Introduction
Advances in biomedical sciences have substantially
contributed to an increase in life expectancy over the past 50
years. However, this progress has led to the necessity for the
treatment and prevention of emerging age-related disorders. As one
of the major targets of senescence, the nervous system is
vulnerable to changes in the environment that arise due to aging,
and is involved in numerous age-dependent neurological deficits,
including pain (1), impaired motor
performance (2), cognitive decline
(3) and dementia (4). In the past century, the majority of
studies about brain aging have focused on the alterations in
neurons and synapses in older individuals; however, little has been
achieved in the prevention of age-related disorders. A number of
neuroimaging studies have found that changes in white matter,
particularly changes in the myelin sheath, may contribute to the
age-dependent functional deficits observed in the nervous system
(5,6). Several species, including humans,
non-human primates and rodents, have been observed to exhibit
age-related myelin breakdown in the nervous system through
ultrastructure electron microscopy studies (7,8).
Certain studies have found that changes in the nerve fibers and
myelin sheath were affected by aging and were likely to have an
important role in the development of age-related cognitive decline
in humans and primates (9,10). Furthermore, the development of
age-related disorders, such as Alzheimer’s and Parkinson’s
diseases, has been associated with alterations in the myelin sheath
in the aging brain (11,12). However, the genetic mechanism
underlying these age-related alterations in the myelin sheath has
not yet been fully elucidated.
Although a number of quantitative studies have
investigated the morphological changes that appear in the nervous
system with age, they have mainly focused on the alterations in
peripheral nerve trunks (13,14),
whilst studies on the myelin sheath in the central nervous system
(CNS) remain limited.
In the present study the morphological changes in
the optic nerve of male Sprague Dawley rats were analyzed at
varying time-points between birth and senescence. Age-related
profiling of the myelin sheath in the optic nerves of rats was
established using microarray hybridization to determine the
molecular changes underlying aging in the CNS.
Materials and methods
Animals
Male Sprague Dawley albino rats (obtained from the
Laboratory Animal Center, The Fourth Military Medical University,
Xi’an, China) ranging in age from postnatal day (PND) 5 to PND 360
were used. The rats were housed in plastic cages with access to
food and water ad libitum and maintained on a 12-h
light/dark cycle at room temperature (22–26°C). The experimental
protocol was approved by the Institutional Animal Care and Use
Committee of The Fourth Military Medical University (Permit no.
SCXK2007-007), and the present study was performed in accordance
with the National Institutes of Health (NIH) Guide for the Care and
Use of Laboratory Animals (NIH Publications No. 80-23).
Electron microscopy analysis
Five rats per group were infused with 2.5%
glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4) following anesthetization with sodium pentobarbital (80
mg/kg; Sigma, St. Louis, MO, USA). The optic nerves were collected
and post-fixed using 1% OsO4 in 0.1 M sodium cacodylate
buffer for 2 h at room temperature and then dehydrated in an
ascending acetone series. The osmicated tissue blocks were further
embedded in Epon-812 (Serva, Heidelberg, Germany) and trimmed under
the light microscope. Ultrathin sections (50–70 nm) were cut
perpendicularly to the axis of nerve fibers using a diamond knife
on an LKB-11800 ultramicrotome (LKB, Stockholm, Sweden) and
collected by copper grids (300 mesh). The ultrathin sections were
stained with uranyl acetate and lead citrate and then observed
under an electron microscope (EM; Hitachi, Tokyo, Japan).
Microphotograph images were captured at the same time.
Histopathological evaluation
Morphometric evaluation of myelinated fibers (MFs)
was performed by measuring ≥200 individual MFs from the sets of
photographs selected from five rats at each time-point. The
age-related pathological alterations of the myelin sheath were
quantified using fiber pathological grading and counting, which
were established in our previous study (15). The damaged MFs were classified into
three grades according to the severity and extent of destruction,
and the percentage of damaged nerve fibers was calculated. The
gradings were as follows: I, slight pathological changes, including
myelin lamina rarefaction and focal demyelination or vacuolization,
with the axon being less affected; II, moderate pathological
changes, including myelin lamina reticulation, focal demyelination,
vacuolization and axonal changes, such as increased electron
density, lipofuscin deposition and glycogen granules; III, severe
pathological changes, including marked myelin damage or disruption,
accompanied by axonal degeneration and loss.
