Introduction
Following high-level spinal cord injury (SCI),
defined broadly as injury above the plane of the chest,
complications of the cardiovascular, respiratory and urological
systems with highly varying severities have been reported in acute
and long-term SCI patient care (1,2).
Immediately following SCI, the majority of patients exhibit
post-traumatic hypotension coupled with a parallel decline in
cardiac output (CO), which may be associated with direct myocardial
injury (3). However, the
association between myocardial injury and SCI remains
controversial, with different studies reporting varying severities
of myocardial ultrastructural change following SCI (4,5).
Thus, therapeutics that limit the degree and severity of myocardial
ultrastructural change in SCI patients are highly desirable in
order to reduce cardiovascular symptoms in the acute phase of
SCI.
The current gold standard method for the detection
of myocardial injury is measuring serum levels of cardiac troponin
T (cTnT) and troponin I (cTnI), the cardiac regulatory proteins
responsible for the control of actin and myosin interactions by
calcium mediation (6). Although
troponins are expressed by the cardiac and skeletal muscles of
healthy individuals, the levels of cardiac troponins remain
undetectably low under normal circumstances. However, extremely
small degrees of cardiac necrosis may be detected using monoclonal
antibodies to epitopes of cTnI and cTnT with little
cross-reactivity for skeletal troponins, thereby indicating
myocardial injury within 4–12 h of injury (7). Furthermore, troponin levels may peak
several days following injury (7).
Combined, testing of cTnI and the mitochondrial enzymes serum
creatine kinase (CK) and creatine kinase (CK-MB) may allow for
cost-effective and accurate myocardial injury assessment, however,
these tools may not fully indicate myocardial infarction risk and
ultrastructural change leading to coronary plaque rupture or
occlusion (7). Thus, clinical
management and peri- and postoperative strategies for cardiac
protection are required for patients with any indication of
myocardial injury.
Phosphocreatine (PCr), also referred to as creatine
phosphate (CP), is an important resource for rapid mobilization of
reserves of high-energy phosphates in skeletal muscles, including
muscles of the heart and has recently been employed for
perioperative cardioprotection (8,9).
Furthermore, recent studies have indicated that CaSR, a G
protein-coupled receptor superfamily C family member, may be used
to indicate the degree of myocardial cell damage leading to
infarction (10,11). Wang et al (10) suggested that CaSR mRNA and protein
expression were significantly upregulated with the severity of
myocardial damage, suggesting that CaSR may be a useful biomarker
for myocardial ischemia and reperfusion injury. Similarly, Chen
et al (11) confirmed the
upregulation of CaSR expression in a rat model of SCI. Thus, PCr
may be a useful agent for myocardial protection following injuries,
including SCI.
The present study assesses the cardioprotective
effects of PCr following SCI by examining changes in CaSR mRNA and
protein expression as well as ultrastructural changes in myocardial
tissues in rats. This study provides preliminary evidence for the
use of PCr in developing clinical cardioprotective agents.
Materials and methods
Study design
Healthy adult male SD rats (n=54) weighing 250–300 g
were purchased from the Animal Laboratory of the Fuzhou General
Hospital of the Nanjing Military Area Command (Fuzhou, Fujian,
China) and housed in the Animal Breeding Room of the Institute of
Hypertension Research, The First Affiliated Hospital of Fujian
Medical University (Fuzhou, Fujian, China) under 12/12 h light/dark
conditions at 25–30°C with ad libitum access to food and
water. A group of 6 rats was subjected to sham operation without
SCI (sham operation group). The remaining rats were divided into
equal groups and subjected to C7 SCI injury by Allen’s method with
or without treatment by abdominal injection of PCr (200 mg/kg) at
6, 12, 24 or 48 h (SCI + treatment and SCI-only groups,
respectively; 6 rats/group/time point). For treated (SCI +
treatment groups) and untreated (SCI-only groups) right apical
tissues were sampled 2 h following treatment at each treatment time
interval. All animal experiments were conducted in compliance with
all national and state guidelines and were approved by the Animal
Use Committee of Fujian Medical University.
SCI model establishment
Rats had no access to food or water for 12 h prior
to the procedure. Each rat was fixed in place on an arched table
and anesthetized by intraperitoneal injection of 2% pentobarbital
sodium (50 mg/kg). The injury site was shaved and disinfected with
10% povidone-iodine. A midline incision was made to expose the C7
spinous process (5 cm) and a longitudinal incision in the skin and
subcutaneous tissue was made to expose the approximate region of
the C6 to T1 spinous process and lamina. In the sham operation
group, a similar operation was performed to expose the C7 region,
however, SCI was not performed.
In the SCI + treatment and SCI-only groups, SCI was
established according to the Allen’s method (12). Briefly, the C7 dural surface was
padded with a plastic gasket along the curvature of the spinal
cord. A 10 g weight was delivered by vertical free-fall along a 5
cm hollow glass tube, impacting the C7 region of the spinal cord.
Successful models exhibited immediate darkening of the spinal cord,
fluttering of the lower extremities, body retraction and spasticity
in the tail muscles. Immediately following SCI, bleeding at the
surface of the spinal cord was stopped using a gelatin sponge and
skin and muscle were sutured. Following each surgery, rats were
treated with intraperitoneal injection of 5 ml of normal saline (40
ml/kg) and intramuscular injection of penicillin (200,000 U)
twice/day for 2 days. Successful models were housed as previously
described and provided daily artificial urination and defecation
care.
