Introduction
Heart failure (HF) is a major health and economic
burden worldwide, and its prevalence is continuously increasing
(1). The key pathophysiological
process that ultimately results in heart failure is cardiac
remodeling in response to chronic pathological stresses, such as
hypertension and myocardial ischemia (2). Cardiac remodeling, which involves
myocyte hypertrophy along with interstitial cell proliferation and
extracellular matrix remodeling, induces structural and functional
changes, mainly in the left ventricle (3). Initially, cardiac remodeling is a
beneficial compensatory process, which reduces cardiac wall stress
and increases cardiac output, but remodeling ultimately results in
the inability of the heart to meet hemodynamic demands. Despite a
number of important therapeutic advances in the treatment of
symptomatic HF, the prevalence, mortality and cost associated with
HF continue to increase (4).
Therefore, the development of novel therapeutic strategies to
attenuate cardiac remodeling and prevent heart failure is an urgent
goal for the biomedical community.
Sanguinarine, derived from the root of
Sanguinaria canadensis and other poppy fumaria species, has
been demonstrated to exert widespread pharmacological actions,
including antimicrobial, antitumor and anti-inflammatory responses
(5–8). In particular, sanguinarine was
reported to possess cardioprotective outcomes, such as
antihypertension, antiplatelet and positive inotropic effects
(9). However, the influence of
sanguinarine on cardiac remodeling was unknown.
The aim of the present study was to investigate
whether sanguinarine improved cardiac hypertrophy and fibrosis in
mice.
Materials and methods
Materials and animal models
Sanguinarine (>98%) was ordered from Shanghai
Winherb Medical S&T Development Co., Ltd. (Shanghai, China).
Adult male C57BL/6 mice, aged 8–10 weeks, were purchased from the
Institute of Laboratory Animal Science, Chinese Academy of Medical
Sciences (Beijing, China) and acclimated for one week prior to
experimental use. The mice were randomly assigned to four groups
(Veh-Sham, SAN-Sham, Veh-AB, SAN-AB). Sanguinarine suspensions were
freshly prepared for the animal experiments using 0.5%
carboxymethyl cellulose solution. The suspensions were administered
to the mice at a constant volume of 1 ml/100 g body weight by oral
gavage once a day. The control group was administered the same
volume of liquid comprised solely of the vehicle solution (0.5%
carboxymethyl cellulose). Aortic banding (AB) was performed as
described previously (10).
Treatment with 5 mg/kg/day sanguinarine or vehicle from one week
after AB surgery or sham surgery for seven weeks allowed for
critical evaluation. All animal procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
publication no. 85-23, revised 1996) and approved by the
Institutional Animal Care and Use Committee at Renmin Hospital,
Wuhan University (Wuhan, China). All surgeries and subsequent
analyses were performed in a blinded manner.
Echocardiographic and hemodynamic
evaluation
Cardiac function was determined by transthoracic
echocardiography and hemodynamic analysis as described previously
(11). Echocardiography was
performed in mice anesthetized with 1.5% isoflurane, using a
MYLAB™ 30CV with a 10-MHz linear-array ultrasound
transducer (Esaote S.p.A, Genoa, Italy). The left ventricle (LV)
dimensions were assessed in the parasternal short-axis view.
End-systole and end-diastole were defined as the phases in which
the smallest or largest areas of the LV were obtained,
respectively. For hemodynamic measurements, a microtip catheter
transducer (SPR-839; Millar Instruments, Houston, TX, USA) was
inserted into the right carotid artery and advanced into the left
ventricle of mice anesthetized with 1.5% isoflurane. The signals
were continuously recorded using a Millar Pressure-Volume system
(MPVS-400; Millar Instruments) and the data were processed by PVAN
data analysis software (Millar Instruments).