Microarray hybridization
RNA was extracted from the optic nerves of male
normal Sprague Dawley albino rats grouped according to 10
age-points (between PND 5 and PND 360). In order to obtain
sufficient RNA for one array hybridization, between three and six
samples from one group were pooled as one biological replicate.
Independent hybridizations of three biological replicates were
performed for each time-point. Following homogenization, total RNA
was extracted using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) in accordance with the
manufacturer’s instructions. The quantity and quality of total RNA
were assessed by measuring the absorbance at 260 and 280 nm and by
gel electrophoresis. Approximately 1 μg total RNA was converted to
biotin-labeled cRNA and hybridized to the Agilent-014879 whole rat
genome microarray 4×44K G4131K (Agilent Technologies, Inc., Santa
Clara, CA, USA) in accordance with the manufacturer’s
instructions.
Quantitative polymerase chain reaction
(qPCR)
For all tissues, 1 μg total RNA was treated with
DNase I (Invitrogen Life Technologies) and used to generate cDNA
using a BioRT Two Step Reverse Transcription kit (BioER, Hangzhou,
China). Resulting cDNA (200 ng) was used as a template for qPCR and
RT-PCR in each reaction. The qPCR was performed using the Applied
Biosystems 7900HT Fast Real-Time PCR system with the associated
Sequence Detection System software version 2.2.2 (Applied
Biosystems™, Foster City, CA, USA). The RT-PCR was performed using
a 2× Taq PCR mix kit (Hangzhou Bioer Technology Co., Ltd.,
Hangzhou, Zhejiang, China). The qPCR and RT-PCR reactions were
performed as follows: 95°C for 10 sec, 40 cycles of 95°C for 5 sec,
and 60°C for 34 sec. Amplification levels were normalized to
expression levels of β-actin for each sample.
Data analysis
Following normalization, probes that showed
significant alterations in expression profiling during the life
span were filtered using a one-way analysis of variance (ANOVA)
(P<0.0001). A total of 3,826 genes were selected for further
analysis. Hierarchical clustering was performed in those rat
transcripts that were expressed differently in the optic nerve
among different ages. Each cluster was analyzed based on the gene
ontology (GO) function (16). GO
annotations were taken from the GO database version 2.2.11.
Hypergeometric distributions were used to detect over- or
under-represented biological process terms in the studied set
compared with the population set. Probabilities obtained using
hypergeometric distributions were corrected by Bonferroni
correction for the test on multiple GO functions. In order to
decrease the number of GO terms, only biological process ontology
terms at four, five and six levels were considered. Terms with
Bonferroni-corrected P-values <1×10−6 were considered
to be significant.
Results
Age-related structural alterations in the
myelin sheath in the optic nerve of rats
To determine the effects of aging on the structure
of the myelin sheath and the MFs, ultrathin sections prepared from
the optic nerve of rats aged between PND 5 and 360 were analyzed
under an EM. Marked age-related alterations were observed in the
myelin sheaths and MFs of rat optic nerves (Fig. 1A).
In the optic nerve of rats, the process of
myelination started after PND 5 and was not fully completed by PND
14. At PND 14 nearly half the fibers in the optic nerve appeared to
be unmyelinated axons. The oligodendrocytes of this developing
period were activated and contained little heterochromatin, which
contributed to the relatively pale appearance of the
oligodendrocyte nuclei under the EM. By PND 28, myelination was
complete and nearly all the axons were surrounded by a myelin
sheath. In this period, the heterochromatin in the nuclei of the
oligodendrocytes increased, which is a typical characteristic of
ordinary oligodendrocytes. Marked breakdown of myelin occurred in
the optic nerve of rats at PND 140, including the myelin tubercles,
the general separation of myelin lamellae and the degeneration of
axons. Similar but more extensive deterioration of the myelin was
observed in the optic nerves of rats at PND 240 and 360. Severe
decompaction of lamellae made the myelin appear wave-like. The
matrix between the MFs also underwent a severe decline after PND
240 (Fig. 1A).
