Introduction
Stroke is a life-threatening disease with a high
incidence, high disability and high mortality rate, causing heavy
economic burden to society and families. Cerebral infarction
accounts for 80% of the total number of strokes (1). In the pathophysiological process of
cerebral infarction, the recovery of the peripheral blood supply is
an important factor for nerve cell prognosis (2). The clinical treatments for cerebral
infarction, include thrombolysis, anticoagulation, blood pressure
regulation and microcirculation improvement. The improvement and
increase of blood supply in ischemic brain tissue, particularly in
the ischemic penumbra, thereby improving energy metabolism of
ischemic brain cells and saving neurons is one of the fundamental
purposes and fundamental approaches for the treatment of cerebral
ischemia.
In recent years, a series of studies have revealed
that good collateral circulation is able to reduce the infarct
volume and reduce the risk of cerebral infarction recurrence
(3,4). Clinical studies have provided
preliminary evidence that promoting collateral circulation is able
to lead to an improved clinical prognosis (5). Cerebral collateral circulation is
blood flow which arrives in the ischemic area through other vessels
(collateral or the newly formed vascular anastomosis) when arteries
in the brain suffer severe stenosis or occlusion. Therefore,
angiogenesis, as an effective method to improve the blood supply to
the brain, is key for improving the prognosis of patients with
stroke, and has become a major focus of study in recent years.
Therapeutic angiogenesis provides a new therapeutic strategy for
revascularization in ischemic stroke (6).
A previous study has demonstrated that a variety of
microRNAs (miRNAs) are involved in the pathophysiological process
of angiogenesis (7). Since the
number of miRNAs is large and the understanding of their roles is
unclear, the role of miRNAs in the regulation of angiogenesis
requires further study and in depth discussion.
Therefore, the present study aimed to examine the
role of miRNA-376b-5p in angiogenesis and the mechanisms mediated
by the hypoxia-inducible factor-1 α (HIF-1α)-vascular endothelial
growth factor (VEGF)-Notch1 pathway in cerebral ischemia. To the
best of our knowledge, the present study provides the first
demonstration that miR-376b-5p is involved in cerebral ischemia
injury and angiogenesis, and also elucidates the regulatory pathway
involved.
Materials and methods
Permanent middle cerebral artery
occlusion (pMCAO) model establishment
This study was approved by the Ethics Committee of
Xiangya Medical College, Central South University (Changsha,
China). All procedures involving animals were performed in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals (NIH Publication no. 86-23, revised 1986). A pMCAO model
was performed according to a study by Longa et al, however,
with certain modifications (8).
The Sprague-Dawley rats were provided by the Experimental Animal
Center of Central South University (Changsha, China). Briefly, 48
male adult Sprague-Dawley rats aged 6–8 weeks old and weighing
250–320 g were anesthetized by intraperitoneal injection of 10%
chloral hydrate (4 ml/kg; Xiangya Hospital of Central South
University). Then, the animals were placed in the supine position
and, through a 2-cm midline incision in the neck, the right common
carotid artery was exposed. Under an operating microscope, the
right external carotid artery was isolated and coagulated distal to
the bifurcation. A 4-0 nylon suture with a round tip was inserted
into the internal carotid artery through the external carotid
artery stump until mild resistance was felt to occlude the origin
of the middle cerebral artery. Then the wound was closed. Sham
surgery animals were anaesthetized and were subjected to artery
isolation without MCAO. Neurological function was evaluated
following surgery by Longa’s score (8). Animals with scores of 1–3 were
included. Following the duration of ischemia for 1, 3 and 7 days,
rats in the MCAO group were anesthetized and perfused
transcardially with sodium chloride followed by 4%
paraformaldehyde. Decapitation was performed to remove the brains
quickly.
TTC staining
Male adult Sprague-Dawley rats were anesthetized by
intraperitoneal injection of 10% chloral hydrate (4 ml/kg) and
decapitation was performed to remove the brains quickly. The brains
were refrigerated at −80°C for 5 min and then cut into sections of
~2 mm thickness. The brain slices were added to 2% TTC (Sigma, St.
Louis, MO, USA) at 37°C for 20 min and then stirred to stain
completely. Following staining, the brain slices were fixed in 4%
paraformaldehyde solution for 24 h.
Immunohistochemistry
The brains were fixed in 4% paraformaldehyde for 24
h prior to being embedded in paraffin. For immunohistochemistry,
serial sections (4 μm) were mounted on slides to dewax and
rehydrate. Following antigen retrieval under high pressure in
citrate buffer, goat serum (ZSGB-BIO Company, Beijing, China) was
used to inhibit nonspecific staining and 3% hydrogen peroxide was
added to inhibit endogenous peroxidase activity. The slides were
incubated with primary antibodies [chicken polyclonal to von
Willebrand factor (vWF) antibody; Abcam, Cambridge, MA, USA, 1:200]
at 37°C for 1 h followed by incubation with a secondary antibody
[goat anti-chicken IgY (HRP), Abcam]. The horseradish peroxidase
reaction was detected using the DAB staining kit (Maixin, Fuzhou,
Fujian, China) according to the manufacturer’s instructions. The
slides were counterstained with hematoxylin and eosin. All the
slides were visualized under a microscope (E200; Nikon, Tokyo,
Japan).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from American Type Culture Collection (Manassas, VA,
USA). The cells in the normal group were maintained in DMEM
(Gibco-BRL, Grand Island, NY, USA), supplemented with 10% newborn
calf serum (Invitrogen Life Technologies, Carlsbad, CA, USA) at
37°C in a humidified 5% CO2, 95% O2
atmosphere. The medium was adjusted every 2–3 days and the cells
were routinely split into two cultures after 4 days. The cells in
the hypoxia group were maintained in DMEM without serum at 37°C in
a humidified 5% CO2, 94% N2, 1% O2
atmosphere.
Transfection
Prior to transfection of the miR-376b-5p
mimic/inhibitor and HIF-1α shRNA, a pre-experiment was performed to
select the most effective HIF-1α shRNA fragment. HUVECs were
transfected using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer’s instructions. After 6 h, the cells
were washed and maintained in the cultures for at least 24 h for
further analysis.
MTT assay
HUVECs were seeded into 96-well plates and allowed
to grow for the appropriate times, and then 10 μl of MTT solution
(0.5 mg/ml; Beyotime, Shanghai, China) was added to each well and
incubated at 37°C for 4 h. DMSO (150 μl; Sigma) was added and
incubated for 15 min to dissolve the formazan crystals. The
absorbance was measured at 570 nm by a microplate reader (ELx800NB;
BioTek Instruments, Inc., Winooski, VT, USA).
Tube formation assay
The tube formation assay was performed using HUVECs
on Matrigel. BD Matrigel matrix (200 μl; BD Biosciences, Franklin
Lakes, NJ, USA) was added to 24-well plates on ice. Then, it was
incubated at 37°C in 5% CO2 for 30 min to allow gel
formation. HUVECs were digested and the cells were suspended at a
density of 4×105/ml. Following gel solidification for 30
min, 50 μl of cell suspension was added to each well and the cell
culture medium was compensated to 1 ml. The plates were incubated
at 37°C in 5% CO2. Following incubation for 2, 4 and 8
h, tube formation was observed using an inverted microscope (TS100;
Nikon). Image-Pro Plus 5.0 (BD Biosciences) software was used and
10 different visual fields were selected to analyze the tube
lengths.
Transwell migration assay
The cell migration experiments were performed using
a 6-well Transwell system (8 μm; Corning, Inc., Corning, NY, USA).