Histological analysis
Excised hearts from mice which were sacrificed by
cervical vertebra dislocation were arrested in diastole with 10%
KCl, weighed, fixed by perfusion with 10% formalin and embedded in
paraffin. The hearts were cut transversely close to the apex to
visualize the left and right ventricles. Several sections of each
heart (4–5 μm thick) were prepared, stained with hematoxylin and
eosin (H&E) and picrosirius red (PSR) to determine the myocyte
cross-sectional area and collagen deposition, and were measured
using a quantitative digital image analysis system (Image Pro-Plus,
version 6.0; Media Cybernetics, Inc., MD, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from various organs using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
reverse-transcribed to complementary DNA, and analyzed by qPCR
using the LightCycler 480 SYBR Green 1 Master mix (Roche
Diagnostics, Mannheim, Germany) and the LightCycler 480 qPCR system
(Roche Diagnostics). The target gene mRNA expression levels were
normalized to those of the internal control GAPDH mRNA and
presented relative to the control group.
Western blotting
Protein samples denatured in SDS sample buffer (125
mmol/l Tris-HCl, pH 6.8, 50% glycerol, 2% SDS, 5% mercaptoethanol
and 0.01% bromophenol blue) were subjected to SDS-PAGE and blotted
onto Immobilon-FL transfer membranes (Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h and
were subsequently incubated with primary antibodies against
interleukin (IL)-1β, IL-6 (R&D Systems, Minneapolis, MN, USA)
and T-IκBα, T-NF-κBp65, P-IκBα, P-NF-κBp65 (all from Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C. Following
three washes in TBST, the membranes were incubated with anti-mouse
or anti-rabbit IgG (LI-COR Biosciences, Lincoln, NE, USA) for 1 h.
Bands were quantified by the Odyssey infrared imaging system
(LI-COR Biosciences) to detect protein expression levels. The
specific protein expression levels were normalized to those of a
GAPDH control (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
for the total cell lysate.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences among groups were determined by two-way