Considering the non-statistical alterations in the
g-ratio distribution in the optic nerve of rats among different
ages (data not shown), grading and counting methods established in
our previous study (15) were used
to evaluate the age-related changes in the myelin sheath. Grading
classification of the pathological changes in the myelin sheath
showed the age dependence of the breakdown of the myelin sheath
(Fig. 1B and C). The percentage of
nerve fibers with pathological alterations in the myelin sheath
increased significantly in the optic nerves of aging and aged rats,
reaching 25.1% at PND 360.
Gene expression data collection and
validation
Using the rat whole genome expression microarray, 30
samples of rat optic nerves, from 10 time-points between PND 5 and
360, were analyzed. The coefficients of variation in the data from
30 bio-chips were found to be between 5.9 and 14.8%, whilst the
detection rates of each array were between 71.3 and 91.2%.
Following normalization, the age-related gene expression profiles
were established for the subsequent bioinformatic analyses. To
validate the microarray data, the age-related expression levels of
certain myelin-associated genes were analyzed using reverse
transcription PCR (RT-PCR) and qPCR.
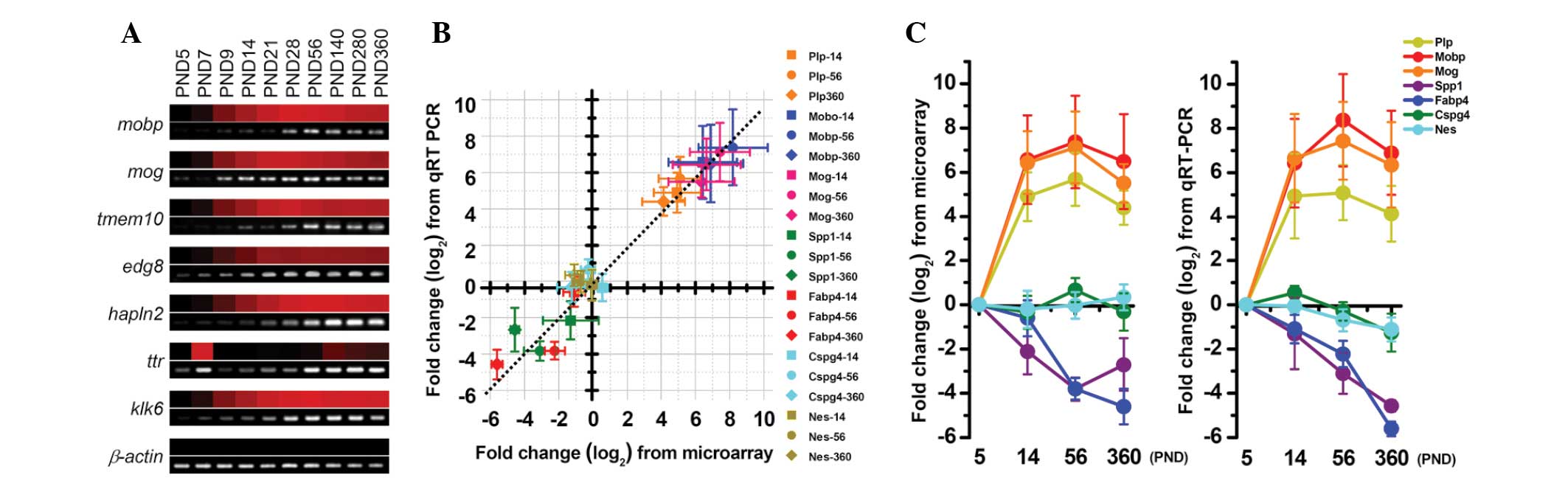
The expression levels of certain transcripts from
the microarray data, which were normalized against the PND 5
expression levels, were validated using RT-PCR; the results
obtained from these two methods showed concordance (Fig. 2A). Similarly, the expression data
from the microarray and the qPCR analysis were also consistent. The
slope of the line of best fit between the data from the array and
the qPCR was nearly one (Fig. 2B),
and the expression of all selected myelin-associated genes showed
similar age-related alterations in the microarray and qPCR data
(Fig. 2C).