Animal treatment
Rats in the SCI + treatment groups were treated at
6, 12, 24 or 48 h following SCI with intraperitoneal injection of
PCr (200 mg/kg). Rats in the sham operation group and SCI groups
were treated with intraperitoneal injection of the equivalent
volumes of normal saline. A midline and longitudinal incision in
the skin and subcutaneous tissue was made 2 h following treatment
administration, to expose the chest organs and the pericardium was
cut to expose the heart. Myocardial tissues from the right apical
structures were collected using ophthalmic scissors. Samples were
placed in sterile tubes and stored by freezing at −80°C.
Simultaneously, the abdominal aorta was exposed and arterial blood
(~2 ml) was sampled by scalp needle expiration and then stored in
refrigerated tubes.
Assessment of serum cTnI, CK and CK-MB
levels
The level of cTnI was determined using an AU2700
automated analyzer provided by Olympus (Tokyo, Japan). The levels
of CK and CK-MB were measured using an ADVIA Centaur automated
analyzer provided Olympus (Tokyo, Japan).
Ultrastructural examination by TEM
A portion of each apical sample was cut into 1×1×1
mm cubes with a surgical-grade blade and fixed immediately in 0.1 M
PBS (pH 7.2) with 3% glutaraldehyde and 1.5% paraformaldehyde
provided by the Laboratory of Electron Microscopy of Fujian Medical
University (Fuzhou, Fujian, China) at 4°C for at least 2 h. Samples
were then rinsed with 0.1 M PBS (3 times), fixed with 1% osmium
tetroxide and 1.5% kalium ferrocyanatum at 4°C for 2 h and rinsed
again 3 times in 0.1 M PBS. Then, samples were subjected to graded
acetone dehydration by 50% alcohol for 10 min, 70% alcohol
saturated with uranyl acetate dye at 4°C overnight, 90% alcohol for
10 min, 90% alcohol-acetone for 10 min, 90% acetone for 10 min and
3 rounds of 100% anhydrous acetone for 10 min. Samples were then
saturated with anhydrous acetone and epoxy 618 embedding medium
(1:1) for 1.5 h and embedded in pure 618 embedding medium at 35°C
for 3 h, with polymerization conditions of 35°C for 12 h, 45°C for
12 h and 60°C for 3 days. Resultant samples were then sectioned
into ultra-thin sections with an ultra-thin slicer (70–80 nm),
stained with uranyl acetate and lead citrate for 5 min and washed
thoroughly with distilled water. Samples were observed using an
EM208 YEM microscope (Philips, Amsterdam, Netherlands).
qRT-PCR detection of CaSR mRNA
expression
Samples of ~0.1 g were ground at low temperatures
and total RNA was extracted with TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). RNA purity was determined at 260
and 280 nm (A260/280). cDNA was synthesized using a Revert Aid
First Strand cDNA Synthesis kit (Fermentas, Vilnius, Lithuania).
Specific mRNA quantification was performed by real-time PCR using
SYBR Premix Ex TaqII (Takara Bio, Inc., Dalian, Liaoning, China) in
a real-time PCR system (Thermal Cycler Dice, USA), according to the
manufacturer’s instructions. qRT-PCR detection was conducted with
primers provided by Dalian Biological Engineering Co., Ltd.
(Shanghai, China): CaSR forward primer, 5′-TTCGGCATCAGCT TTGTG-3′,
reverse primer 5′-TGAAGATGATTTCGTCTTCC-3′ and amplification
fragment length was 38 bp; GAPDH forward primer 5′-CTCAACTACATG
GTCTACATG-3′, reverse primer 5′-TGGCATGGACTGTGGTCATGAG-3′ and
amplification fragment length was 420 bp. 2−ΔΔCt was
calculated to represent the relative mRNA expression of target
genes, as previously described (13).
Western blot analysis of CaSR protein
expression
Samples of ~0.1 g were ground and subjected to
treatment with protein RIPA lysis buffer (BioTeke Biotechnology
Co., Ltd., Beijing, China). The supernatant was collected following
centrifugation at 14,167 × g and 4°C for 5 min. Protein
quantification was conducted using a BCA Protein Assay kit (BioTeke
Biotechnology Co., Ltd.). For western blot analyses, equal amounts
of protein were separated by 10% SDS-PAGE and electrophoretically
transferred onto polyvinylidene fluoride (PVDF) membranes
(Invitrogen, Grand Island, NY, USA). Non-specific sites on each
blot were blocked with 5% milk powder diluted in TBS with 0.05%
Tween (TBST). Following overnight incubation with rabbit anti-CaSR
polyclonal antibody and rabbit anti-β-actin monoclonal antibody
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), each blot was
washed three times with TBST buffer. Blots were then incubated with
HRP-labeled goat anti-rabbit secondary antibody (Beijing Zhongshan
Golden Bridge Corporation, China) for 1 h. Proteins were detected
using Ultra ECL reagent (BioTeke Biotechnology Co., Ltd.). Band
intensity was quantified using Quantity One 4.6.2 software
(Bio-Rad, Hercules, CA, USA). CaSR expression was normalized to
β-actin.
Statistical analysis
All data were reported as the means ± SD. All data
were analyzed with SPSS v.11.5 for Windows (SPSS, Inc., Chicago,
IL, USA). Statistical significance was evaluated by one-way
analysis of variance (ANOVA) and with the Dunnett t-test for post
hoc analysis. P<0.05 were considered to indicate a statistically
significant difference.