analysis of variance followed by Tukey’s post hoc test. Comparisons
between two groups were performed using an unpaired Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sanguinarine protects against cardiac
hypertrophy induced by pressure overload
To investigate the effects of sanguinarine on
cardiac hypertrophy, all wild-type mice were subjected to AB
surgery or sham surgery with or without oral sanguinarine
administration from one week after surgery.
In the present study, sanguinarine inhibited the
development of cardiac hypertrophy after eight weeks of AB. The
beneficial effects of sanguinarine treatment were indicated by
significant increases in LV ejection fraction and LV fractional
shortening, and significant reductions in LV diastole, LV systole,
interventricular septal thickness at end-diastole, interventricular
septal thickness at end-systole, LV posterior wall thickness at
end-diastole, LV posterior wall thickness at end-systole, and the
heart weight (HW)/body weight (BW), lung weight/BW and HW/tibia
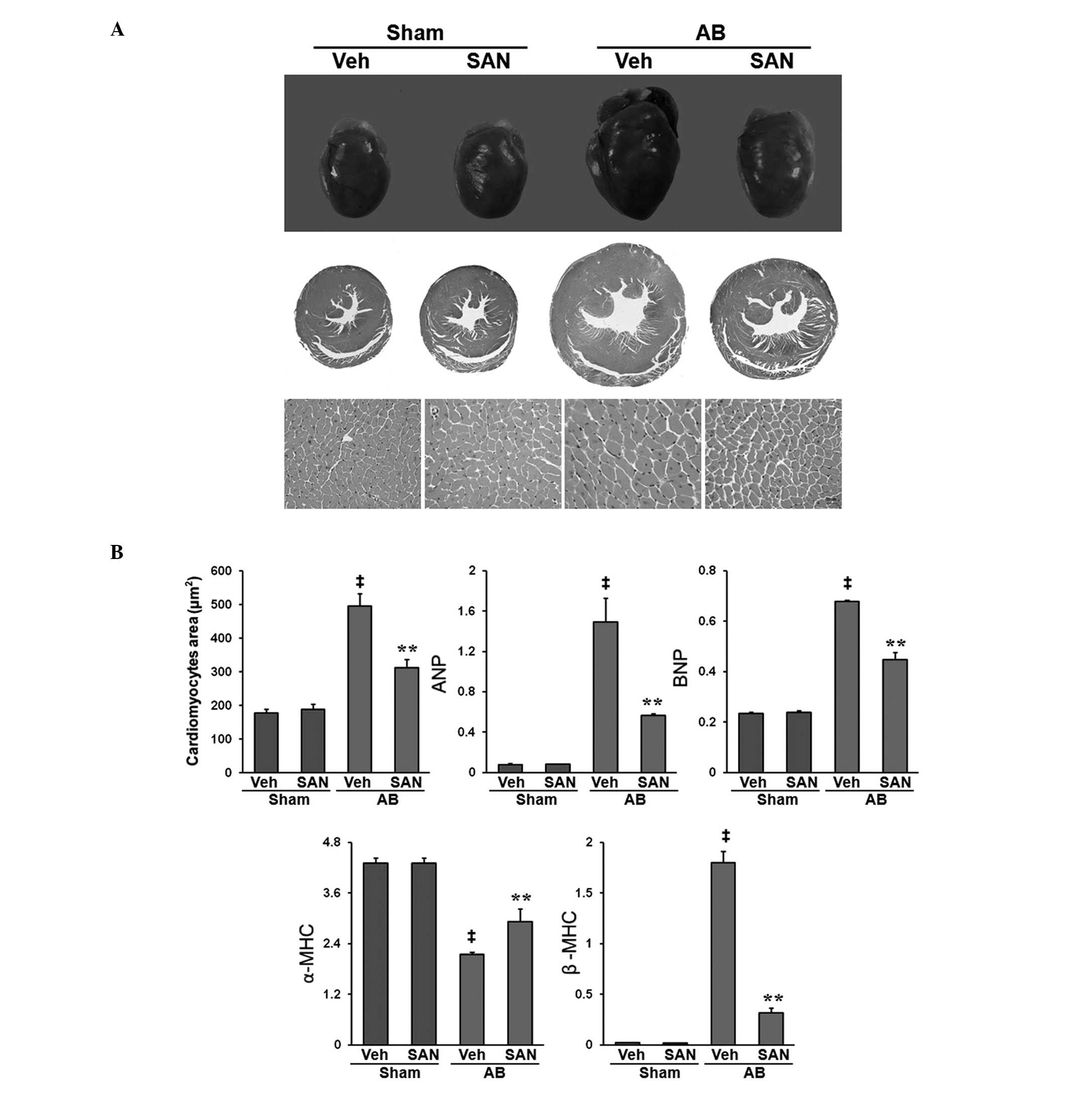
length ratios compared with the vehicle AB group (Tables I and II). Gross heart and H&E staining
indicated that the mice that received oral administration of
sanguinarine exhibited markedly reduced cardiac hypertrophy and
myocyte cross-sectional areas (Fig.
1A). The expression levels of AB-mediated hypertrophic markers,
including atrial natriuretic peptide, brain natriuretic protein and
β-myosin heavy chain (β-MHC) were significantly reduced in the mice
treated with sanguinarine whereas those of α-MHC were increased
(P<0.01; Fig. 1B) compared with
the expression levels in the Vehicle-AB mice. These results suggest
that sanguinarine negatively regulated the extent of the cardiac
hypertrophy and dysfunction induced by pressure overload.
 | Table IEchocardiographic and hemodynamic
parameters of the effects of sanguinarine on cardiac remodeling
induced by AB in wild-type mice. |
Table I
Echocardiographic and hemodynamic
parameters of the effects of sanguinarine on cardiac remodeling
induced by AB in wild-type mice.