Revealing transcriptional changes
underlying optic nerve lifespan
Using high-throughput one-way ANOVA, 3,826 genes
that showed differences in expression at each time-point were
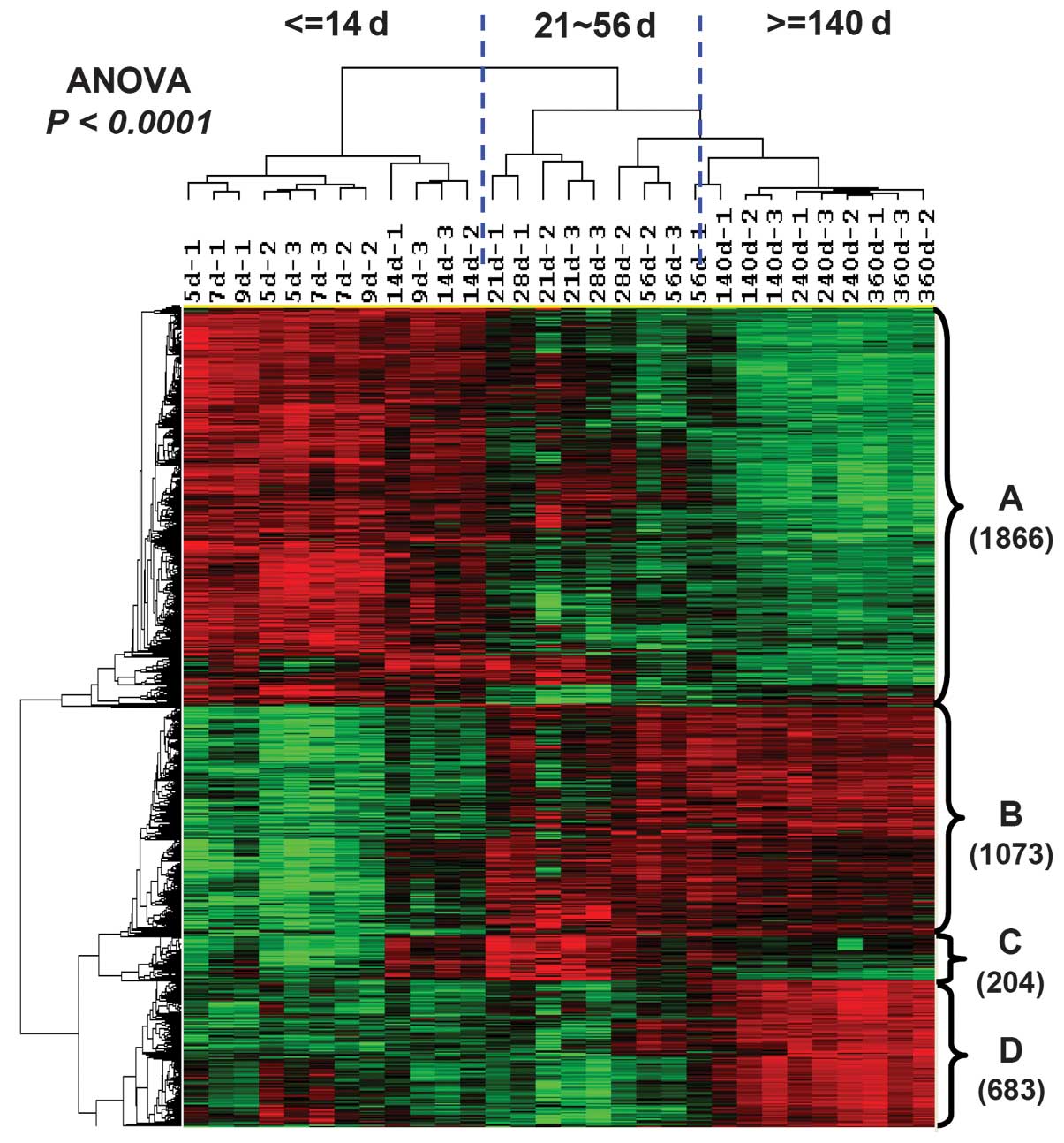
identified (P<0.0001). Hierarchical clustering analysis was used
on these differentially expressed genes to investigate the
categorized characteristics of lifespan and gene expression
(Fig. 3). The results suggested
that the lifespan of the optic nerve in rats could be divided into
three stages: The myelin development period between PND 5 and 14;
the maintenances of mature myelin sheath between PND 21 and 56; and
the aging process of the myelin sheath, starting from PND 140.