Results
Serum cTnI, CK and CK-MB levels are
reduced by PCr treatment
The serum cTnI, CK and CK-MB levels in treated and
untreated SCI rats were significantly higher than those of the sham
operation group rats at all time points (P<0.05). In the
SCI-only group, levels of cTnI decreased from 6 to 48 h, with 48 h
values significantly greater than those of the sham operation group
(P<0.05). Serum CK content in the SCI-only and the SCI +
treatment group were highest at 24 h and levels at 48 h remained
significantly greater than those of the sham operation group
(P<0.05). CK-MB levels in the SCI-only and SCI+treatment group
were highest at 12 h and levels at 48 h remained significantly
greater than those of the sham operation group (P<0.05). At all
time points, rats in the SCI + treatment group exhibited
significantly lower cTnI, CK and CK-MB levels than the SCI-only
group (P<0.05; Table I).
 | Table ISerum cTnI, CK and CK-MB levels. |
Table I
Serum cTnI, CK and CK-MB levels.
| Sham operation group
(n=6) | SCI-only groups | SCI + treatment
groups |
|---|
|
|
|---|
| 6 h (n=6) | 12 h (n=6) | 24 h (n=6) | 48 h (n=6) | 6 h (n=6) | 12 h (n=6) | 24 h (n=6) | 48 h (n=6) |
|---|
| cTnI | 0.004±0.002*$ | 0.056±0.001*&# | 0.031±0.002*&# | 0.026±0.001*&# | 0.011±0.003*&# | 0.047±0.003&$@ | 0.023±0.002&$@ | 0.018±0.006&$@ | 0.009±0.001&$@ |
| CK | 520.521±
122.135*$ | 3005.021±
632.173*&# | 4089.032±
348.006*&# | 5307.124±
256.781*&# | 2344.124±
901.346*&# | 2781.324±
456.093&$@ | 3012.115±
783.289&$@ | 5012.112±
478.387&$@ | 1988.09±
456.326&$@ |
| CK-MB | 552.833±
98.053*$ | 1150.092±
309.325*&# | 2713.121±
349.489*&# | 1345.032±
387.587*&# | 564.083±
793.328*&# | 1023.013±
893.389&$@ | 2012.398±
674.398&$@ | 1012.144±
498.236&$@ | 1249.932±
345.908&$@ |
Ultrastructure abnormalities and necrosis
in myocardial cells induced by SCI are relieved by PCr
treatment
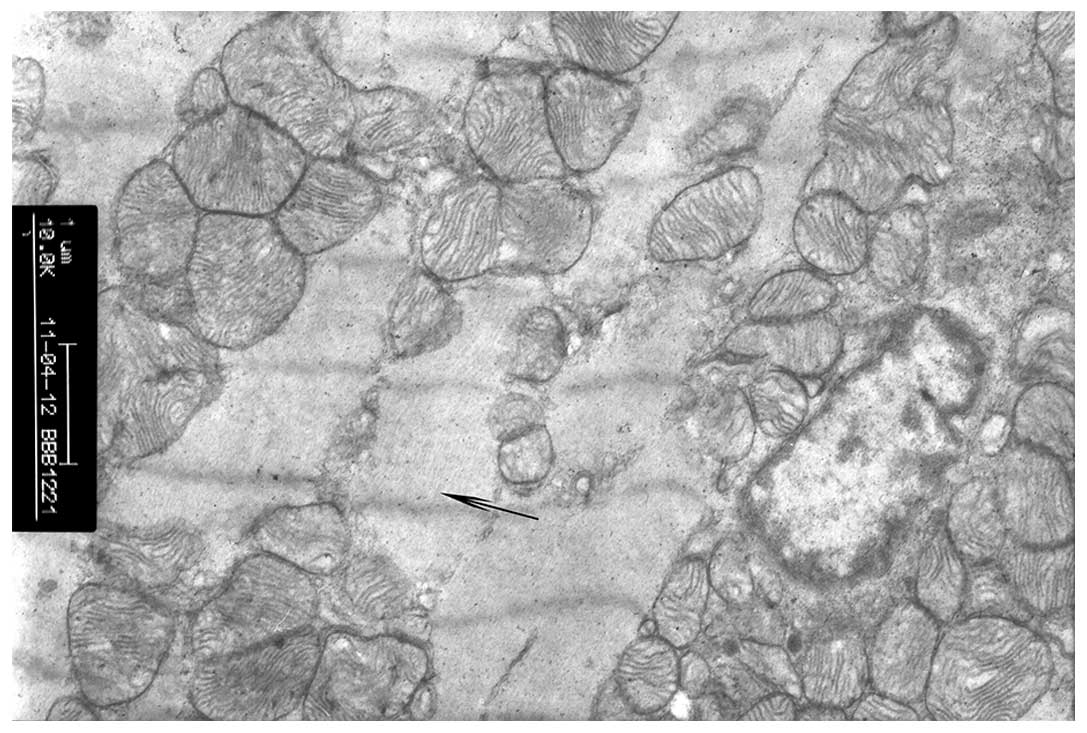
Sham operation group
In the sham operation group, myocardial cells
exhibited normal nucleus and nucleolus regions, cardiac myofibrils
were arranged in neat rows, sarcomeres were clear and the
mitochondrial membrane was intact (Fig. 1).
