Introduction
Prostate cancer (PCa) is the second leading cause of
increased cancer incidence and cancer-associated mortality among
males in the United States (1). In
2010, the incidence of new prostate cancer cases was estimated to
be 217,730, resulting in 32,050 mortalities (2). The majority of patients with advanced
PCa develop bone metastases and suffer from long-term skeletal
morbidity, involving pain, pathological fractures and spinal cord
compression, which has a significant impact on the quality of life
of the patient. Therefore, studying the molecular mechanisms
underlying PCa bone metastasis is considered essential for the
prevention and treatment of PCa.
Peroxisome proliferator-activated receptor (PPAR) γ
regulates the expression of genes involved in the control of lipid
metabolism and insulin sensitivity via ligand-activated
transcriptional activity (3).
PPARγ ligands include naturally occurring fatty acids,
15-deoxy-delta12,14-prostaglandin J2 (PGJ2) and thiazolidinediones
(TZDs), such as troglitazone and rosiglitazone (RSG) (4). The activation of PPARγ by TZDs and
other ligands has been shown to inhibit proliferation and invasion,
as well as induce apoptosis and cell cycle arrest, in prostate and
other cancer cells through PPARγ-dependent and -independent
pathways (5–9). Therefore, PPARγ is recognized as a
relevant target for cancer therapy.
The present study aimed to investigate the effect of
PPARγ activation by ligands on tumor cell migration and invasion.
C-X-C chemokine receptor type 4 (CXCR4) has been reported to
mediate proliferation, invasion and metastasis of tumor cells;
therefore, it was hypothesized that RSG may modulate the expression
of CXCR4 and inhibit the migration and invasion of prostate cancer
cells.
Materials and methods
Cell culture
PC-3 human prostate carcinoma cells were initially
stored frozen in our laboratory (Department of Orthopedics, Tonji
Medical College, Wuhan, China) prior to cultivation in RPMI-1640
medium (Hyclone Laboratories, Inc., Logan, UT, USA) supplemented
with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA),
1×105 U/l penicillin and streptomycin (Hyclone
Laboratories, Inc.) in an incubator at 37°C with 5%
CO2.
Cell migration assay
Cell motility was assessed using an in vitro
wound healing assay. PC-3 cells were plated in a six-well plate and
grown until confluent. Monolayers of confluent PC-3 cells were then
scarred, and the repair was monitored using an inverted microscope
(Nikon TE200-S; Nikon Corporation, Tokyo, Japan), following 12 h
pretreatment with 10 μM RSG (Sigma Aldrich, St. Louis, MO, USA)
then 24 h exposure to 100 ng/ml CXCL12 (R&D Systems Inc.,
Minneapolis, MN, USA). Wound width was measured at 0 and 24 h
following the start of incubation with or without RSG and in the
absence or presence of CXCL12. Experiments were performed in
triplicate and data are presented as the mean ± standard
deviation.
Cell invasion assay
The invasive potential of PC-3 cells was quantified
using a Matrigel™-coated Transwell system, as described previously
(10). The chamber (Corning Inc.,
Corning, NY, USA) contained a polycarbonate membrane filter with a
pore size of 8 μm, which was coated with Matrigel and inserted into
a 24-well culture plate. PC-3 cells were then seeded in the top
chamber of the Matrigel. Following pre-incubation with or without
RSG (10 μM) for 12 h, Transwell chambers were then placed into the
wells of a 24 well plate, in which either basal medium or basal
medium supplemented with 100 ng/ml CXCL12 was added for 24 h. After
48 h incubation, PC-3 cells on the upper surface of the filters
were removed using cotton swabs. Cells that had invaded the lower
surface of the membrane were fixed using methanol and stained with
crystal violet. Each experiment was performed in triplicate and
cells were counted in five fields in each well using light
microscopy. The invasive ability of PC-3 cells was assessed
relative to the invasive ability of the untreated control
cells.