| Parameter | Veh-Sham | SAN-Sham | Veh-AB | SAN-AB |
|---|
| Number | 8 | 8 | 8 | 8 |
| HR (beats/min) | 513±11 | 516±17 | 517±9 | 510±13 |
| IVSd (mm) | 0.67±0.01 | 0.67±0.01 | 0.82±0.02a | 0.78±0.01a |
| LVd (mm) | 3.65±0.03 | 3.81±0.04 | 5.21±0.02a | 4.56±0.13ac |
| LVPWd (mm) | 0.67±0.01 | 0.68±0.02 | 0.83±0.02a | 0.76±0.02ab |
| IVSs (mm) | 1.03±0.02 | 1.05±0.02 | 1.26±0.03a | 1.13±0.03ac |
| LVs (mm) | 2.05±0.03 | 2.29±0.05 | 4.00±0.13a | 3.19±0.14ac |
| LVPWs (mm) | 1.04±0.02 | 1.06±0.02 | 1.24±0.02a | 1.16±0.03ab |
| LVEF (%) | 81.00±0.76 | 77.88±1.11 | 52.88±1.33a | 65.00±1.74ac |
| LVFS (%) | 43.38±0.63 | 41.50±0.78 | 23.38±0.78a | 30.75±1.22ac |
| ESP (mmHg) | 102.85±2.38 | 102.69±2.29 | 144.41±3.04a | 141.11±8.73a |
 | Table IIGeneral data on the effects of
sanguinarine on cardiac remodeling induced by AB in wild-type
mice. |
Table II
General data on the effects of
sanguinarine on cardiac remodeling induced by AB in wild-type
mice.
| Parameter | Veh-Sham | SAN-Sham | Veh-AB | SAN-AB |
|---|
| Number | 10 | 10 | 10 | 10 |
| BW (g) | 28.42±0.42 | 28.99±0.44 | 29.00±0.41 | 28.39±0.46 |
| HW (mg) | 122.20±2.80 | 120.60±1.73 | 236.60±6.40a | 170.50±7.79ab |
| LW (mg) | 141.50±3.25 | 139.40±3.19 | 166.30±6.04a | 143.40±3.77b |
| HW/BW (mg/g) | 4.30±0.08 | 4.17±0.08 | 8.18±0.27a | 5.99±0.21ab |
| LW/BW (mg/g) | 4.99±0.12 | 4.82±0.12 | 5.76±0.27a | 5.05±0.09b |
| HW/TL (mg/mm) | 6.66±0.13 | 6.43±0.10 | 12.65±0.32a | 9.09±0.40ab |