The cells were digested, following washing with phosphate-buffered
saline, and counted and resuspended in media without serum to
obtain a cell density of 5×104/ml. Cell media with fetal
bovine serum (1 ml; Invitrogen Life Technologies) was added to the
lower chamber and 2 ml of cell suspension was added to the
Transwell plates. Following incubation for 12, 24 and 48 h, the
upper chamber was fixed in 95% ethanol for 15 min and stained with
hematoxylin for 10 min. The cells were counted under the TS100
microscope.
Quantitative polymerase chain reaction
(qPCR)
Total RNAs were isolated from cerebral infarction or
HUVECs using TRIzol reagent (Invitrogen Life Technologies).
According to the manufacturer’s instructions, 2 μg of total RNA was
used for reverse transcription using a cDNA synthesis kit
(RevertAid™ First Strand cDNA Synthesis kit; Fermentas, Vilnius,
Lithuania). The samples were quantified by qPCR in a 7300 Sequence
Detection system (Applied Biosystems, Foster City, CA, USA) using
an SYBR-Green PCR kit (Applied Biosystems). The relative expression
level was calculated by the comparative CT method. The sequences of
the amplified miRNA transcripts were as follows: rno-miR-376b-5p,
5′-GUGGAUAUUCCUUCUAUGGUUA-′3; hsa-miR-376b-5p,
5′-CGUGGAUAUUCCUUCUAUGUUU-3′.
Western blot analysis
The protein samples were loaded onto SDS-PAGE
(Beyotime) and separated by electrophoresis. Then, proteins were
transferred onto a PVDF membrane (EMD Millipore Corporation,
Billerica, MA, USA) and incubated in 1% bovine serum albumin
(Amresco Inc., Solon, OH, USA) at 37°C for 1 h. The blots were
probed with specific primary antibodies (rabbit polyclonal to
Notch1 and rabbit polyclonal to VEGFA, Abcam; rabbit monoclonal to
HIF-1α, EMD Millipore Corporation; mouse monoclonal to GAPDH, Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and then the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody at 37°C for 1 h. The signals were visualized
using a chemiluminescence-based detection system (ECL Western
Blotting kit; Pierce Biotechnology, Inc., Rockford, IL, USA).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPPS, Inc., Chicago, IL, USA). Student’s t-test was used
for analysis. Data are representative of at least three independent
experiments and are presented as the means ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
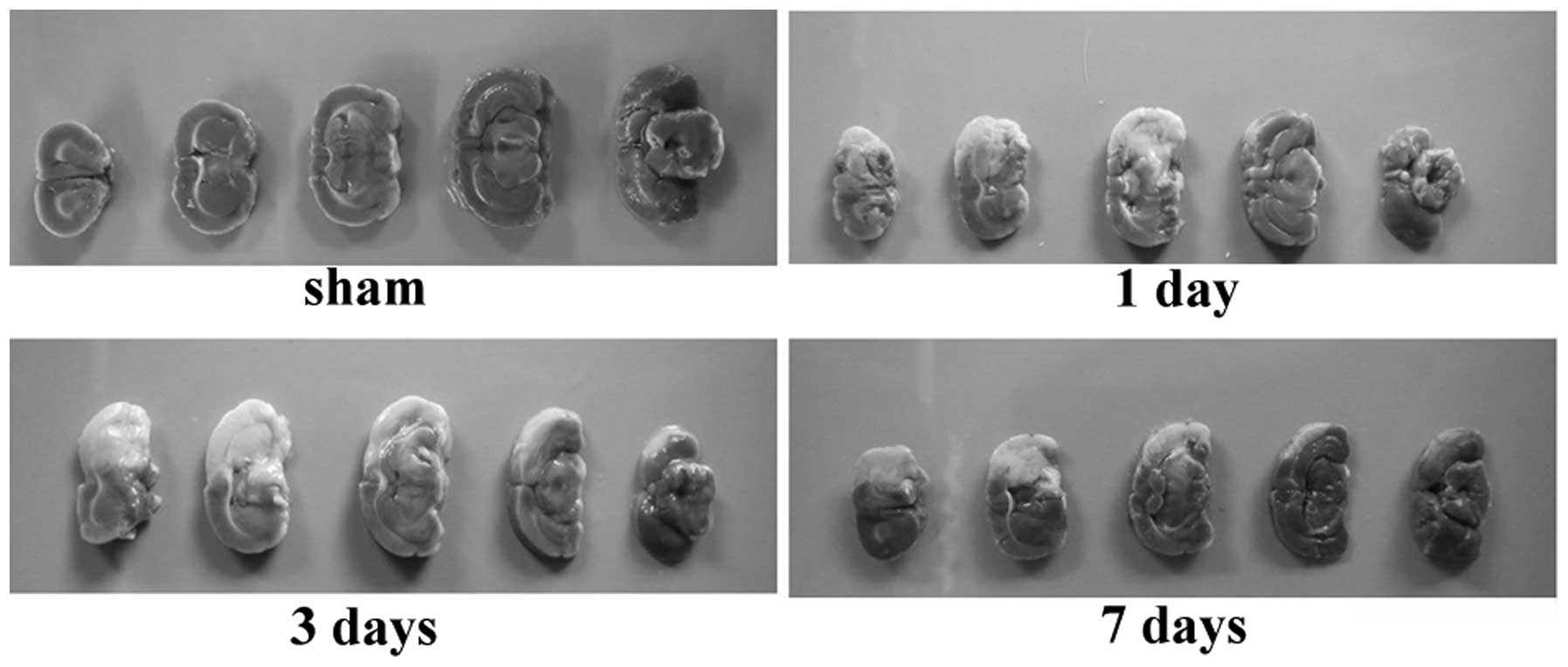
TTC staining certifies that the pMCAO
model was established successfully
As shown in Fig. 1,
TTC staining demonstrated that in the pMCAO groups, the infarction
area was white and the normal brain tissue was red (dark grey in
Fig. 1). The infarction scope in
the cerebral tissue was consistent with the blood-supply region
controlled by the middle cerebral artery. The brain tissue in the
sham group was uniformly red. The pMCAO model was established
successfully.