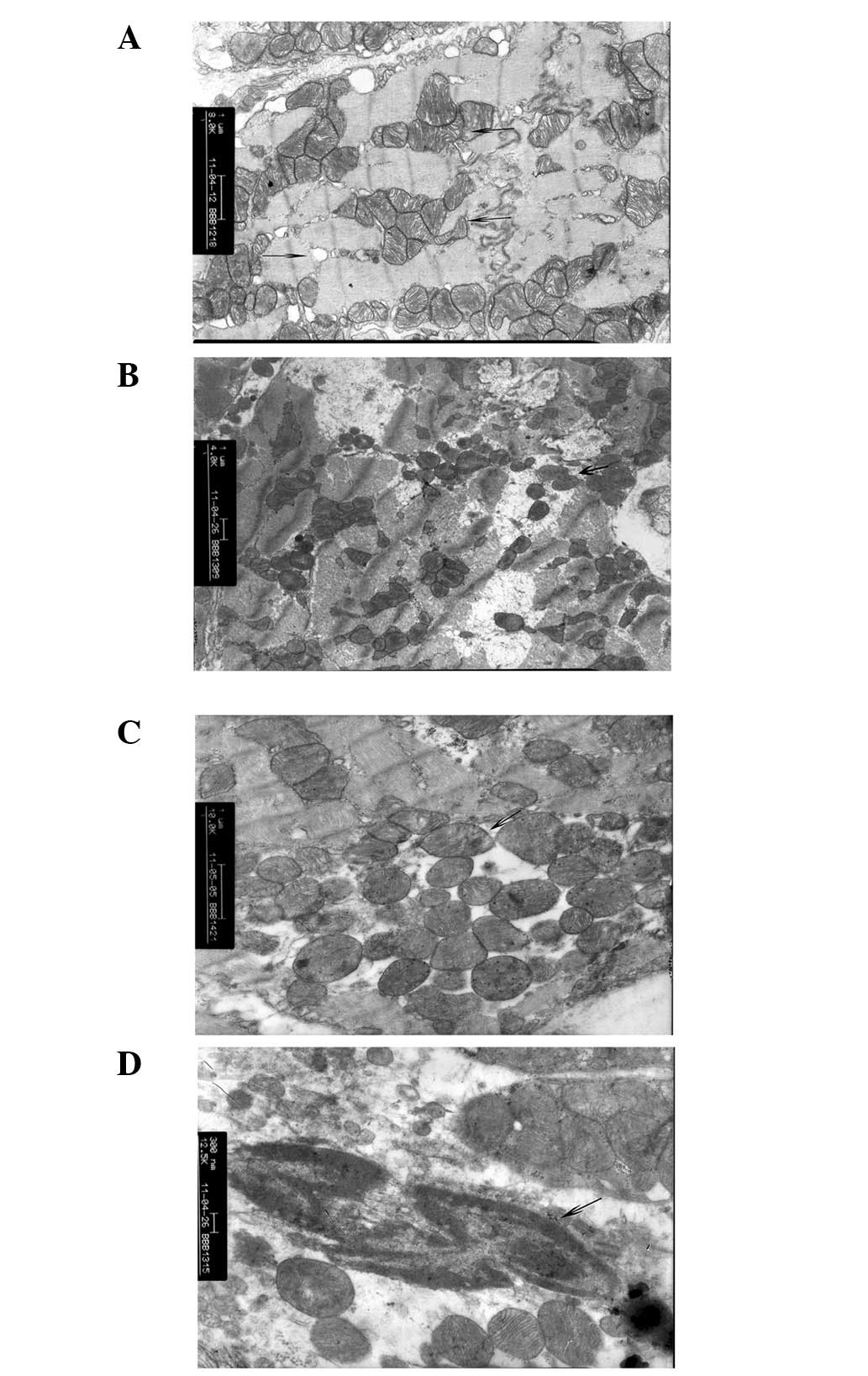
SCI-only groups
From 6 to 48 h, progressive necrotic change was
observed in myocardial cells of the SCI-only groups. In the 6
h-SCI-only group, the visible portion of the cardiac sarcoplasm
exhibited edema, myofibril dissolution, fracture, swelling of the
membrane and slight membrane breakage. Additionally, these cells
exhibited significant displacement of the mitochondria, cellular
contents and other damage indicative of necrosis, however, overall
cellular structure remained intact without significant swelling or
vacuole degeneration. The basic ridge structure was clear and rich
with glycogen granules in the mitochondria (Fig. 2A). By 12 h (12 h-SCI-only group),
the structure of visible myocardial filaments had dissolved,
mitochondrial swelling was apparent and cristae dissolution in
vacuoles indicated necrotic processes. Furthermore, nuclear
chromatin at 12 h was marginalized, with highly coiled chromatin in
the nucleus and cavitation in vacuolar structures with apoptotic
morphological changes in the myocardium (Fig. 2B). By 24 h (24 h-SCI-only group),
changes in myocardial apoptotic morphology were readily apparent,
including highly coiled chromatin in the nucleus (marginalization),
high degrees of nuclear chromatin condensation and apparent
cavitation in numerous vacuolar structures. Furthermore, there were
a large number of broken myocardial filaments, indicating
significant myocardial cell damage (Fig. 2C). By 48 h (48 h-SCI-only group),
necrotic changes in myocardial cell structure were commonplace,
including complete, or near complete, myofilament dissolution and
sarcomere fault, significant mitochondrial swelling, dissolution of
mitochondrial cristae and fusion and deposition of electron dense
particles in cavitation. Furthermore, the cell membrane was visibly
swollen with slight ruptures in multiple regions and interconnected
myocardial cells of the intercalated disc were apparent throughout
the intercellular space (Fig.
2D).