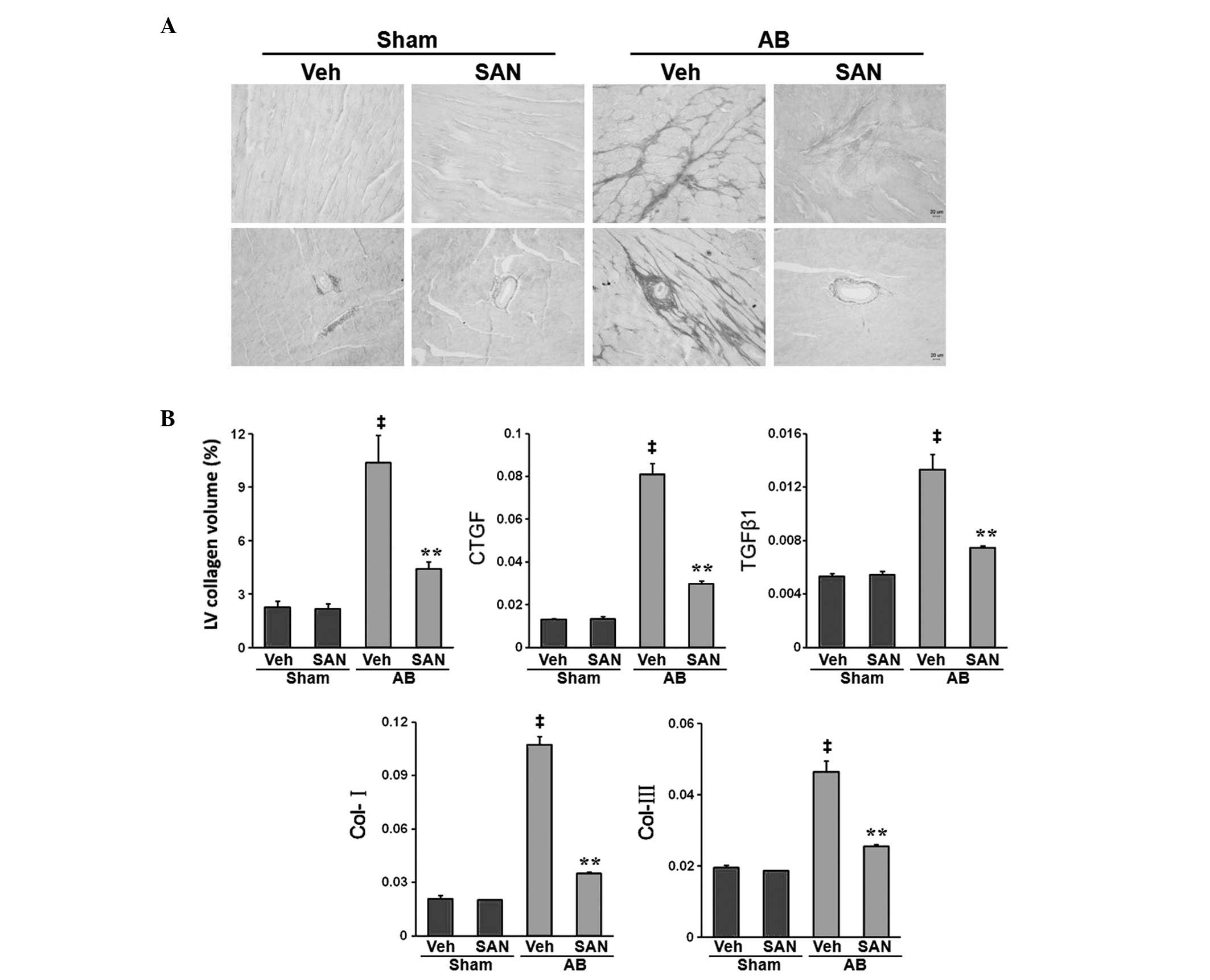
Sanguinarine inhibits cardiac fibrosis
induced by pressure overload
To determine the extent of fibrosis in the heart,
paraffin-embedded sections were stained with PSR. Marked
perivascular and interstitial fibrosis was detected using PSR
staining in vehicle-fed and sanguinarine-fed mice that were
subjected to AB (Fig. 2A).
However, the extent of cardiac fibrosis and the LV collagen volume
fraction were markedly reduced in sanguinarine-fed mice (Fig. 2A). Subsequent analysis of the mRNA
expression levels of fibrotic mediators, such as transforming
growth factor-β1 (TGFβ1), connective tissue growth factor (CTGF),
collagen I and collagen II, demonstrated a reduced response to
fibrosis in sanguinarine-fed mice (Fig. 2B). These results suggest that
sanguinarine inhibited the cardiac fibrosis induced by pressure
overload.
Sanguinarine inhibits myocardial nuclear
factor (NF)-κB activation in response to pressure overload
To investigate the molecular mechanisms by which
sanguinarine inhibits cardiac remodeling, the effects of
sanguinarine on the NF-κB signaling pathway were examined. As
expected, AB induced a significant increase in expression levels of
IL-1β, IL-6, IκBα and NF-κB p65, in the hearts of the vehicle-fed
mice in comparison with those receiving sham surgery (Fig. 3A and B). However, the pressure
overload-induced activation was markedly inhibited in the hearts of
the sanguinarine-fed mice (Fig. 3A and
B).