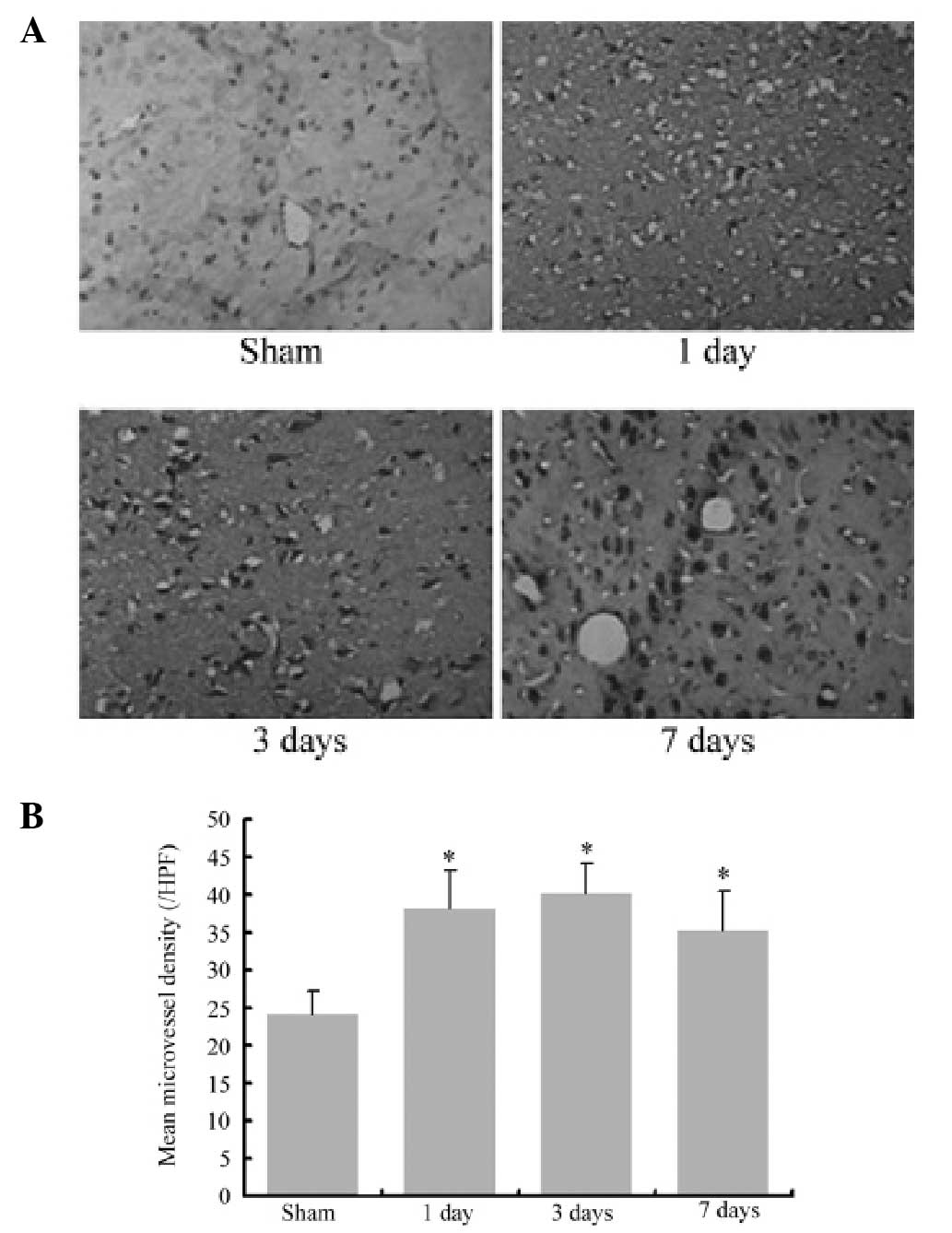
vWF expression increases following
MCAO
vWF immunohistochemistry images of cerebral
infarction following MCAO are shown in Fig. 2. vWF-positive cells were confined
to the endothelial cells in microvessels of the infarction area.
The results demonstrated that the vWF-positive cell number
significantly increased following MCAO compared with the sham group
(P<0.05). These data suggested that angiogenesis was induced
following cerebral ischemia.
miR-376b-5p is downregulated following
MCAO
To examine the expression changes of miR-376b-5p in
cerebral ischemia, qPCR was used to analyze the relative mRNA
expression level of miRNA-376b-5p following MCAO. The relative mRNA
level in the sham group was designated as 1 and it was revealed
that miRNA-376b-5p was gradually downregulated 1 day (0.79±0.03)
after MCAO (P<0.05) and then decreased rapidly to the minimum 7
days (0.06±0.01) after MCAO (P<0.01). Therefore, miRNA-376b-5p
appears to be involved in cerebral ischemia.
Alterations in the mRNA and protein level
of HIF-1α, VEGFA and Notch1 following MCAO
To investigate the basis for angiogenesis following
cerebral ischemia, the present study examined the relative
expression of HIF-1α, VEGFA and Notch1 at the mRNA and protein
level following MCAO. As shown in Fig.
3, qPCR and western blotting results indicated that the mRNA
and protein expression level in the sham group was low, and it was
significantly increased from baseline 1 day after MCAO (P<0.05)
and continued to increase up to 7 days (P<0.01). In addition,
the results demonstrated that the alterations in VEGFA and Notch1
at the mRNA and protein level were significantly increased from 1
day after MCAO (P<0.05) and remained at high levels until 7 days
(P<0.01). These data indicated that HIF-1α, VEGFA and Notch1 are
involved in angiogenesis following cerebral ischemia.
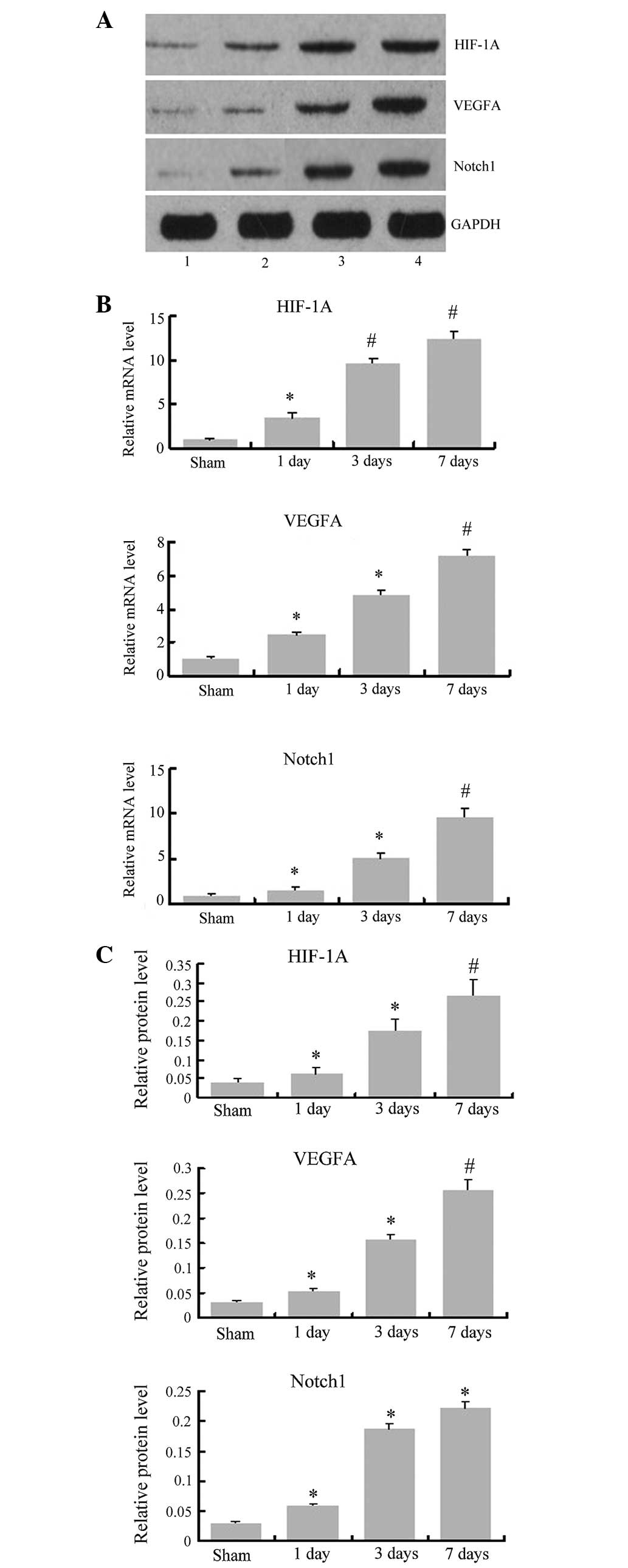 | Figure 3Changes in HIF-1α, VEGFA and Notch1
at the mRNA and protein levels following MCAO. (A) Representative
images of western blot analysis for HIF-1α, VEGFA, Notch1 and GAPDH
at the indicated time points following MCAO. 1, sham; 2, MCAO 1
day; 3, MCAO 3 days; 4, MCAO 7 days. (B) Relative mRNA levels for
HIF-1α, VEGFA and Notch1, normalized to β-actin. (C) Relative
protein expression levels for HIF-1α, VEGFA and Notch1, normalized
to GAPDH. *P<0.05, compared with the sham group.