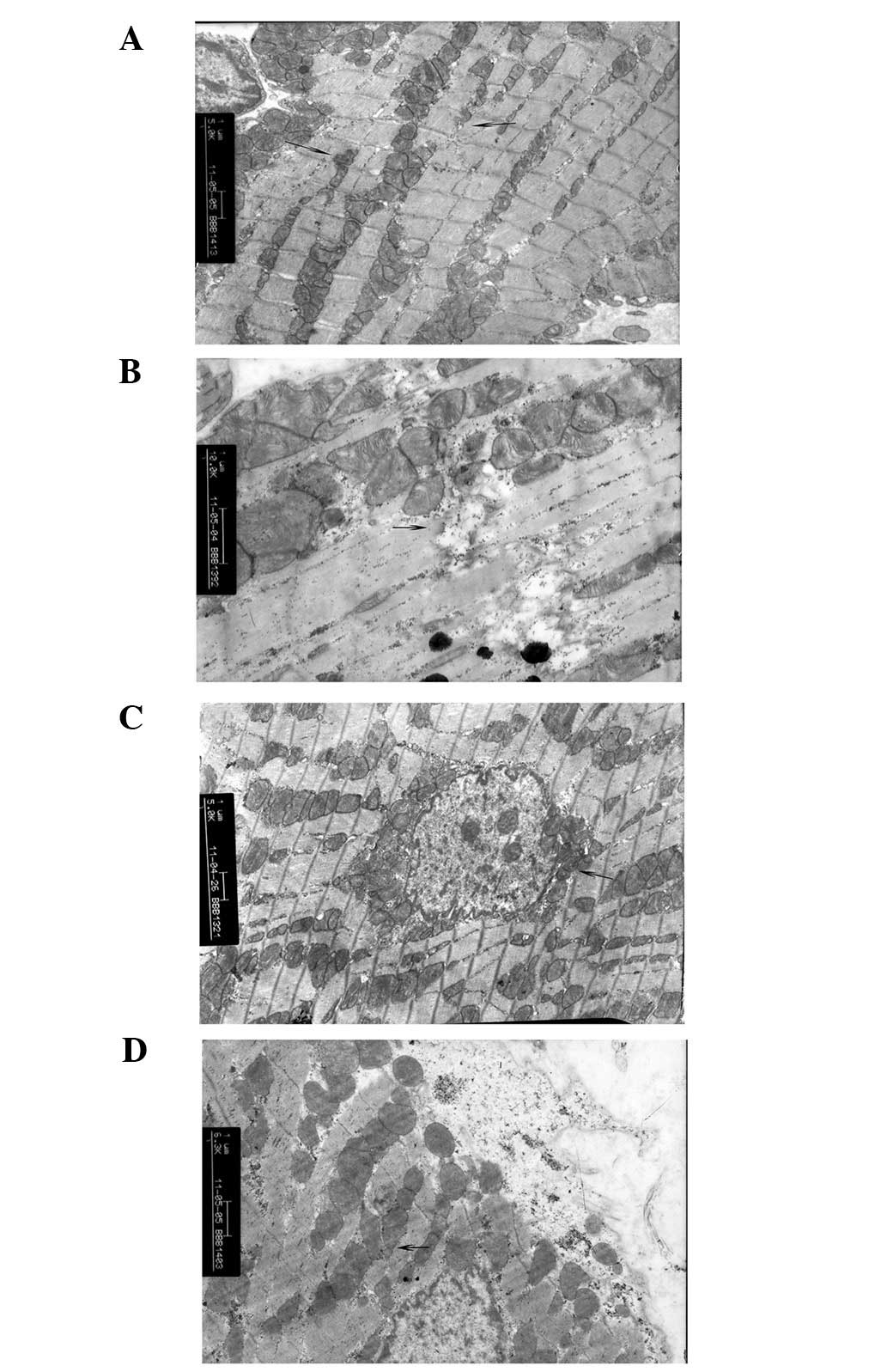
SCI + treatment groups
From 6 to 48 h, progressive necrotic change was
relieved in the SCI + treatment groups compared with the SCI-only
groups. In the 6 h-SCI + treatment group, the majority of the
cardiac muscle fibers were neatly arranged and myocardial filaments
were clear. Furthermore, the sarcomere structure was clear, only
slight swelling of the cell membrane was apparent, mitochondria
were displaced and occasionally condensed, however generally
intact, no swelling and vacuolar degeneration was present and the
basic ridge structure was clear and rich with glycogen granules in
the mitochondria (Fig. 3A). By 12
h (12 h SCI + treatment group), the structure of the nucleus,
mitochondria, myocardial fibers and sarcomere were basically
intact, although some dissolution of filaments was apparent
(Fig. 3B). By 24 h (24 h SCI +
treatment group), the structure of the majority of the myocardial
cells and filament sarcomeres remained intact and mitochondrial
structure remained normal, although slight edema was observed in
the sarcoplasmic region of myocardial cells. Notably, dissolution
of myofibrils was apparent by 24 h, with fracture, collapse, cell
swelling and slight breakage along with displaced and condensed
mitochondria, however, these features were less prominent than in
the 24 h-SCI-only group. Additionally, the ridge structure was
defined and intact, with only slight cavitation (Fig. 3C). By 48 h (48 h-SCI + treatment
group), the majority of the myocardial cell nuclei and sarcomere
structures remained notably more complete than in the 48 h SCI-only
group. Furthermore, myofilaments were predominantly intact,
mitochondria structure was generally normal and no swelling or
vacuolar degeneration was observed. Only slight dissolution of the
mitochondrial crest, indicated by fusion or electron dense particle
deposition was apparent, revealing minimal cavitation. Connections
between myocardial cell structures of the intercalated disc
remained intact, with only a small fraction of myocardial cells
exhibiting sarcoplasmic edema, myofibril dissolution, membrane
swelling and slight bursting. While certain mitochondrial positions
were shifted, none appeared to have punctured the plasma membrane
and minimal necrosis was observed (Fig. 3D).
