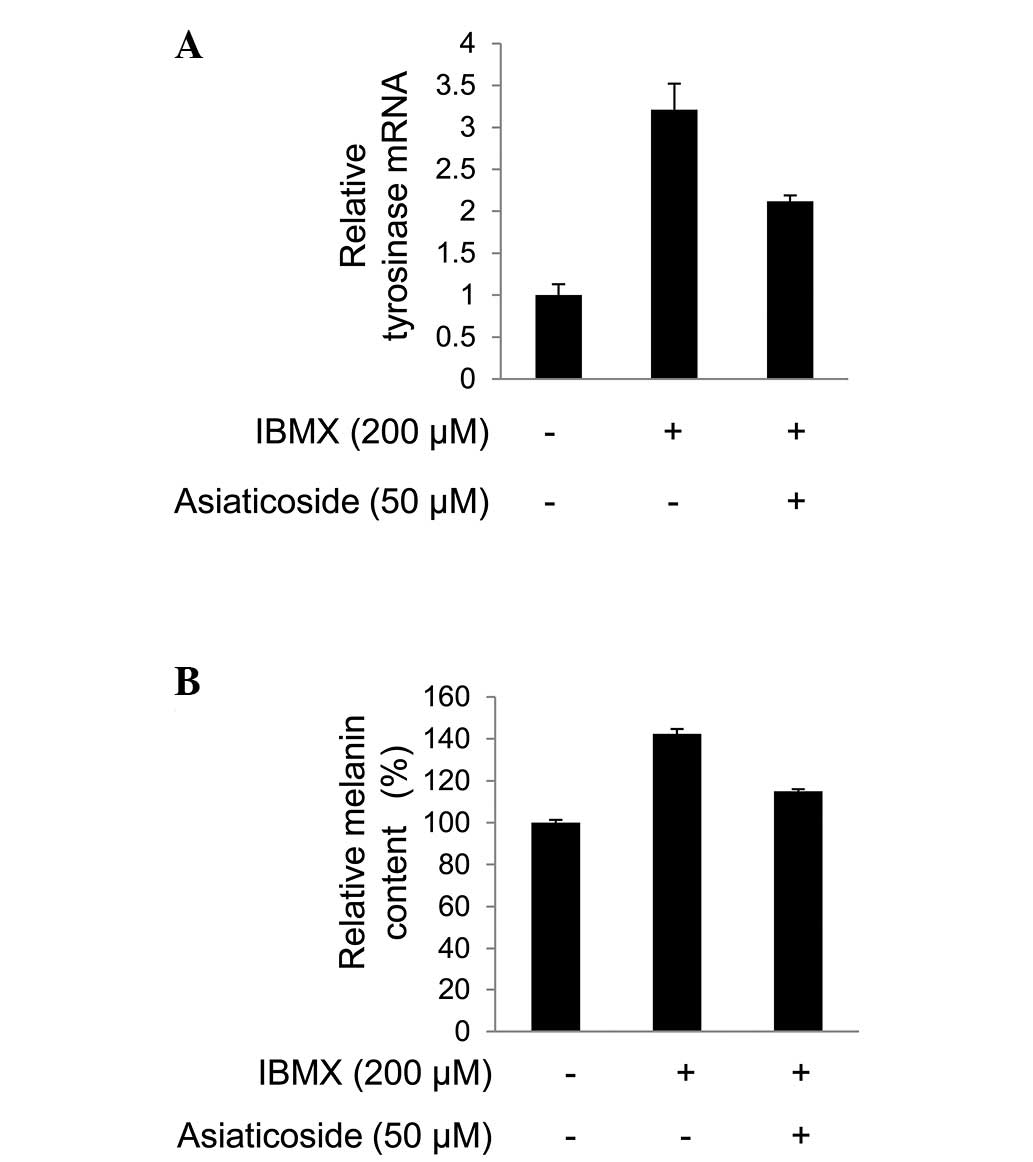
In addition, asiaticoside repressed the
melanogenesis induced by IBMX by reducing the level of tyrosinase
mRNA (Fig. 3A). Consistent with
this, the melanin content was decreased by asiaticoside in the
B16F10 cells undergoing IBMX-induced melanogenesis (Fig. 3B). The decrease in tyrosinase mRNA
is common in MITF-repressed melanocytes, as MITF transcriptionally
regulates tyrosinase (5).
Furthermore, IBMX, a cyclic adenosine monophosphate (cAMP)
phosphodiesterase inhibitor, induces the level of expression in
MITF target genes by increasing the level of cAMP (18). Therefore, MITF activity was
assessed in the present study.