#P<0.01, compared with the sham group. MCAO, middle
cerebral artery occlusion; HIF-1α, hypoxia-inducible factor-1 α;
VEGFA, vascular endothelial growth factor A. |
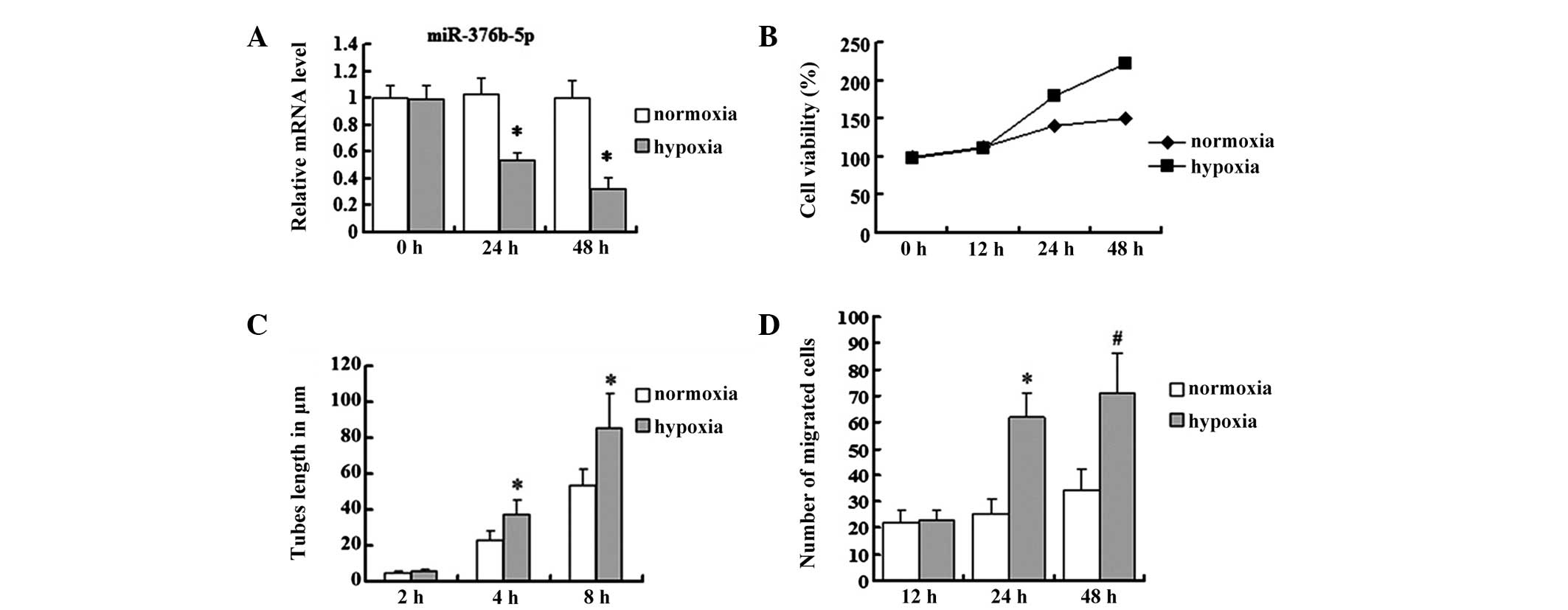
Hypoxia downregulates miR-376b-5p in
HUVECs
miR-376b-5p was suggested to be downregulated in rat
cerebral ischemia. HUVECs were used as an in vitro model to
further examine whether hypoxia regulates the expression of
miR-376b-5p. HUVECs were grown either in normoxia or hypoxia for
different time periods (24 or 48 h) and alterations in miR-376b-5p
levels were determined by qPCR. The results were consistent with
the in vivo study; miR-376b-5p levels were decreased in
hypoxia for 24 and 48 h compared with those in normoxia (P<0.05;
Fig. 4A).
Hypoxia induces angiogenesis in
HUVECs
In the in vivo study, angiogenesis was
induced following MCAO and the role of hypoxia in angiogenesis
in vitro was further investigated. To examine whether
hypoxia was able to affect the viability of HUVECs, an MTT assay
was performed. Compared with the normoxia group, the viability of
HUVECs was repressed in the hypoxia group at 12 h and then
increased up to 48 h (Fig. 4B).
Since tube formation and cell migration were important processes in
angiogenesis, the tube length and migrated cell number of HUVECs in
hypoxia were further examined. The present study revealed that
differences in tube lengths at 2 h between the normoxia and hypoxia
group were not significant (4.73±0.88 vs. 5.69±1.02 μm; P>0.05);
however, with time, the lengths of the tubes increased in the
hypoxia group at 4 and 8 h compared with the normoxia group (4 h:
37.31±7.98 vs. 22.97±5.12 μm; 8 h: 85.43±19.11 vs. 53.32±9.12 μm;
P<0.05) (Fig. 4C). The cell
migration ability was investigated by Transwell migration assay and
it was revealed that the difference in the migration cell number
between normoxia and hypoxia groups was not clear at 12 h (22±5 vs.
23±4; P>0.05); however, at 24 and 48 h, the migration cell
number in the hypoxia group significantly increased compared with
the normoxia group (24 h: 62±9 vs. 25±6; P<0.05; 48 h: 71±15 vs.
34±8; P<0.01) (Fig. 4D).