Western blot analysis
Cells were grown on six-well culture plates, washed
with 1X phosphate-buffered saline (PBS) and harvested in lysis
buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
Na2EDTA, 1 mM ethylene glycol tetraacetic acid (EGTA),
1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1
mM Na3VO4, 1 μg/ml leupeptin and 1 mM
phenylmethanesulfonyl fluoride (Cell Signaling Technology Inc.,
Beverly, MA, USA). The cell extractions were collected and
centrifuged at 10,000 × g for 10 min at 4°C before the supernatants
were collected as cell lysates. Equal concentrations of total cell
lysate were resolved using 10% SDS-PAGE (Boster, Wuhan, China) and
transferred to a polyvinylidene fluoride (PVDF) transfer membrane
(Merck Millipore, Billerica, MA, USA). Non-specific binding sites
were blocked using 5% non-fat dry milk in 1X Tris-buffered saline
containing 0.1% Tween 20 (TBST; Boster), followed by incubation
with primary antibodies against the proteins of interest in 3%
bovine serum albumin (BSA; Boster)-TBST [phosphorylated Akt
(p-Akt), CXCR4] or 5% non-fat dry milk-TBST (Akt, β-actin).
Subsequently, the membranes were incubated with an appropriate
secondary antibody (horseradish peroxidase-conjugated goat
anti-mouse or anti-rabbit immunoglobulin G; Wuhan Boster Biological
Technology Ltd, Wuhan, China). Immunoblots were visualized using
SuperSignal™ West Pico Chemiluminescent substrate (Thermo Fisher
Scientific, Waltham, MA, USA). The antibodies raised against human
Akt, p-Akt (Ser473) and β-actin (rabbit monoclonal IgG) were
purchased from Cell Signaling Technology. The antibody raised
against human CXCR4 (rabbit polyclonal IgG) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Quantitative polymerase chain reaction
(qPCR) analysis
PC-3 cells were plated in six-well plates for all
experiments and allowed to grow for 48 h prior to any treatment.
GW9662 (Santa Cruz Biotechnology, Inc.) was added 2 h prior to any
other treatment. Total RNA was extracted using TRIzol®
reagent according to the manufacturer’s instructions (Invitrogen
Life Technologies, Carlsbad, CA, USA). A total of 1 μg RNA was
reverse-transcribed according to the kit’s instructions (Invitrogen
Life Technologies). qPCR was performed using TransStart Green qPCR
SuperMix (TransGen Biotech Co., Beijing, China) using an iQ5
Sequence Detection System (Bio-Rad Laboratories Inc., Berkley, CA,
USA). Results were normalized to those obtained for GAPDH. Each
sample was analyzed in triplicate and the experiment was repeated
twice. The theshold cycle (CT) value (the cycle number at which the
fluorescence crosses the threshold) was measured and
2−ΔCT (ΔCT = CT − CTGAPDH) was defined as the
quantity of the amplified fragment. The primer sequences are listed
in Table I.
 | Table IPrimer sequences for qPCR
analysis. |
Table I
Primer sequences for qPCR
analysis.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| GAPDH |
CCATGAGAAGTATGACAACAGCC |
GGGTGCTAAGCAGTTGGTG |
| CXCR4 |
TGCCCACCATCTACTCCATCA |
AGGATGACCAATCCATTGCCC |
| VEGF |
TTACGGTCTGTGTCCAGTGTA |
TTCTCTGTTATGTTGCCAGCC |
| MMP2 |
GATACCCCTTTGACGGTAAGGA |
CCTTCTCCCAAGGTCCATAGC |
| MMP9 |
TGTACCGCTATGGTTACACTCG |
GGCAGGGACAGTTGCTTCT |
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS 11.0 statistics
software (SPSS, Inc., Chicago, IL, USA). Statistical significance
was analyzed using one-way analysis of variance. Where significance
was observed, a Dunnett’s post-hoc test was used to determine the
statistical significance of the differences between the treated-
and untreated-groups, with a value of P<0.05 considered to
indicate a statistically significant difference.