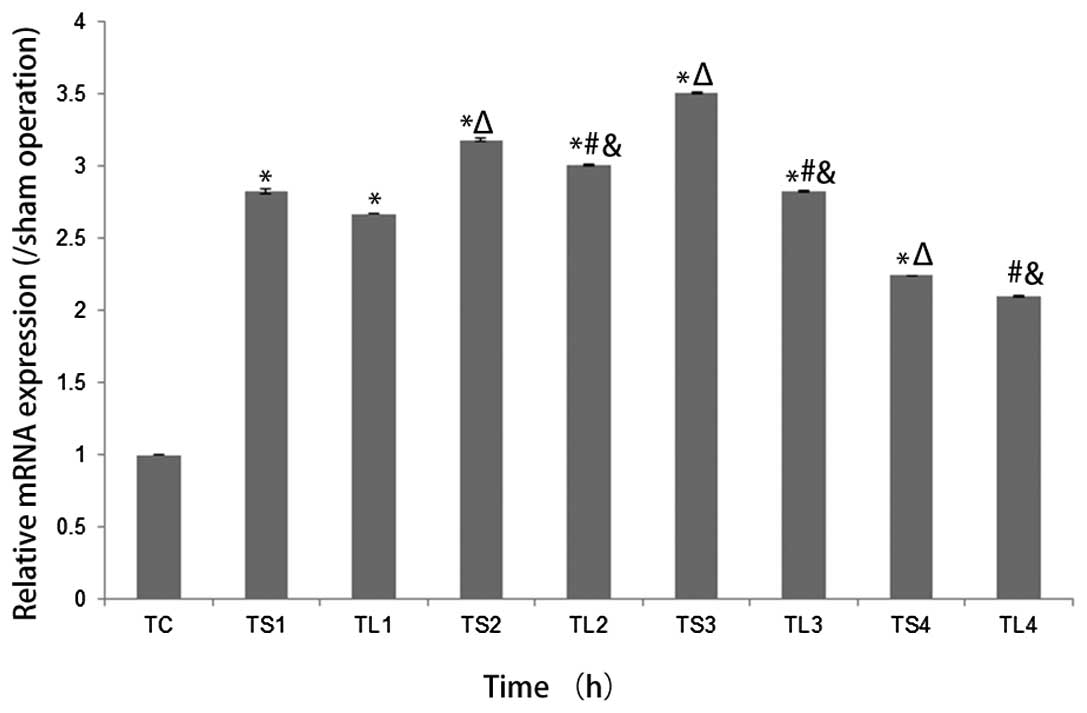
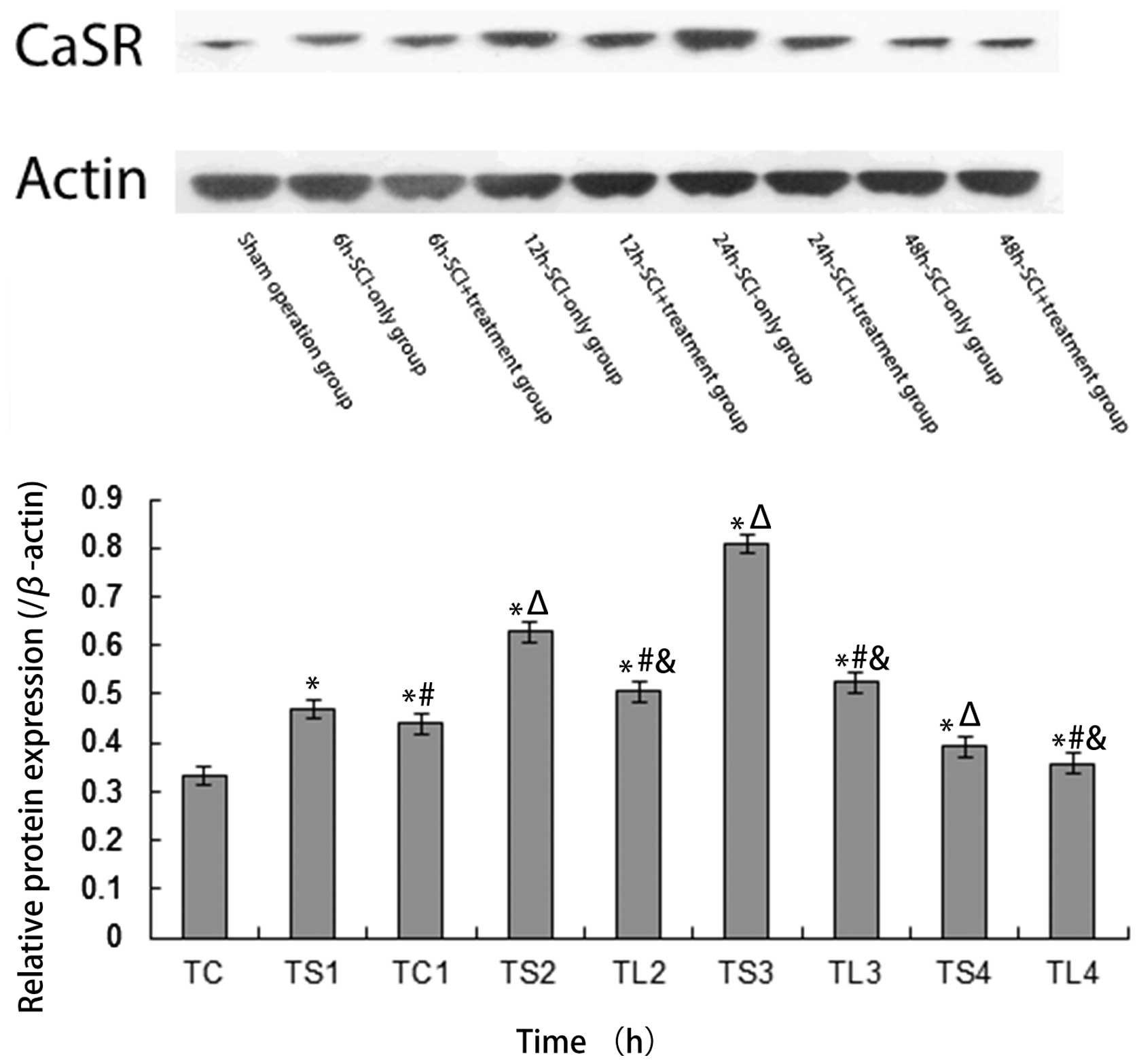
CaSR mRNA and protein levels are reduced
following PCr treatment
All SCI-only and SCI + treatment groups’ CaSR mRNA
expression levels at all time intervals were significantly higher
than values observed in the sham operation group (all P<0.05),
with SCI + treatment groups exhibiting significantly lower CaSR
mRNA expression levels than that in the SCI-only groups at all time
intervals (all P<0.05). In the SCI-only group, CaSR mRNA
expression increased from 6 to 24 h then decreased to 48 h,
however, the SCI + treatment group exhibited increases up to 12 h
and decreases thereafter (all P<0.05). Relative CaSR mRNA
expression among the sham operation group, SCI-only groups and SCI
+ treatment groups are displayed in Fig. 4. A similar trend in relative CaSR
protein expression determined by western blotting is displayed in
Fig. 5. Relative CaSR protein
expression among the sham operation group, SCI-only groups at 6,
12, 24 or 48 h and SCI+treatment groups at 6, 12, 24 or 48 h
following SCI were 0.331±0.021, 0.470±0.004, 0.627±0.008,
0.808±0.012, 0.392±0.005, 0.439±0.011, 0.505±0.007, 0.524±0.005 and
0.358±0.023, respectively.