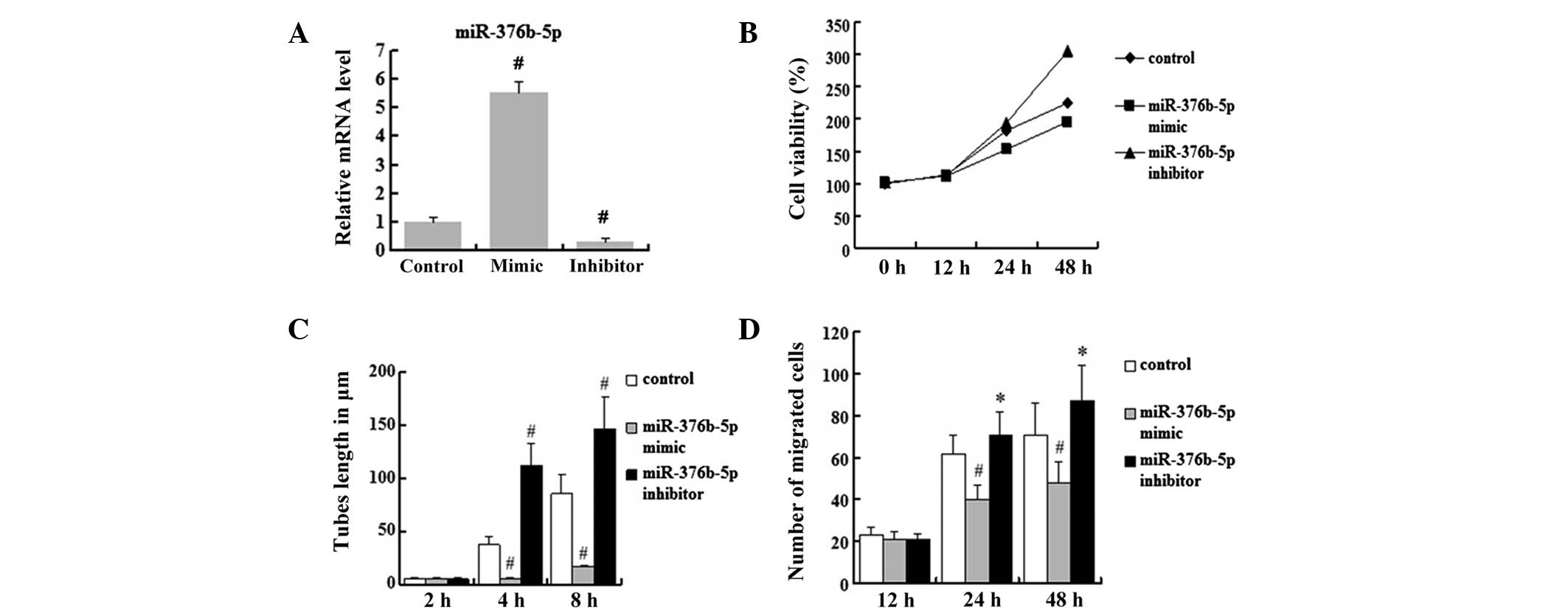
miR-376b-5p represses angiogenesis in
vitro
To further characterize the involvement of
miR-376b-5p in angiogenesis following cerebral ischemia, the
miR-376b-5p mimic and the miR-376b-5p inhibitor were transfected
into HUVECs to study whether the expression level of miR-376b-5p
was able to affect the process of angiogenesis in response to
ischemia injury. The transfection efficiency of the miR-376b-5p
mimic group and the miR-376b-5p inhibitor group was ~80%. The
relative mRNA level of miR-376b-5p in hypoxic HUVECs transfected
with negative control miRNA (miR-NC) was designated to be 1. The
mRNA level of miR-376b-5p increased significantly in miR-376b-5p
mimic-transfected HUVECs (5.54±0.38) compared with
miR-NC-transfected cells (P<0.01), and the level of miR-376b-5p
mRNA decreased significantly in the miR-376b-5p inhibitor group
(0.3±0.13) compared with the miR-NC group (P<0.01) (Fig. 5A). Using HUVECs in hypoxia with
miR-NC-transfected cells as the control, the data suggested that
the miR-376b-5p mimic was able to significantly repress
hypoxia-induced increases in the cell viability of HUVECs; however,
the miR-376b-5p inhibitor enhanced hypoxia-induced increases in the
cell viability of HUVECs (Fig.
5B). These results indicated that miR-376b-5p represses the
cell viability of HUVECs in hypoxia. Next, the present study
investigated the tube lengths in HUVECs and revealed that the
miR-376b-5p mimic and miR-376b-5p inhibitor did not affect tube
formation at 2 h (P>0.05). However, with time, the tube lengths
decreased in the miR-376b-5p mimic group at 4 and 8 h compared with
the miR-NC group (P<0.01) while the miR-376b-5p inhibitor
reversed this decrease (P<0.01) (Fig. 5C). In addition, the present study
examined whether the expression level of miR-376b-5p was able to
affect cell migration. The results illustrated that the miR-376b-5p
mimic significantly decreased the migration cell number compared
with the hypoxia group (P<0.01) and this effect was reversed by
the miR-376b-5p inhibitor (P<0.05) (Fig. 5D). Overall, these results suggest
that miR-376b-5p represses angiogenesis in hypoxic HUVECs.
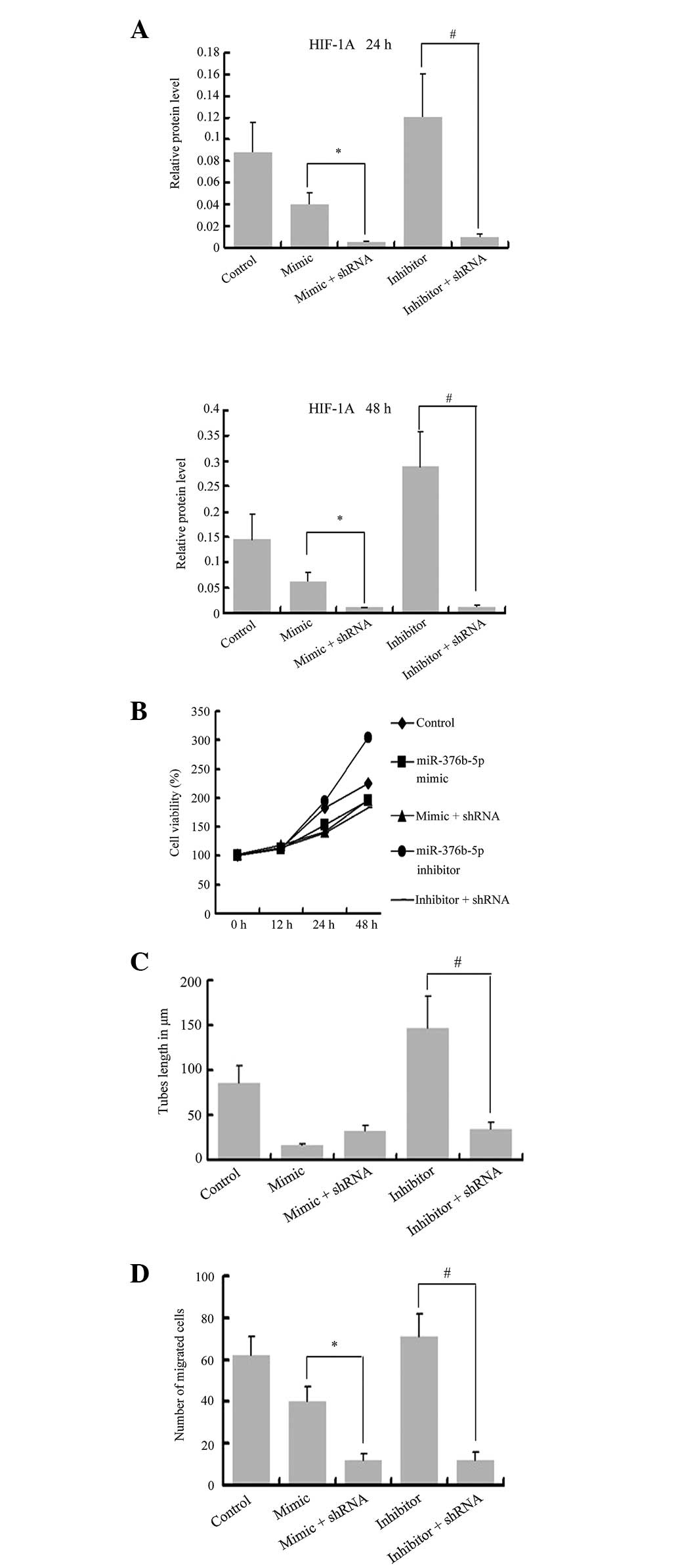
miR-376b-5p represses angiogenesis via
the HIF-1α target gene
To further examine the role of miR-376b-5p in
angiogenesis during hypoxia and its mechanism of regulation, the
present study predicted the targets of miR-376b-5p by PicTar
(http://pictar.mdc-berlin.de/) and
focused on HIF-1α. The protein expression of HIF-1α was rare in
normoxia, however, it was induced increasingly under hypoxia
(0.035±0.005 vs. 0.146±0.049; P<0.05). shRNA against HIF-1α was
transfected into hypoxic HUVECs, as shown in Fig. 6A and, following transfection,
HIF-1α significantly decreased at the protein level (P<0.01).