Results
RSG suppresses CXCR4 mRNA and protein
levels in prostate cancer cells
A previous study has shown that CXCR4 expression is
significantly higher in human PCa tissue than in hyperplastic
prostate tissues (11). This
finding suggests that CXCL12 may exhibit an autocrine regulatory
role via its receptor, CXCR4, in the regulation of PCa cell
migration, invasion and metastasis (12). Therefore, the initial
investigations in the present study focused on the effect of RSG on
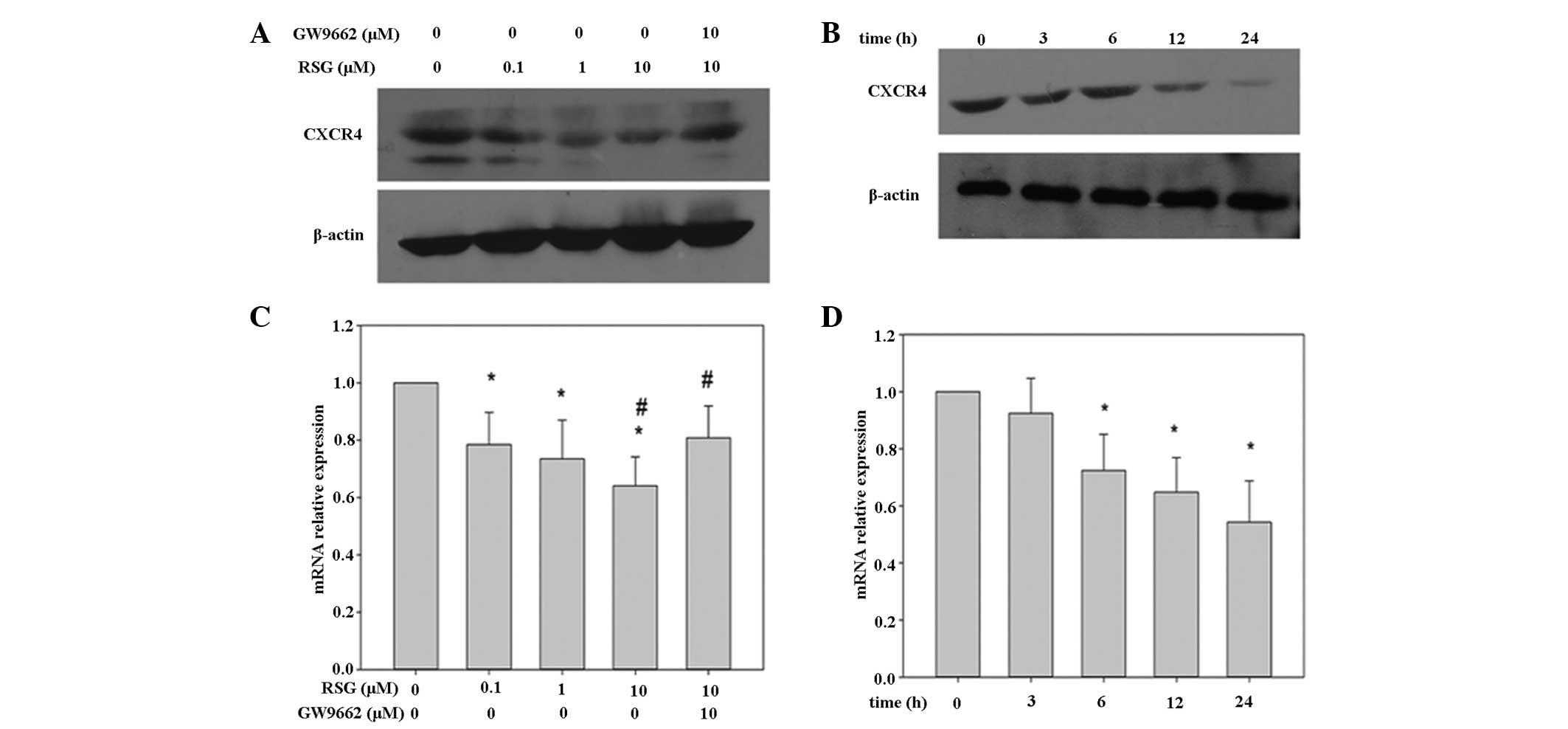
the expression of CXCR4 in PC-3 cells. It has been reported that
TZDs activate PPARα and PPARδ receptors at concentrations >10 μM
(13); therefore, concentrations
of ≤10 μM were utilized in the present investigations. It was
observed that when PC-3 cells were incubated with various RSG
concentrations for 24 h, or for various time periods with 10 μM
RSG, the expression of CXCR4 was suppressed in a dose- and time-
dependent manner, respectively (Fig.
1A and B). This downregulation was not due to a decrease in
cell viability, as ~90% of PC-3 cells were viable under these
conditions (data not shown).
It was hypothesized that the suppression of CXCR4
expression may occur at the transcriptional level. Therefore,
following PC-3 cell treatment with RSG for various time-periods,
the mRNA was extracted for qPCR analysis. As shown in Fig. 1D, RSG was found to downregulate
CXCR4 mRNA expression in a time-dependent manner, with a
significant reduction observed from 6 h following exposure. CXCR4
mRNA expression was inhibited at all RSG doses tested, with 22, 27
and 36% inhibition observed following 12 h exposure to 0.1, 1 and
10 μM RSG, respectively (Fig
1C).