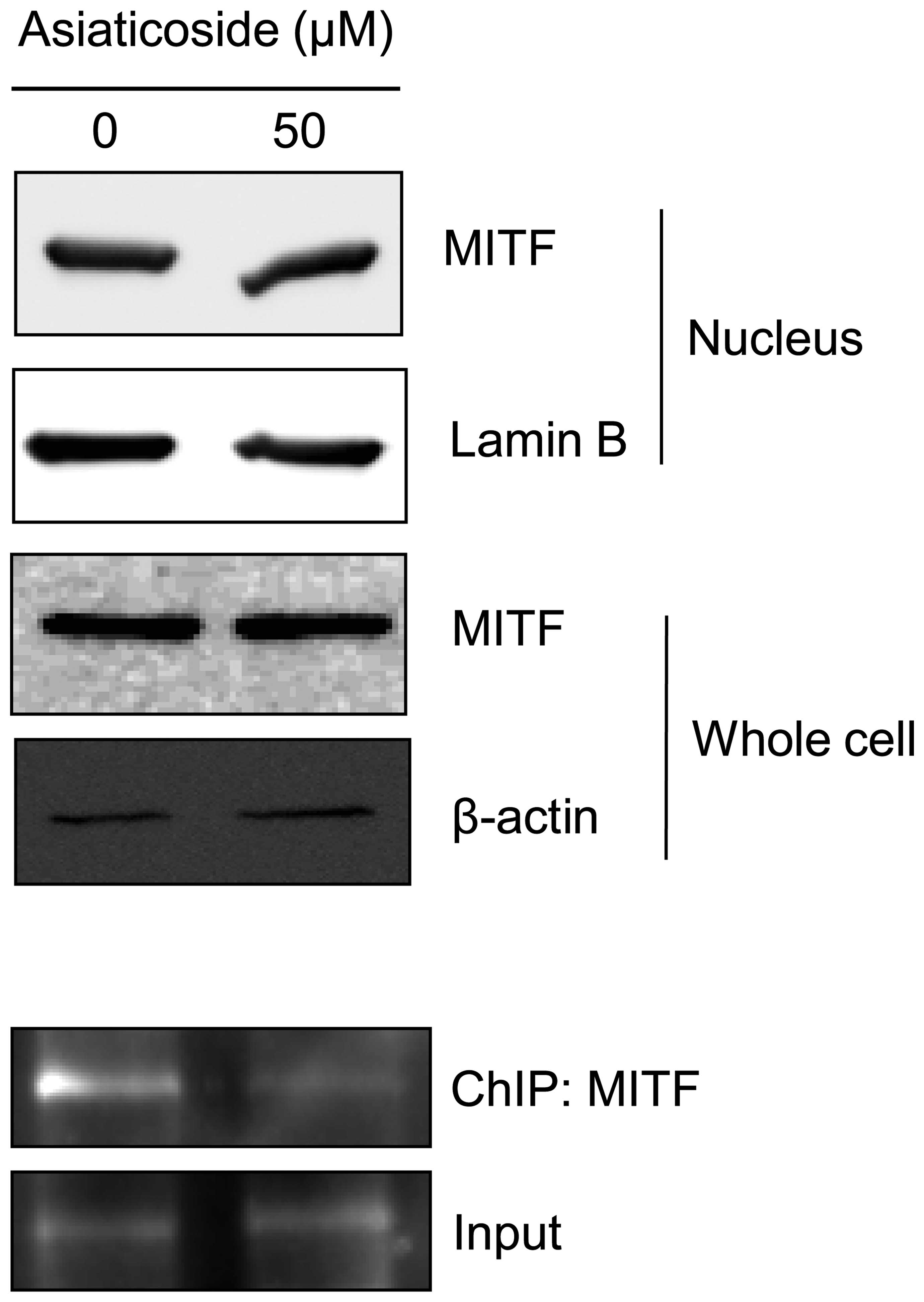
Asiaticoside decreases tyrosinase mRNA
expression by repressing the DNA binding affinity of MITF
As described in the introduction, MITF is a key
regulator of melanogenesis and a possible target for the treatment
of hyperpigmentation diseases (6,7).