The MTT assay revealed that cell viability in the shRNA + inhibitor
group was lower than that of the inhibitor group and the hypoxia
group at 24 and 48 h (Fig. 6B).
The tube formation experiment demonstrated that the length of tubes
at 8 h in the shRNA + inhibitor group was decreased compared with
the inhibitor group, also the tube lengths in the shRNA groups were
decreased compared with the hypoxia group (Fig. 6C). Furthermore, the migration cell
number in the shRNA groups decreased at 24 h compared with the
mimic/inhibitor groups and hypoxia group (Fig. 6D). The same results were also
observed in those at 4 h (data not shown). All these data suggested
that miR-376b-5p is able to directly bind to the HIF-1α gene, which
is important in angiogenesis.
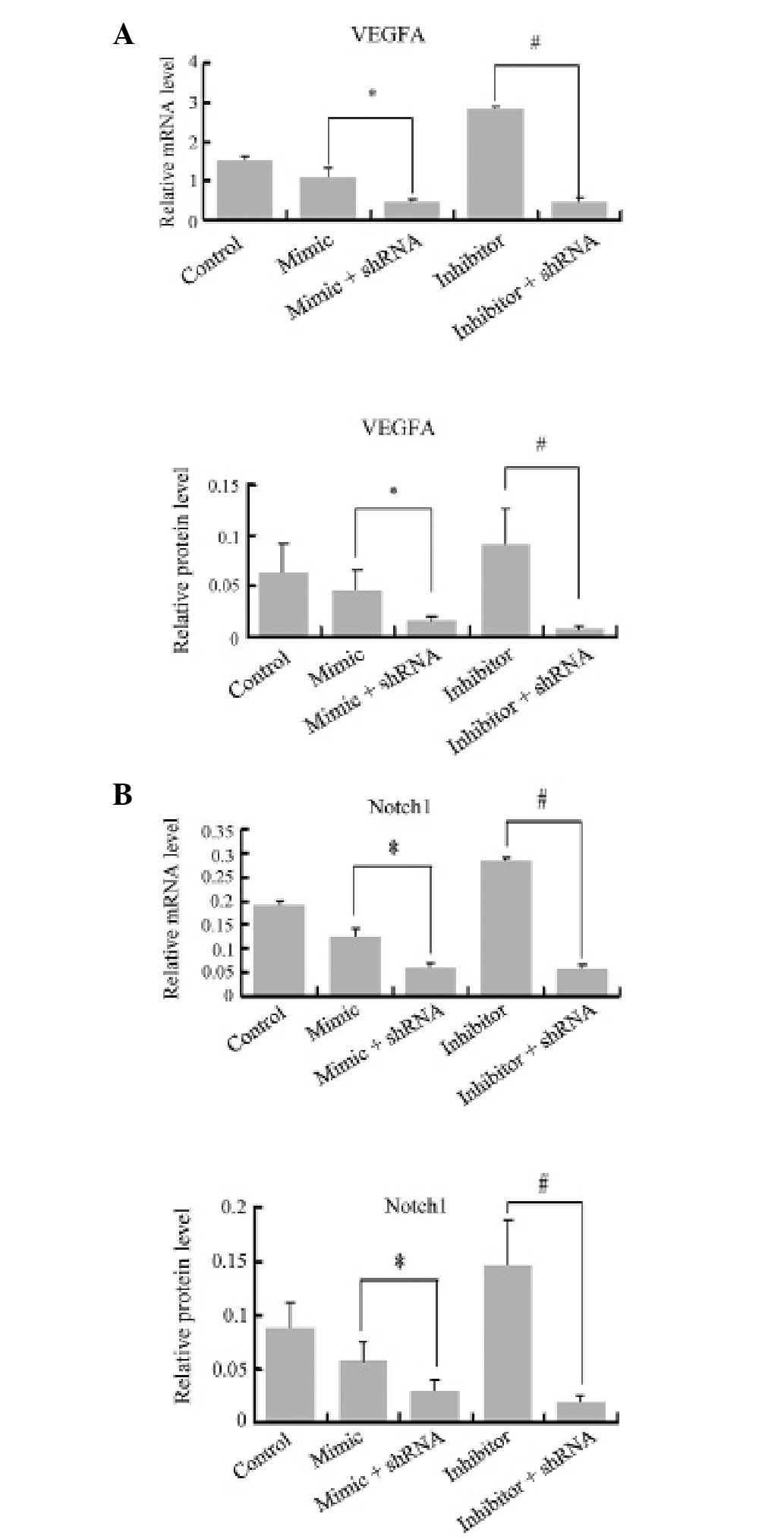
miR-376b-5p represses the expression of
angiogenesis via the HIF-1α mediated VEGFA-Notch1 pathway
To confirm that miR-376b-5p represses angiogenesis
during hypoxia through the HIF-1α-VEGFA-Notch1 pathway, VEGFA and
Notch1 relative mRNA and protein levels were also measured by qPCR
and western blot analysis. VEGFA relative mRNA and protein level in
the hypoxia group were higher than in the normoxia group
(0.049±0.015 vs. 0.137±0.041 for protein; P<0.05). Following
transfection of the mimic or inhibitor for 48 h, the miR-376b-5p
mimic reduced the relative mRNA and protein levels of VEGFA
compared with the miR-NC group (0.070±0.022 vs. 0.118±0.029 for
protein; P<0.05). The miR-376b-5p inhibitor significantly
enhanced hypoxia-induced increases in the relative mRNA and protein
levels of VEGFA (0.246±0.074 vs. 0.118±0.029 for protein;
P<0.05). The mRNA and protein levels of Notch1 also increased in
the hypoxia group compared with the normoxia group (0.055±0.015 vs.
0.140±0.040 for protein; P<0.05) and the miR-376b-5p mimic
reduced the relative mRNA and protein levels of Notch1 to low
levels (P<0.05). The miR-376b-5p inhibitor had an opposite
effect and the relative mRNA and protein levels of Notch1 were
significantly increased in the miR-376b-5p inhibitor group compared
with that in the miR-NC group (NC, 0.128±0.033; mimic, 0.086±0.028;
inhibitor, 0.201±0.062 for protein; P<0.05). The relative mRNA
and protein levels of VEGFA in the shRNA groups were lower than
those of the mimic/inhibitor groups, and they were also lower than
those in the hypoxia group (Fig.
7A). The results also demonstrated that the relative mRNA and
protein levels of Notch1 decreased in the shRNA groups compared
with the mimic/inhibitor groups and hypoxia group (Fig. 7B). These results suggest that
miR-376b-5p downregulates VEGFA and Notch1 expression at the mRNA
and protein levels and HIF-1α mediated this effect.
Discussion
miRNAs are ~22 nt long non-coding RNAs, which are
widely present in eukaryotic organisms. They control mRNA levels
and function by binding to the 3′-UTR (9–13).
The binding of miRNAs is considered to either degrade the mRNAs or
repress translation (14–15). miRNAs are involved in numerous
complex physiological processes, including growth and development,
organogenesis, cell proliferation and apoptosis. Kulshreshtha et
al first reported that hypoxia was able to induce expression
changes in miRNAs in cells in 2007 (16). Then, a number of studies revealed
that the expression profiles of miRNAs also altered following
ischemia and hypoxia in certain organs, including the retina,
myocardium and hippocampus (17–19).