Clustering analysis also divided those differentially expressed
genes into four subsets according to their expression tendency
(Fig. 3). Cluster A included 1,866
transcripts only expressed at high levels during the early
development period. Transcripts in cluster B (1,073 transcripts)
had low expression levels in development, but had high expression
following the mature period. Transcripts in cluster C (204
transcripts) were upregulated during the maintenance period of the
mature myelin sheath, but had low expression during the aging
period. Cluster D contained 683 transcripts that were identified as
aging-specific genes (peak expression emerged only after PND
140).
Aging predominantly affects genes
involved in lipid biosynthesis, immune response and transmitter
transportation
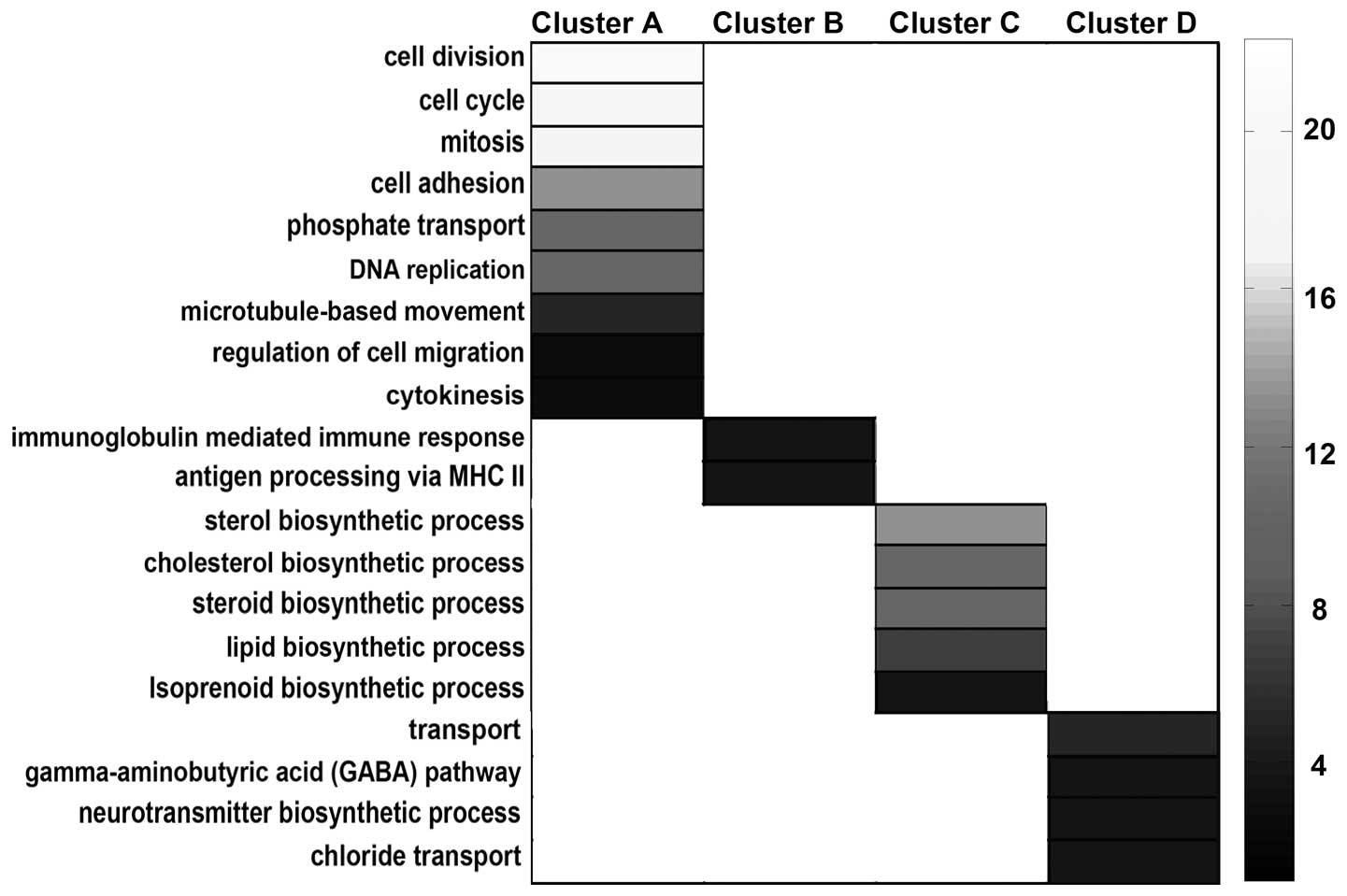
Functional enrichment analysis, along with GO
annotation, was used to determine the functions of the genes in
each cluster subset. As the results suggest, genes in cluster A,
which were highly expressed only during the developmental period,
were predominantly involved in proliferation, including the
biological processes of cell division, cell cycle, mitosis and DNA
replication (cluster A; Fig. 4).