Therefore, in the present study, it was hypothesized that the
asiaticoside-dependent inhibition of melanogenesis could be
regulated by MITF transactivation activity. To determine whether
asiaticoside regulates MITF, the translocation and protein
expression of MITF in asiaticoside-treated B16F10 cells was
monitored. Asiaticoside did not regulate MITF expression in the
whole cell lysates. In addition, the nuclear translocation of MITF
was unchanged following incubation with asiaticoside. However,
asiaticoside treatment altered the DNA binding affinity of MITF in
the B16F10 cells (Fig. 4).
Discussion
C. asiatica extracts are used for treating
psoriasis and wounds of the skin (19,20).
C. asiatica extracts promote wound healing through
production of collagen (21). In a
previous study, Saraf et al (16) reported that hydroalcoholic extracts
of Curcuma caesia (rhizome), Areca catechu (seeds),
Centella asiatica (leaves), Cinnamon zeylanicum
(dried bark), and Tamarindus indica (fruit pulp) reduce
melanin content, as measured by mexameter. However, the study
indicated that herbal extracts enhance photoprotection by
increasing the sun protection factor value (16). Therefore, the present study
evaluated the effects of C. asiatica extracts on
melanogenesis in melanocytes.
As shown in Fig. 1,
TECA decreased the melanin content in the B16F10 cells in a
concentration-dependent manner. Melanin is produced by a multi-step
metabolic conversion of L-tyrosine to melanin (22). The metabolic synthesis of melanin
is performed by enzymes such as tyrosinase, TRP1 and TRP2 (23). Tyrosinase is the major enzyme
involved in the melanin metabolic pathway (23). In the present study, asiaticoside
inhibited melanogenesis by the inhibition of tyrosinase protein and
mRNA expression (Fig. 2A and B).
In IBMX-induced melanogenesis, tyrosinase transcription is
regulated by MITF (24).
Therefore, to determine the effect of asiaticoside on MITF, the
expression levels, translocation and DNA binding affinity of MITF
protein were assessed in the present study. Notably, as shown
Fig. 4, asiaticoside inhibits
melanogenesis by interfering with the DNA binding of MITF.
Similarly, Um et al (25)
reported that
{1-[2-(4-chloro-phenoxy)-ethyl]-1H-benzoimidazol-2-ylsulfanyl}-acetic
acid inhibits melanogenesis by interfering with direct MITF binding
to E-box DNA. In addition, MITF DNA binding is inhibited by PIAS3,
a protein inhibitor of activated signal transduction and activator
of transcription 3 (26).
In conclusion, these results indicate that TECA, and
in particular, asiaticoside, inhibited melanogenesis by regulating
the DNA binding affinity of MITF. Furthermore, the melanin content
was reduced by decreasing the level of expression in tyrosinase and
MITF target genes. Therefore, the present study revealed that
asiaticoside is a novel candidate for melanogenesis inhibition
through repression of DNA binding to MITF.
Acknowledgements
The present study was supported by the Konkuk
University research support program.
References
|
1
|
Park HY, Kosmadaki M, Yaar M and Gilchrest
BA: Cellular mechanisms regulating human melanogenesis. Cell Mol
Life Sci. 66:1493–1506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsukamoto K, Jackson IJ, Urabe K, Montague
PM and Hearing VJ: A second tyrosinase-related protein, TRP-2, is a
melanogenic enzyme termed DOPAchrome tautomerase. EMBO J.
11:519–526. 1992.PubMed/NCBI
|
|
3
|
Negroiu G, Dwek RA and Petrescu SM:
Tyrosinase-related protein-2 and -1 are trafficked on distinct
routes in B16 melanoma cells. Biochem Biophys Res Commun.
328:914–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vachtenheim J and Borovanský J:
‘Transcription physiology’ of pigment formation in melanocytes:
central role of MITF. Exp Dermatol. 19:617–627. 2010.
|
|
6
|
Wan P, Hu Y and He L: Regulation of
melanocyte pivotal transcription factor MITF by some other
transcription factors. Mol Cell Biochem. 354:241–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi X, Zhao G, Zhang H, et al: MITF-siRNA
formulation is a safe and effective therapy for human melasma. Mol
Ther. 19:362–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacKay D and Miller AL: Nutritional
support for wound healing. Altern Med Rev. 8:359–377. 2003.
|
|
9
|
Brinkhaus B, Lindner M, Schuppan D and
Hahn EG: Chemical, pharmacological and clinical profile of the East
Asian medical plant Centella asiatica. Phytomedicine.