Although RSG was found to affect CXCR4 expression in
PC-3 cells, the mechanism by which this was achieved was unclear.
To determine whether the reduction in expression of CXCR4 by RSG
was dependent upon the activation of PPARγ, PC-3 cells were treated
with the PPARγ antagonist, GW9662. The downregulation of CXCR4 mRNA
and protein expression induced by RSG in PC-3 cells was attenuated
upon addition of GW9662 (Fig. 1).
These results indicate that RSG is capable of downregulating CXCR4
expression in a manner dependent upon PPARγ activation. In
combination, these data suggest that RSG may inhibit CXCR4
expression in PC-3 cells, in a PPARγ-dependent manner.
RSG inhibits CXCL12-induced migration in
PC-3 cells
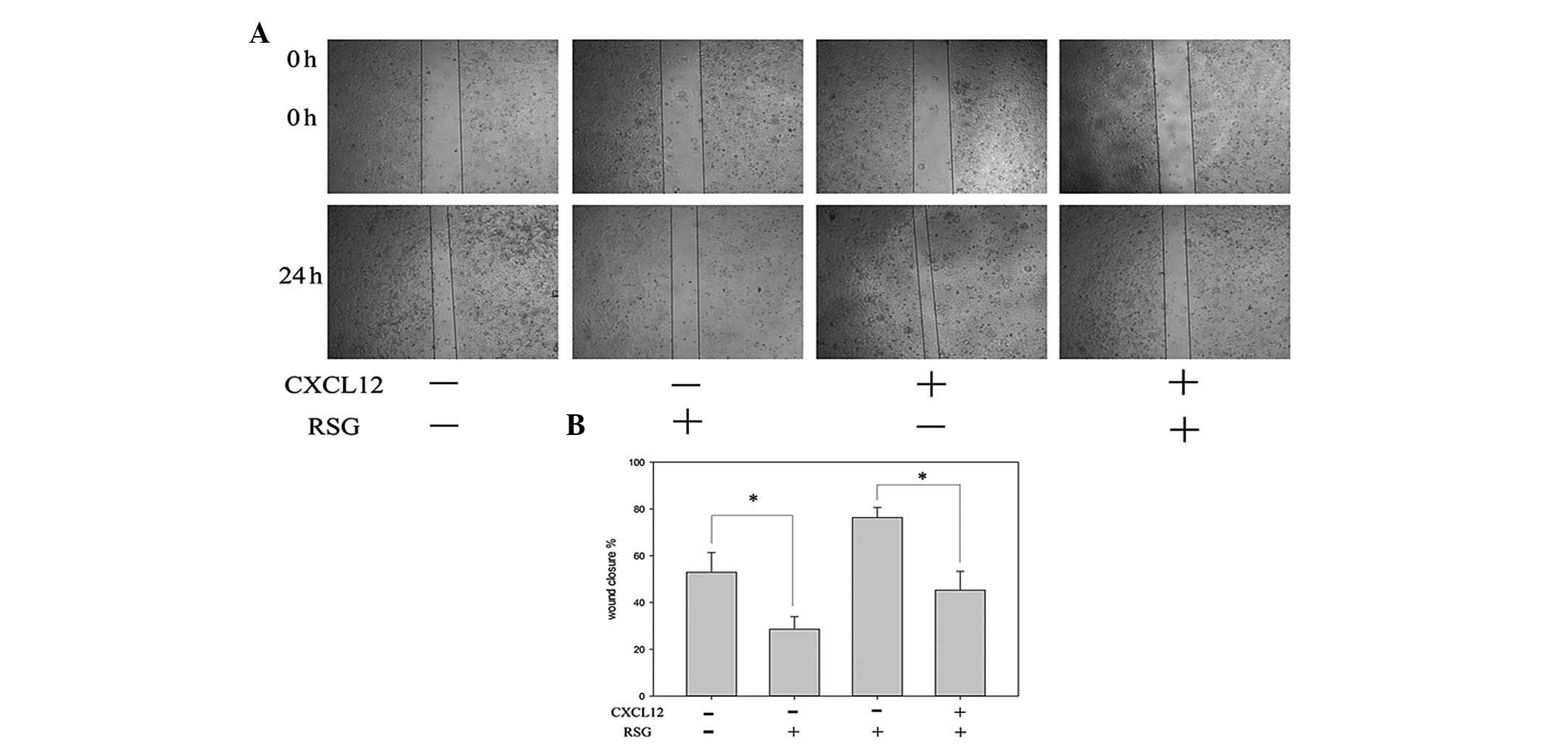
This study further investigated a potential
correlation between RSG-induced downregulation of CXCR4 and PCa
cell migration. An in vitro wound healing assay revealed
that PC-3 cells migrated more rapidly upon treatment with CXCL12
and that this effect was abolished upon treatment with RSG
(Fig. 2).