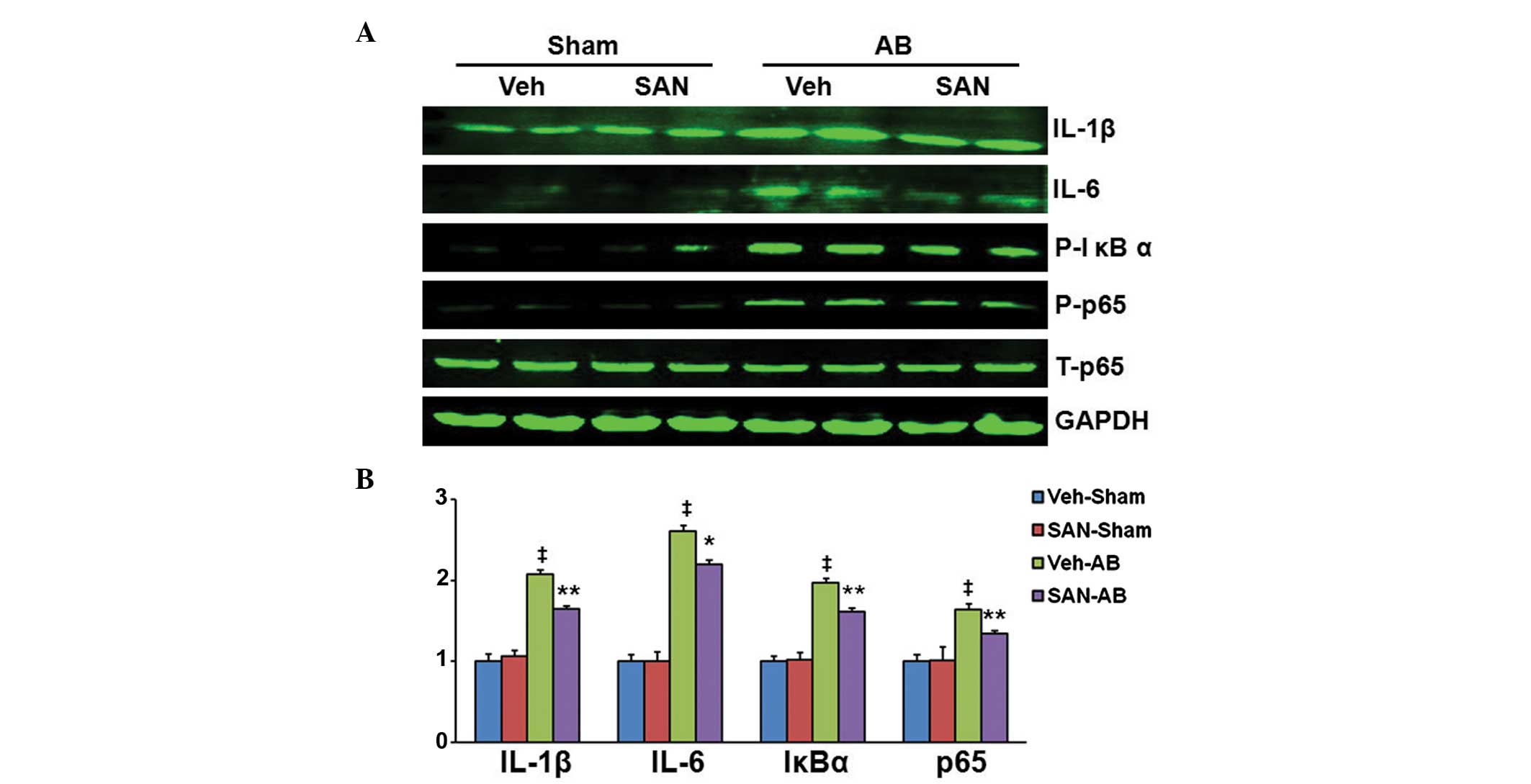 | Figure 3Reversal effect of sanguinarine on the
myocardial NF-κB signaling pathway in response to pressure
overload. (A) Phosphorylation of proteins associated with IL-1β,
IL-6, IκBα, NF-κBp65 in the left ventricular myocardium of AB or
Sham mice (n=6). (B) Quantitative measurements of IL-1β, IL-6,
P-IκBα, P-NF-κBp65 protein relative to GAPDH. Values are presented
as the mean ± standard error of the mean. ‡P<0.01
compared with the Vehicle-Sham group; *P<0.05 and
**P<0.01 compared with the Vehicle-AB group. NF-κB,
nuclear factor-κB; IL, interleukin; Sham, sham surgery; AB, aortic
banding; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Veh,
vehicle; SAN, sanguinarine. |
Discussion
Progress has been achieved in understanding the
molecular and cellular processes of heart failure, but this disease
remains a predominant cause of illness and mortality in the aging
society. Novel treatments that target disease mechanisms at the
cellular and whole-organ level are required to halt and reverse the
consequences of cardiac remodeling, which include cardiac
hypertrophy and fibrosis. The present study used a mechanical
overload-induced cardiac remodeling paradigm. The main findings
were as follows: Sanguinarine inhibited the cardiac hypertrophy,
fibrosis and dysfunction induced by pressure overload; sanguinarine
blocked the myocardial NF-κB signaling pathway and inflammatory
activation in response to pressure overload. Thus, to the best of
our knowledge sanguinarine was shown for the first time, to
effectively protect against cardiac remodeling and dysfunction.