The other three clusters, which showed more marked alterations in
the aging period, were significantly enriched for genes involved in
the immune response, lipid biosynthesis and transmitter
transportation, indicating that these three biological processes
were the most affected in optic nerve aging (Fig. 4). The induction of immune processes
in the mature and aging periods of the myelin sheath included the
adaptive immune response and antigen processing and the
presentation of exogenous peptide (cluster B; Fig. 4). By contrast, transcripts encoding
proteins involved in lipid, sterol and isoprenoid biosynthesis
exhibited very low expression in the aging period (cluster C;
Fig. 4). The aging-specific genes
were found to be transmitter transport genes, particularly those
involved in inhibitory transmitter transportations, including the
γ-aminobutyric acid pathway and the chloride transport processes
(cluster D; Fig. 4).
Discussion
To the best of our knowledge, the present study
provides the first genome-wide view of changes in gene expression
in the optic nerve of aging rats, along with a comparison of myelin
sheath structure. The expression database, covering 10 time-points
between development and senescence, is likely to be a valuable
resource for further research on the effects of aging on the myelin
sheath.
Using the pathological fiber grading and counting
method established in our previous study (15), the degree of myelin disruption in
the optic nerve of aging rats was quantified, and it was found that
the pathological alterations in the myelin sheath were
age-dependent. As early as PND 140, mild pathological changes were
observed in the myelin sheath in the rat optic nerve fibers. More
severe pathological alterations were observed in rats at PND 240,
with further deterioration in older rat optic nerves. Similar
age-related myelin breakdown has been described in the optic nerve
and other regions of the CNS in rhesus monkeys (10,17,18).
Although few studies have focused on the effects of aging on the
CNS of rats, a number of studies have found similar marked
abnormalities of the myelin sheath in the peripheral nervous system
(PNS) of aged rats (14,19).
The first genome-wide profiling of age-related
changes in peripheral nerves was published in 2012, which indicated
an upregulation of immune response transcripts and a downregulation
of lipid metabolism transcripts in aging mice (20). By comparing the results from the
present study with the previous gene expression profile of the PNS,
the genetic mechanisms underlying the age-related myelin sheath
decline in the CNS and PNS were able to be identified (20). Although the same GOs were enriched
in these two profiles, fewer overlapping genes were found in the
two datasets, which suggests differences in aging regulation among
different species.
The observed upregulation of transcripts involved in
immune response processes following the mature period is in
accordance with previous studies, which demonstrated an increase in
inflammatory responses during the aging process in the brain
(21,22) and the accumulation of macrophages
in aging peripheral nerves (13).
Our previous data also strongly suggested that there was a
correlation between the age-related microglia activation and the
age-related myelin breakdown in nearly all parts of the CNS of rats
(23). Furthermore, an increase in
the number of macrophages has been observed in animal models of
inherited neuropathies, and macrophages and/or microglia have been
shown to have a pathogenic impact on the myelin sheath through
chemokine signaling-mediated demyelination (24). Notably, the activation of the
complement cascade has been observed in the brain of aging monkeys
(25,26) and the inactivation of the
complement cascade has been indicated to facilitate the
regeneration of injured nerves (27). These studies further support the
relevance of immune response processes as potential targets for
drug development for the prevention of age-related myelin
breakdown.
The significant over-representation of transcripts
involved in lipid synthesis among the downregulated genes in the
aging period indicate the importance of this biological process in
the vulnerability of the myelin sheath to aging. Lipids constitute
>70% of myelin membranes and are required in large quantities
for myelin assembly (28). The low
expression levels of lipid metabolism genes observed after PND 140
are consistent with the detected myelin breakdown in the optic
nerve of aging rats (Fig. 1). A
similar downregulation of lipid synthesis genes was also identified
in the aging profiling of the peripheral nerves of mice and in the
profiling of peripheral myelin protein 22-, SREBF chaperone- or
Lipin 1-knockout mice (20). The
results of the present study, combined with those of previous
reports, demonstrate the importance of oligodendrocyte lipids as
markers of myelin sheath integrity and function, not only in the
period of myelin development, but also during myelin decline.