Discussion
The present study demonstrated that treatment with
intraperitoneal injection of 200 mg/kg PCr following SCI in rats
preserved myocardial ultrastructure, reduced CaSR mRNA and protein
expression levels, and produced consistently lower cTnI, CK and
CK-MB levels when administered within the first 48 h following
injury. Furthermore, myocardial necrosis, capsular swelling and
abnormal mitochondrial characteristics were notably reduced in all
rats treated with PCr, which may preserve function of these tissues
and have beneficial cardioprotective effects. Thus, PCr merits
further study as a potentially useful clinical agent for
cardioprotection in the acute stages of SCI.
Varying degrees of myocardial injury and necrosis
have been reported following SCI and these mechanisms are generally
controversial and poorly characterized (5). Since cardiovascular failure and
dysfunction remain leading causes of mortality and disability in
patients with SCI, numerous potentially cardioprotective compounds
have been recently investigated in animal models, including FPTIII,
MG132 or Ang-(1–7) (14).
Unfortunately, the majority of successful treatments require
immediate administration, generally directly following the initial
SCI assault or within the first 6 h. Preliminary studies in the
last two decades have indicated that post-SCI administration of
PCr, however, is able to effectively improve functional heart
contractile recovery, lower diastolic pressure and mediate abnormal
myocardial enzyme release relatively late in the acute SCI period
(15). Similarly, it has been
reported that phosphorous-containing compounds, including PCr, are
capable of reducing demyelination in nerve fibers following SCI,
resulting in overall improvement in systemic nervous and
cardiovascular function (16).
Furthermore, a benefit to cerebral tissues, including reduced
morphological change and apoptosis, has been demonstrated using PCr
during cerebral ischemic reperfusion injury (17), which may be analogous to
cardiological damage during SCI. The current study, which examines
rats within the first 48 h following SCI, provides additional
supporting evidence that PCr may have beneficial cardioprotective
effects by limiting abnormal changes in cell ultrastructure.
It is well established that administration of PCr is
safe, reaching its peak activity within 20 to 40 min following
intramuscular administration or 30 to 120 min following intravenous
administration in humans (8).
Furthermore, PCr stays active in the human body for up to 250 min
following administration (8). It
has been suggested that PCr has a similar activity in rats
(17), which was used as a basis
for the 2 h post-treatment sampling time selected for this study.
In addition to providing energy during this period, Liu et
al (9) demonstrated that PCr
administration during the acute phase following injury has a
beneficial protective effect on mitochondrial membranes, consistent
with the results observed in the current study. Furthermore,
upregulation of CaSR, raising intracellular Ca2+ levels,
has been demonstrated to cause calcium overload and endoplasmic
reticulum imbalance (18). As a
result, elevation of CaSR associated with SCI may indirectly
increase myocardial cell apoptosis by increasing stress on the
membranes of the endoplasmic reticulum, which further increases G
protein-phospholipids enzyme C-inositol triphosphate signaling and
intracellular Ca2+ ion release (18). Thus, the beneficial effects of PCr
treatment in reducing CaSR levels in the present study may be
central to the mechanism by which PCr reduces overall morphological
change and apoptosis in myocardial tissues following SCI and other
traumatic injuries.
Mechanistically, administration of PCr has been
previously demonstrated to act through several different
intracellular mechanisms involving inhibition of
lysophosphoglyceride accumulation in the ischemic myocardium and
cardiac cell sarcolemma preservation by PCr-related zwitterionic
interactions as well as ATP regulation and inhibition of adenine
nucleotide degradation (15). The
current study provided novel ultrastructural support for these
mechanisms, suggesting that the myocardium and sarcolemma are,
indeed, preserved structurally and functionally by PCr
administration throughout the acute period, even later (up to 48 h)
following the initial SCI incident. Furthermore, PCr may also act
to maintain the extracellular ATP levels and increase cell
penetrance of key compounds required to sustain cellular
electrolyte balance (15),
consistent with findings in the serum that support this sustained
extracellular action. These mechanisms, however, require further
verification in larger and more extensive biochemical studies prior
to their confirmation.