Several studies have observed that sanguinarine
inhibited the NF-κB signaling pathway and inflammation to execute
its biological effects (8,12). Chronic inflammation is a hallmark
of HF, and inflammatory mediators are important in processes within
cardiac remodeling, including cardiomyocyte hypertrophy,
alterations in fetal gene expression levels and interstitial
fibrosis (13,14). Inhibition of the NF-κB signaling
pathway ameliorates chronic infusion of angiotensin II (AngII) or
pressure overload-induced myocardial inflammation and cardiac
hypertrophy (15,16). Pressure overload promotes cardiac
fibroblast proliferation and extracellular matrix accumulation,
thereby exacerbating cardiac fibrosis and subsequent heart failure.
NF-κB inhibition may suppress pressure overload or AngII-induced
CTGF expression and TGFβ-Smad signaling pathway activation, to
decrease the extent of cardiac fibrosis and improve heart function
(14,17). In the present study, sanguinarine
was identified to attenuate cardiac remodeling via inhibiting the
activation of NF-κB and downstream pro-inflammatory cytokines,
including IL-1β and IL-6.
To the best of our knowledge, this demonstrates for
the first time that sanguinarine is effective in inhibiting cardiac
remodeling and preserving heart function in banding mice. In
addition, these results provide experimental evidence for the
application of sanguinarine in the treatment of cardiac remodeling
and heart failure.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81270303 and 81300104) and
the Planning Project of Innovative Experiment of Wuhan University’s
Undergraduate (grant nos. S2013746).