Furthermore, it was found in the present study that the
downregulation of lipid metabolism transcripts was paralleled by
the reduction in myelin-associated proteins, including myelin basic
protein, myelin-related oligodendrocyte basic protein and myelin
oligodendrocyte glycoprotein, which have been previously shown to
be downregulated in the CNS (23)
and PNS of aging rats (29). It is
possible that the age-related reduction in cholesterol metabolism
genes resulted in the decreased expression of myelin protein genes,
as previously observed in a mouse mutant affecting glial
cholesterol biosynthesis (30).
These observations, together with those of previous reports
describing the role of local lipid metabolism in myelin development
and function (30,31), strongly indicate that alterations
in lipid synthesis transcripts contribute to the age-related
pathophysiological changes observed in the myelin sheath.
The upregulation of the transcripts involved in the
neurotransmitter transport process, particularly the inhibitory
neurotransmitter pathway, was quite novel. Further studies
investigating the interrelation between the neurotransmitter
transport processes and the age-related myelin decline are
required, as, to the best of our knowledge, no previous studies
have reported this until now.
In conclusion, in the present study the
morphological changes in the myelin sheath in the optic nerve of
rats were analyzed at 10 time-points throughout life. Marked
alterations in the myelin sheath were observed in the optic nerves
of aging and aged rats, which became aggravated with age.
Age-related profiling of the myelin sheath in the optic nerves of
rats was established using microarray hybridization at 10
time-points throughout life, between birth and senescence. In the
present analysis 3,826 transcripts associated with age-induced
alterations in the myelin sheath in the optic nerve were
identified. It was also found that the most significantly altered
biological processes in aging were lipid metabolism, the immune
response and transmitter transport. This indicates that the
downregulation of lipid synthesis genes and the upregulation of
immune genes and neurotransmitter transport genes in aging may
provide a genetic basis for the age-related alterations observed in
the myelin sheath.
Acknowledgements
This study was supported by grants from the Major
State Basic Research Development Program of China (973 Program)
(nos. 2011CB504100 and 2013BAI04B04) and the National Natural
Science Foundation of China (no. 81171049).
References
|
1
|
Mold JW, Vesely SK, Keyl BA, Schenk JB and
Roberts M: The prevalence, predictors, and consequences of
peripheral sensory neuropathy in older patients. J Am Board Fam
Pract. 17:309–318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattay VS, Fera F, Tessitore A, et al:
Neurophysiological correlates of age-related changes in human motor
function. Neurology. 58:630–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyberg L, Lövdén M, Riklund K,
Lindenberger U and Bäckman L: Memory aging and brain maintenance.
Trends Cogn Sci. 16:292–305. 2012. View Article : Google Scholar
|
|
4
|
Bartzokis G, Lu PH and Mintz J: Human
brain myelination and amyloid beta deposition in Alzheimer’s
disease. Alzheimers Dement. 3:122–125. 2007.PubMed/NCBI
|
|
5
|
Sherin JE and Bartzokis G: Human brain
myelination trajectories across the lifespan: implications for CNS
function and dysfunction. Handbook of the Biology of Aging. Masoro
EJ and Austad SN: 7th edition. Academic Press; San Diego, CA: pp.
333–346. 2011, View Article : Google Scholar
|
|
6
|
Kochunov P, Thompson PM, Lancaster JL, et
al: Relationship between white matter fractional anisotropy and
other indices of cerebral health in normal aging: tract-based
spatial statistics study of aging. Neuroimage. 35:478–487. 2007.