7:427–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
da Rocha MD, Viegas FP, Campos HC,
Nicastro PC, Fossaluzza PC, Fraga CA, Barreiro EJ and Viegas C Jr:
The role of natural products in the discovery of new drug
candidates for the treatment of neurodegenerative disorders II:
Alzheimer’s disease. CNS Neurol Disord Drug Targets. 10:251–270.
2011.PubMed/NCBI
|
|
11
|
Defillipo PP, Raposo AH, Fedoce AG,
Ferreira AS, Polonini HC, Gattaz WF and Raposo NR: Inhibition of
cPLA2 and sPLA2 activities in primary cultures of rat cortical
neurons by Centella asiatica water extract. Nat Prod Commun.
7:841–843. 2012.PubMed/NCBI
|
|
12
|
Belcaro G, Maquart FX, Scoccianti M,
Dugall M, Hosoi M, Cesarone MR, Luzzi R, Cornelli U, Ledda A and
Feragalli B: TECA (Titrated Extract of Centella asiatica):
new microcirculatory, biomolecular, and vascular application in
preventive and clinical medicine. A status paper. Panminerva Med.
53(3 Suppl 1): 105–118. 2011.
|
|
13
|
Gohil KJ, Patel JA and Gajjar AK:
Pharmacological review on Centella asiatica: a potential
herbal cure-all. Indian J Pharm Sci. 72:546–556. 2010.
|
|
14
|
Soo Lee Y, Jin DQ, Beak SM, Lee ES and Kim
JA: Inhibition of ultraviolet-A-modulated signaling pathways by
asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur J
Pharmacol. 476:173–178. 2003.PubMed/NCBI
|
|
15
|
Bonte F, Dumas M, Chaudagne C and Meybeck
A: Influence of asiatic acid, madecassic acid, and asiaticoside on
human collagen I synthesis. Planta Med. 60:133–135. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saraf S, Chhabra SK, Kaur CD and Saraf S:
Development of photochemoprotective herbs containing cosmetic
formulations for improving skin properties. J Cosmet Sci.
63:119–131. 2012.PubMed/NCBI
|
|
17
|
Hosoi J, Abe E, Suda T and Kuroki T:
Regulation of melanin synthesis of B16 mouse melanoma cells by 1
alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res.
45:1474–1478. 1985.PubMed/NCBI
|
|
18
|
Essayan DM: Cyclic nucleotide
phosphodiesterases. J Allergy Clin Immunol. 108:671–680. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tenni R, Zanaboni G, De Agostini MP, Rossi
A, Bendotti C and Cetta G: Effect of the triterpenoid fraction of
Centella asiatica on macromolecules of the connective matrix
in human skin fibroblast cultures. Ital J Biochem. 37:69–77.
1988.
|
|
20
|
Maquart FX, Chastang F, Simeon A,
Birembaut P, Gillery P and Wegrowski Y: Triterpenes from
Centella asiatica stimulate extracellular matrix
accumulation in rat experimental wounds. Eur J Dermatol. 9:289–296.
1999.
|
|
21
|
Suguna L, Sivakumar P and Chandrakasan G:
Effects of Centella asiatica extract on dermal wound healing
in rats. Indian J Exp Biol. 34:1208–1211. 1996.
|
|
22
|
Letellier S, Garnier JP, Spy J and
Bousquet B: Determination of the L-DOPA/L-tyrosine ratio in human
plasma by high-performance liquid chromatography. Usefulness as a
marker in metastatic malignant melanoma. J Chromatogr B Biomed Sci
Appl. 696:9–17. 1997. View Article : Google Scholar
|
|
23
|
Ando H, Kondoh H, Ichihashi M and Hearing
VJ: Approaches to identify inhibitors of melanin biosynthesis via
the quality control of tyrosinase. J Invest Dermatol. 127:751–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SY, Jin ML, Kim YH, Kim Y and Lee SJ:
Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by
inactivating CREB and MITF signaling pathways. Arch Dermatol Res.
303:737–744. 2011.
|
|
25
|
Um JM, Kim HJ, Lee Y, Choi CH, Hoang
Nguyen D, Lee HB, Shin JH, Tai No K and Kim EK: A small molecule
inhibitor of Mitf-E-box DNA binding and its depigmenting effect in
melan-a cells. J Eur Acad Dermatol Venereol. 26:1291–1297. 2012.
View Article : Google Scholar
|
|
26
|
Levy C, Nechushtan H and Razin E: A new
role for the STAT3 inhibitor, PIAS3: a repressor of microphthalmia
transcription factor. J Biol Chem. 277:1962–1966. 2012. View Article : Google Scholar : PubMed/NCBI
|