References
|
1
|
Shah AM and Mann DL: In search of new
therapeutic targets and strategies for heart failure: recent
advances in basic science. Lancet. 378:704–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gjesdal O, Bluemke DA and Lima JA: Cardiac
remodeling at the population level - risk factors, screening, and
outcomes. Nat Rev Cardiol. 8:673–685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barry SP and Townsend PA: What causes a
broken heart - molecular insights into heart failure. Int Rev Cell
Mol Biol. 284:113–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houser SR, Margulies KB, Murphy AM,
Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin
JD, Sussman MA and Koch WJ; American Heart Association Council on
Basic Cardiovascular Sciences, Council on Clinical Cardiology and
Council on Functional Genomics and Translational Biology. Animal
models of heart failure: a scientific statement from the American
Heart Association. Circ Res. 111:131–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adhami VM, Aziz MH, Mukhtar H and Ahmad N:
Activation of prodeath Bcl-2 family proteins and mitochondrial
apoptosis pathway by sanguinarine in immortalized human HaCaT
keratinocytes. Clin Cancer Res. 9:3176–3182. 2003.PubMed/NCBI
|
|
6
|
Dinkova-Kostova AT: Phytochemicals as
protectors against ultraviolet radiation: versatility of effects
and mechanisms. Planta Med. 74:1548–1559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Stefano I, Raspaglio G, Zannoni GF,
Travaglia D, Prisco MG, Mosca M, Ferlini C, Scambia G and Gallo D:
Antiproliferative and antiangiogenic effects of the
benzophenanthridine alkaloid sanguinarine in melanoma. Biochem
Pharmacol. 78:1374–1381. 2009.PubMed/NCBI
|
|
8
|
Niu X, Fan T, Li W, Huang H, Zhang Y and
Xing W: Protective effect of sanguinarine against acetic
acid-induced ulcerative colitis in mice. Toxicol Appl Pharmacol.
267:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackraj I, Govender T and Gathiram P:
Sanguinarine. Cardiovasc Ther. 26:75–83. 2008.
|
|
10
|
Zong J, Deng W, Zhou H, Bian ZY, Dai J,
Yuan Y, Zhang JY, Zhang R, Zhang Y, Wu QQ, et al:
3,3′-Diindolylmethane protects against cardiac hypertrophy via
5′-adenosine monophosphate-activated protein kinase-alpha2. PLoS
One. 8:e534272013.
|
|
11
|
Deng W, Zong J, Bian Z, Zhou H, Yuan Y,
Zhang R, Guo H, Zhang Y, Shen D, Li H and Tang Q: Indole-3-carbinol
protects against pressure overload induced cardiac remodeling via
activating AMPK-alpha. Mol Nutr Food Res. 57:1680–1687. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pěnčiková K, Kollár P, Müller Závalová V,
Táborská E, Urbanová J and Hošek J: Investigation of sanguinarine
and chelerythrine effects on LPS-induced inflammatory gene
expression in THP-1 cell line. Phytomedicine. 19:890–895.
2012.PubMed/NCBI
|
|
13
|
Dai J, Shen DF, Bian ZY, Zhou H, Gan HW,
Zong J, Deng W, Yuan Y, Li F, Wu QQ, et al: IKKi deficiency
promotes pressure overload-induced cardiac hypertrophy and
fibrosis. PLoS One. 8:e534122013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma Y, Chen Y, Yang Y, Chen B, Liu D, Xiong
Z, Zhang C and Dong Y: Proteasome inhibition attenuates heart
failure during the late stages of pressure overload through
alterations in collagen expression. Biochem Pharmacol. 85:223–233.
2013. View Article : Google Scholar
|
|
15
|
Kawano S, Kubota T, Monden Y, Kawamura N,
Tsutsui H, Takeshita A and Sunagawa K: Blockade of NF-kappaB
ameliorates myocardial hypertrophy in response to chronic infusion
of angiotensin II. Cardiovasc Res. 67:689–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Yin R, Ernest R, Li Y, Zhelyabovska
O, Luo J, Yang Y and Yang Q: Liver X receptors are negative
regulators of cardiac hypertrophy via suppressing NF-kappaB
signalling. Cardiovasc Res. 84:119–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai Y, Yu SS, Chen TT, Gao S, Geng B, Yu
Y, Ye JT and Liu PQ: EGCG inhibits CTGF expression via blocking
NF-kappaB activation in cardiac fibroblast. Phytomedicine.
20:106–113. 2013. View Article : Google Scholar : PubMed/NCBI
|