View Article : Google Scholar
|
|
7
|
Verdú E, Ceballos D, Vilches JJ and
Navarro X: Influence of aging on peripheral nerve function and
regeneration. J Peripher Nerv Syst. 5:191–208. 2000.
|
|
8
|
Peters A: The effects of normal aging on
myelinated nerve fibers in monkey central nervous system. Front
Neuroanat. 3:112009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hinman JD and Abraham CR: What’s behind
the decline? The role of white matter in brain aging. Neurochem
Res. 32:2023–2031. 2007.
|
|
10
|
Peters A and Kemper T: A review of the
structal alterations in the cerebral hemispheres of the aging
rhesus monkey. Neurobiol Aging. 33:2357–2372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartzokis G: Alzheimer’s disease as
homeostatic responses to age-related myelin breakdown. Neurobiol
Aging. 32:1341–1371. 2011.
|
|
12
|
Bohnen NI and Albin RL: White matter
lesions in Parkinson disease. Nat Rev Neurol. 7:229–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ceballos D, Cuadras J, Verdú E and Navarro
X: Morphometric and ultrastructural changes with ageing in mouse
peripheral nerve. J Anat. 195:563–576. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma AK, Bajada S and Thomas PK: Age
changes in the tibial and plantar nerves of the rat. J Anat.
130:417–428. 1980.PubMed/NCBI
|
|
15
|
Xie F, Fu H, Hou JF, Jiao K, Costigan M
and Chen J: High energy diets-induced metabolic and prediabetic
painful polyneuropathy in rats. PLoS One. 8:e574272013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Huang J, Jiang Y, et al: Multi-stage
analysis of gene expression and transcription regulation in C57/B6
mouse liver development. Genomics. 93:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sandell JH and Peters A: Effects of age on
nerve fibers in the rhesus monkey optic nerve. J Comp Neurol.
429:541–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luebke J, Barbas H and Peters A: Effects
of normal aging on prefrontal area 46 in the rhesus monkey. Brain
Res Rev. 62:212–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majeed SK: Survey on spontaneous
peripheral neuropathy in aging rats. Arzneimittelforschung.
42:986–990. 1992.PubMed/NCBI
|
|
20
|
Verdier V, Csárdi G, de Preux-Charles AS,
et al: Aging of myelinating glial cells predominantly affects lipid
metabolism and immune response pathways. Glia. 60:751–760. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ginsberg SD: Expression profile analysis
of brain aging. Brain Aging: Models, Methods, and Mechanisms.
Riddle DR: CRC Press; Boca Raton: pp. 159–185. 2007, View Article : Google Scholar
|
|
22
|
Lee CK, Weindruch R and Prolla TA:
Gene-expression profile of the ageing brain in mice. Nat Genet.
25:294–297. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie F, Zhang JC, Fu H and Chen J:
Age-related decline of myelin proteins is highly correlated with
activation of astrocytes and microglia in the rat CNS. Int J Mol
Med. 32:1021–1028. 2013.PubMed/NCBI
|
|
24
|
Mäurer M, Kobsar I, Berghoff M, Schmid CD,
Carenini S and Martini R: Role of immune cells in animal models for
inherited neuropathies: facts and visions. J Anat. 200:405–414.
2002.PubMed/NCBI
|
|
25
|
Hinman JD, Duce JA, Siman RA, Hollander W
and Abraham CR: Activation of calpain-1 in myelin and microglia in
the white matter of the aged rhesus monkey. J Neurochem.
89:430–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sloane JA, Hinman JD, Lubonia M, Hollander
W and Abraham CR: Age-dependent myelin degeneration and proteolysis
of oligodendrocyte proteins is associated with the activation of
calpain-1 in the rhesus monkey. J Neurochem. 84:157–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramaglia V, Tannemaat MR, de Kok M, et al:
Complement inhibition accelerates regeneration in a model of
peripheral nerve injury. Mol Immunol. 47:302–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chrast R, Saher G, Nave KA and Verheijen
MH: Lipid metabolism in myelinating glial cells: lessons from human
inherited disorders and mouse models. J Lipid Res. 52:419–434.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rangaraju S, Hankins D, Madorsky I, et al:
Molecular architecture of myelinated peripheral nerves is supported
by calorie restriction with aging. Aging Cell. 8:178–191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saher G, Quintes S, Möbius W, et al:
Cholesterol regulates the endoplasmic reticulum exit of the major
membrane protein P0 required for peripheral myelin compaction. J
Neurosci. 29:6094–6104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Verheijen MH, Camargo N, Verdier V, et al:
SCAP is required for timely and proper myelin membrane synthesis.
Proc Natl Acad Sci USA. 106:21383–21388. 2009. View Article : Google Scholar : PubMed/NCBI
|