In a study by Dharap et al (20), the authors profiled miRNAs
following transient MCAO in the adult rat brain. The expression of
238 miRNAs were evaluated between 3 h and 3 days. Compared with the
sham group, eight miRNAs were increased and 12 miRNAs were
decreased in at least four out of five reperfusion time points.
These studies indicate a critical role of miRNAs in controlling
mRNA transcription and translation in the post-ischemic brain.
Among the 12 decreased miRNAs, rno-miR-376b-5p was demonstrated to
be decreased from 3 h to 3 days by miRNA microarray analysis. The
present study focused on the expression of miR-376b-5p using
qPCR.
In our in vivo study, a rat pMCAO model was
constructed and the mRNA expression level of miR-376b-5p in the
cerebral infarct area was observed from 1 to 7 days using qPCR. The
results demonstrated that rno-miR-376b-5p altered following focal
ischemia. The mRNA expression level decreased compared with the
sham group, which was consistent with miRNA microarray analysis in
the study by Dharap et al (20). In our in vitro study, the
mRNA expression level of miR-376b-5p in the hypoxia group was
downregulated compared with the normoxia group. The results in
vivo and in vitro suggested that miR-376b-5p is involved
in the regulation of the pathophysiological process following
ischemic brain injury.
Angiogenesis is a process involving the
proliferating, remodeling and sprouting of endothelial cells and
subsequent formation of new blood vessels from pre-existing
vasculature (21). Certain
insults, including brain trauma and ischemia were able to induce
angiogenesis (22,23).
vWF is a specific surface marker of endothelial
cells. In the current study, for angiogenesis analysis in
vivo, immunocytochemistry was used with the vWF antibody to
label microvessels in the cerebral infarct area following MCAO. The
in vivo results suggested that the mean microvessel density
was increased 1, 3 and 7 days following MCAO, therefore, it
confirmed that responsive angiogenesis was induced following
cerebral ischemia. It has been demonstrated that the proliferation,
migration and morphological differentiation of endothelial cells
are important in angiogenesis. In our in vitro study, the
results suggested that the proliferation, migration and tube
formation of HUVECs were increased under hypoxia. These results
also verified that angiogenesis was induced following hypoxia,
which was consistent with the results in vivo.
Angiogenesis is regulated by activator and inhibitor
molecules and signaling pathways (24). These growth factors and kinases
affect vascular endothelial cells and smooth muscle cells,
stimulating the migration, proliferation and differentiation of
endothelial cells, and then new blood vessels form by the sprouting
of new capillaries.
VEGFA is the most important activator molecule in
the process of angiogenesis. VEGFA is important in angiogenesis
following cerebral ischemia (25).
It has been revealed that the expression of VEGFA is closely
associated with microvessel density and new vascular density in
tissue; VEGFA has been demonstrated to participate in the capillary
formation and improve the blood flood surrounding the infarction
area (26). VEGFA also
demonstrated a neuroprotective effect by improving local
microcirculation, promoting the injured nerve generation and neural
proliferation (27–31).
Numerous studies have confirmed that the Notch
signaling system is important in the proliferation and
differentiation of endothelial cells. There are four Notch genes in
mammals, encoding four Notch receptors, Notch 1, Notch 2, Notch 3
and Notch 4, and five Notch ligands, DII1, DII3, DII4, Jagged l and
Jagged 2. The Notch signaling system is involved in angiogenesis
(32,33). Previous studies have demonstrated
that the Notch signaling pathway is involved in and controls the
formation of new blood vessels accompanied with the VEGFA pathway
(34). VEGFA, as an upstream
regulator of Notch (34), combined
with its receptor VEGFR, then induced the expression of Notch 1 and
its ligand DII4, and the transcription of Notch target genes were
initiated to promote angiogenesis.
The expression of VEGFA is regulated by numerous
factors and hypoxia is the strongest known regulator. HIF-1α is a
nuclear transcription factor induced by hypoxia and its expression
can be found in all mammalian cells during hypoxia. Experimental
studies suggested that following cerebral ischemia, the areas where
the expression of HIF-1α is found is considered to be the region in
chronic hypoxia around the infarct area (35,36).
HIF-1α is important in mediating signal transfer between hypoxia
and angiogenesis. Hypoxic cells induce the expression of VEGFA via
activating HIF-1α (37). Zhang
et al (25) revealed that
following focal cerebral ischemia, the expression of VEGFA and
VEGFR were significantly increased around the infarcted area. The
expression of HIF-1α was also increased around the infarcted area.
Therefore, hypoxia-induced HIF-1α-VEGFA-Notch1 angiogenesis is
suggested to be an important pathway in angiogenesis
regulation.
In the present study, the in vivo and in
vitro results revealed that the expression of HIF-1α at the
mRNA and protein levels were rare in normoxia, however, it was
increasingly induced under hypoxia. In addition, the expression of
VEGFA and Notch1 were upregulated in the hypoxia group at the mRNA
and protein levels compared with the normoxia group. These results
illustrated that hypoxia-induced upregulation of VEGFA and Notch1
is associated with the increased expression of HIF-1α.
Studies regarding the regulatory effect of miRNAs on
angiogenesis are increasing. Certain studies demonstrated that the
expression profiles of a series of miRNAs altered following
ischemia, and certain miRNAs are important in angiogenesis
(20,38,39).
Therefore, identifying miRNA expression profiles targeting
angiogenesis and further studying its regulatory mechanisms are of
great significance for the treatment of stroke.
To further examine the role of miR-376b-5p in
angiogenesis during cerebral ischemia, the miR-376b-5p mimic and
miR-376b-5p inhibitor were transfected into hypoxic HUVECs. The
proliferation, migration and tube formation of HUVECs were measured
for estimating angiogenesis. The results demonstrated that the
miR-376b-5p mimic downregulated angiogenesis and the miR-376b-5p
inhibitor upregulated angiogenesis. These results verified that
miRNA-376b-5p was able to regulate angiogenesis.
A functional link between miRNA expression and
HIF-1α has been identified by certain studies. HIF-1α is able to be
targeted by the miR-17–92 cluster, miR-424 and miR-20b (40–42).
A specific group of miRNAs have been reported to be induced in
response to hypoxia, at least partially via a HIF-1-dependent
mechanism (16).
The present study predicted the targets of
miR-376b-5p by target prediction programs and focused on HIF-1α.
Next, to examine the underlying mechanisms responsible for our
observation, it was suggested that miRNA-376b-5p regulates
angiogenesis via the HIF-1α-VEGFA-Notch1 pathway. HIF-1α was
knocked down by the transfection of shRNA. The results demonstrated
that following transfection, the expression of HIF-1α was low. The
miRNA-376b-5p mimic or miR-376b-5p inhibitor was not able to affect
angiogenesis via HIF-1α, thus the angiogenesis index, including
proliferation, migration, tube formation and the expression of
angiogenesis-related molecules VEGFA/Notch1 was significantly
different from the mimic/inhibitor group. Furthermore, the present
study demonstrated that following shRNA transfection, the
angiogenesis index and the expression of angiogenesis-related
molecules VEGFA/Notch1 were significantly different from the
hypoxia group. It was suggested that since HIF-1α expression was
significantly decreased compared with the hypoxia group; the HIF-1α
mediated signal between hypoxia and angiogenesis was repressed.