Notably, the current study is limited by the
relatively small group and use of a rat animal model, which may
have PCr activity peaks that slightly vary from those of human
patients. However, the cardioprotective benefits of preoperative
PCr have been demonstrated in preliminary studies of human patients
undergoing open heart surgery (19), providing strong evidence that the
currently demonstrated cardioprotective PCr benefit in SCI rats may
similarly be applicable in humans that have experienced SCI.
Furthermore, it has been demonstrated that cellular energy
metabolism, impacted by PCr levels, following SCI is non-linear and
highly variable (20). Thus,
further study is required to determine the optimal dosages and
treatment times for administration of PCr in human patients,
necessitating further clinical study prior to implementation.
The current study demonstrates that PCr has numerous
cardioprotective benefits for increasing energy, reducing
morphological changes and preserving the normal ultrastructural
features in myocardial cells in the acute period (≤48 h) following
SCI. Furthermore, PCr-treated SCI rats exhibited lower CaSR levels
at all studied intervals than rats that underwent SCI without
treatment, suggesting improvements in calcium balance that may
contribute to reduced apoptosis by preserving intracellular
membrane structures in myocardial cells. Thus, early treatment with
PCr may potentially contribute to better functional recovery and
fewer cardiovascular complications, thereby meriting further study
and evaluation for clinical implementation. The full impact of CaSR
regulation and its mechanism following PCr treatment, however,
requires further study.
References
|
1
|
Bach JR: Noninvasive respiratory
management of high level spinal cord injury. J Spinal Cord Med.
35:72–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hagen EM, Faerestrand S, Hoff JM, Rekand T
and Gronning M: Cardiovascular and urological dysfunction in spinal
cord injury. Acta Neurol Scand Suppl. 71–78. 2011. View Article : Google Scholar
|
|
3
|
Guha A and Tator CH: Acute cardiovascular
effects of experimental spinal cord injury. J Trauma. 28:481–490.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu ZD, Shi XY, He XY, Zou Z and Li YK: The
protective effect of propofol on myocardium after cervical spinal
cord injury in rats. Fudan University Journal of Medical Sciences.
35:711–714. 2008.
|
|
5
|
Chen H, Lin J and Lin C: Change of serum
myocardial enzymes and anesthesia processing in the acute phase
paraplegia of cervical spinal cord injury. Journal of Fujian
Medical University. 34:63–64. 2000.
|
|
6
|
Melanson SE, Morrow DA and Jarolim P:
Earlier detection of myocardial injury in a preliminary evaluation
using a new troponin I assay with improved sensitivity. Am J Clin
Pathol. 128:282–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma S, Jackson PG and Makan J: Cardiac
troponins. J Clin Pathol. 57:1025–1026. 2004. View Article : Google Scholar
|
|
8
|
Shi J, Yuan S and Xue Q: Myocardial
protection of phosphocreatine in patients receiving on-pump
coronary artery bypass grafting surgery. Molecule Cardiology of
China. 10:44–47. 2010.
|
|
9
|
Liu Y, Li T and Ren Y: Protective effect
of phosphocreatine on mitochondrial membranes in cardiomyocytes.
Chinese Heart Journal. 16:14–16. 2004.
|
|
10
|
Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang
B and Wu L: Calcium and polyamine regulated calcium-sensing
receptors in cardiac tissues. Eur J Biochem. 270:2680–2688. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Ma C and Zhang X: Role of
myocardial-sensing receptors in a rat model of high-level spinal
cord injury. Chin J Anesthesiol. 31:992–994. 2011.
|
|
12
|
Allen AR: Surgery of experimental lesion
of spinal cord equivalent to crush injury of fracture dislocation
of spinal column a preliminary report. JAMA. 57:878–880. 1911.
View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
14
|
Benter IF, Abul HT, Al-Khaledi G, Renno
WM, Canatan H and Akhtar S: Inhibition of Ras-GTPase farnesylation
and the ubiquitin-proteasome system or treatment with
angiotensin-(1–7) attenuates spinal cord injury-induced cardiac
dysfunction. J Neurotrauma. 28:1271–1279. 2011.
|
|
15
|
Saks VA, Dzhaliashvili IV, Konorev EA and
Strumia E: [Molecular and cellular aspects of the cardioprotective
mechanism of phosphocreatine]. Biokhimiia. 57:1763–1784.
1992.PubMed/NCBI
|
|
16
|
Akino M, O’Donnell JM, Robitaille PM and
Stokes BT: Phosphorus-31 magnetic resonance spectroscopy studies of
pig spinal cord injury. Myelin changes, intracellular pH, and
bioenergetics. Invest Radiol. 32:382–388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang LH, Xia ZY, Zhao B, Wei XD, Luo T and
Meng QT: Phosphocreatine preconditioning attenuates apoptosis in
ischemia-reperfusion injury of rat brain. J Biomed Biotechnol.
2011:1070912011.PubMed/NCBI
|
|
18
|
Jiang CM, Han LP, Li HZ, Xu CQ, Sun YH and
Zhao WM: Expression of calcium sensing receptor during
ischemia/reperfusion myocardial damage and relationship between
CaSR and injury of myocardium. Chinese Journal of Pathophysiology.
6:112007.
|
|
19
|
Cheng SX and Hu QH: Cardioprotective
effect of exogenous phosphocreatine in patients undergoing open
heart surgery. Hunan Yi Ke Da Xue Xue Bao. 26:353–355. 2001.(In
Chinese).
|
|
20
|
Anderson DK, Means ED, Waters TR and
Spears CJ: Spinal cord energy metabolism following compression
trauma to the feline spinal cord. J Neurosurg. 53:375–380. 1980.
View Article : Google Scholar : PubMed/NCBI
|