In conclusion, the present study systematically
demonstrated that miR-376b-5p potently inhibited angiogenesis in
the rat MCAO model in vivo and miR-376b-5p was able to
effectively inhibit the proliferation, migration and tube formation
of HUVECs in vitro. To the best of our knowledge, the
present study demonstrated for the first time that miR-376b-5p
inhibits angiogenesis in HUVECs by targeting the HIF-1α-mediated
VEGFA/Notch1 signaling pathway. In view of the important role of
miRNA in the regulation of angiogenesis, identifying new miRNAs
targeting angiogenesis and further examining its regulatory
pathways and mechanisms, making miRNAs a new target for the
treatment of ischemic diseases, are of important instructive
significance in vascular repair for the clinical treatment of
ischemic stroke.
References
|
1
|
Wang WZ: Neurology. 4th edition. People’s
Medical Publishing House; Beijing: pp. 1302001
|
|
2
|
Zhu XF, Rao ML, Peng J, et al: Dynamis
observed morphologic change of neuron and microcirculation in focal
cerebral ischemia and reperfusion of rat. Chin J Clin Rehabil.
6:1904–1905. 2002.
|
|
3
|
Bang OY, Saver JL, Buck BH, et al; UCLA
Collateral Investigators. Impact of collateral flow on tissue fate
in acute ischaemic stroke. J Neurol Neurosurg Psychiatry.
79:625–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liebeskind DS, Cotsonis GA, Saver JL, et
al; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID)
Investigators. Collaterals dramatically alter stroke risk in
intracranial atherosclerosis. Ann Neurol. 69:963–974. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miteff F, Levi CR, Bateman GA, et al: The
independent predictive utility of computed tomography angiographic
collateral status in acute ischaemic stroke. Brain. 132:2231–2238.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krupinski J, Kaluza J, Kumar P, et al:
Role of angiogenesis in patients with cerebral ischemic stroke.
Stroke. 25:1794–1798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu F, Yang Z and Li G: Role of specific
microRNAs for endothelial function and angiogenesis. Biochem
Biophys Res Commun. 386:549–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longa EZ, Weinstein PR, Carlson S, et al:
Reversible middle cerebral artery occlusion without craniectomy in
rats. Stroke. 20:84–91. 1989. View Article : Google Scholar
|
|
9
|
Fire A, Xu S, Montgomery MK, et al: Potent
and specific genetic interference by double-stranded RNA in
Caenorhabditis elegans. Nature. 391:806–811. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caplen NJ, Parrish S, Imani F, et al:
Specific inhibition of gene expression by small doublestranded RNAs
in invertebrate and vertebrate systems. Proc Natl Acad Sci USA.
98:9742–9747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grishok A, Pasquinelli AE, Conte D, et al:
Genes and mechanisms related to RNA interference regulate
expression of the small temporal RNAs that control C. elegans
developmental timing. Cell. 106:23–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Humphreys DT, Westman BJ, Martin DI, et
al: MicroRNAs control translation initiation by inhibiting
eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc
Natl Acad Sci USA. 102:16961–16966. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing Q, Huang S, Guth S, et al:
Involvement of microRNA in AU-rich element-mediated mRNA
instability. Cell. 120:623–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kulshreshtha R, Ferracin M, Wojcik SE, et
al: A microRNA signature of hypoxia. Mol Cell Biol. 27:1859–1867.
2007. View Article : Google Scholar
|
|
17
|
Wen QQ, Jia YJ, Wang MC, et al: Expression
analysis of microRNA on acute cerebral ischemia in rats. J
Chongqing Univ. 33:23–26. 2005.
|
|
18
|
Roy S, Khanna S, Hussain SR, et al:
MicroRNA expression in response to murine myocardial infarction:
miR-21 regulates fibroblast metalloprotease-2 via phosphatase and
tensin homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen J, Yang X, Xie B, et al: MicroRNAs
regulate ocular neovascularization. Mol Ther. 16:1208–1216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dharap A, Bowen K, Place R, et al:
Transient focal ischemia induces extensive temporal changes in rat
cerebral microRNAome. J Cereb Blood Flow Metab. 29:675–687. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Velazquez OC, Snyder R, Liu ZJ, et al:
Fibroblast-dependent differentiation of human microvaseular
endothelial cells into capillary-like 3-dimensional networks. FASEB
J. 16:1316–1318. 2002.PubMed/NCBI
|
|
22
|
Beck H and Plate KH: Angiogenesis after
cerebral ischemia. Acta Neuropathol. 117:481–496. 2009. View Article : Google Scholar
|
|
23
|
Guo X, Liu L, Zhang M, et al: Correlation
of CD34+ cells with tissue angiogenesis after traumatic
brain injury in a rat model. J Neurotrauma. 26:1337–1344. 2009.
|
|
24
|
Yancopoulos GD: Vascular-specific growth
factors and blood vessel formation. Nature. 407:242–248. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZG, Zhang L, Jiang Q, et al: VEGF
enhances angiogenesis and promotes blood-brain barrier leakage in
the ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abumiya T, Lucero J, Heo JH, et al:
Activated microvessels express vascular endothelial growth factor
and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb
Blood Flow Metab. 19:1038–1050. 1999. View Article : Google Scholar
|
|
28
|
Sondell M, Lundborg G and Kanje M:
Vascular endothelial growth factor has neurotrophic activity and
stimulates axonal outgrowth, enhancing cell survival and Schwann
cell proliferation in the peripheral nervous system. J Neurosci.
19:5731–5740. 1999.
|
|
29
|
Schratzberger P, Schratzberger G, Silver
M, et al: Favorable effect of VEGF gene transfer on ischemic
peripheral neuropathy. Nat Med. 6:405–413. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZJ and He L: Vascular endothelial
growth factor and ischemic cerebrovascular disease. Chin J Clin
Rehabil. 8:131972004.
|
|
31
|
Greenberg DA and Jin K: From angiogenesis
to neuropathology. Nature. 438:954–959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Limbourg FP, Takeshita K, Radtke F, et al:
Essential role of endothelial Notch1 in angiogenesis. Circulation.
111:1826–1832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song Cai-Li, Zhang Feng-Chun, Xu
Ying-Chun, et al: Effect of breast cancer stromal cells on
expression of Wntl, Notchl and β-catenin and migration of MCF-7
cells. Medical Bulletin of Shanghai Jiaotong University.
28:921–924. 2008.
|
|
34
|
Hainaud P, Contrerès JO, Villemain A, et
al: The role of the vascular endothelial growth factor-Delta-like 4
ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and
endothelial cell functions. Cancer Res. 66:8501–8510. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin KL, Mao XO, Nagayama T, et al:
Induction of vascular endothelial growth factor and
hypoxia-inducible factor-1alpHa by global ischemia in rat brain.
Neuroscience. 99:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharp FR, Lu A, Tang Y, et al: Multiple
molecular penumbras after focal cerebral ischemia. J Cereb Blood
Flow Metab. 20:1011–1032. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonauer A, Carmona G, Iwasaki M, et al:
MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeyaseelan K, Lim KY and Armugam A:
MicroRNA expression in the blood and brain of rats subjected to
transient focal ischemia by middle cerebral artery occlusion.
Stroke. 39:959–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taguchi A, Yanagisawa K, Tanaka M, et al:
Identification of hypoxia-inducible factor-1 alpha as a novel
target for miR-17–92 microRNA cluster. Cancer Res. 68:5540–5545.
2008.PubMed/NCBI
|
|
41
|
Ghosh G, Subramanian IV, Adhikari N, et
al: Hypoxia-induced microRNA-424 expression in human endothelial
cells regulates HIF-alpha isoforms and promotes angiogenesis. J
Clin Invest. 120:4141–4154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cascio S, D’Andrea A, Ferla R, et al:
miR-20b modulates VEGF expression by targeting HIF-1 alpha and
STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 224:242–249.
2010.PubMed/NCBI
|