RSG inhibits CXCL12-induced invasion in
PC-3 cells
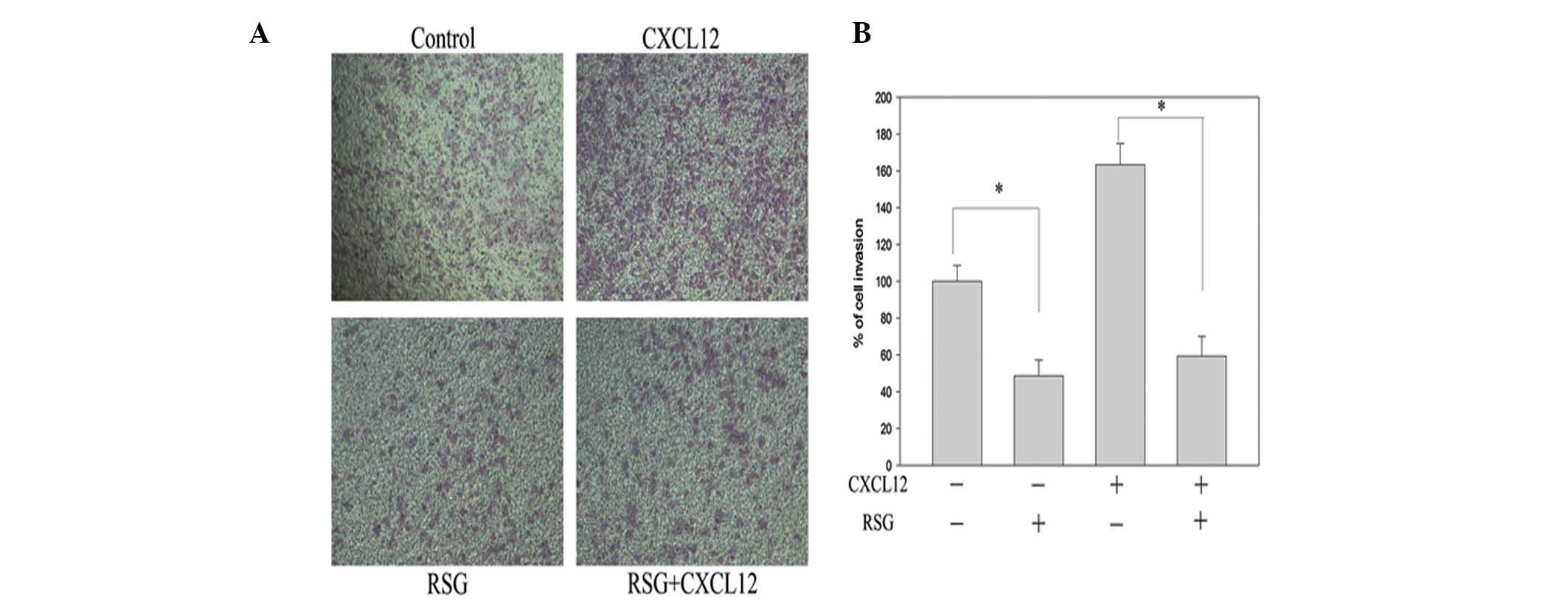
To elucidate the inhibitory effect of RSG upon
CXCL12-induced invasion in PC-3 cells, chamber invasion assays were
performed. RSG was observed to suppress the invasion of PC-3 cells
across the Matrigel-coated filter in a dose-dependent manner, with
10 μM RSG found to inhibit 52% of cell invasion. RSG was also
observed to suppress the CXCL12-induced invasion of the PC-3 cells
(Fig. 3). These results indicate
that RSG markedly inhibits the CXCL12-induced invasion of PC-3
cells.
RSG inhibits CXCL12-induced Akt
activation in prostate cancer cells
It has previously been indicated that the signaling
proteins, PI3K and Akt, may be associated with the expression of
matrix-metalloproteinases (MMPs) and metastasis induction (14). To further elucidate the signal
transduction pathways responsible for CXCR4 expression and PC-3
cell migration and invasion, the activation of Akt, a signaling
component of pathways coupled to G-protein-coupled chemoattractant
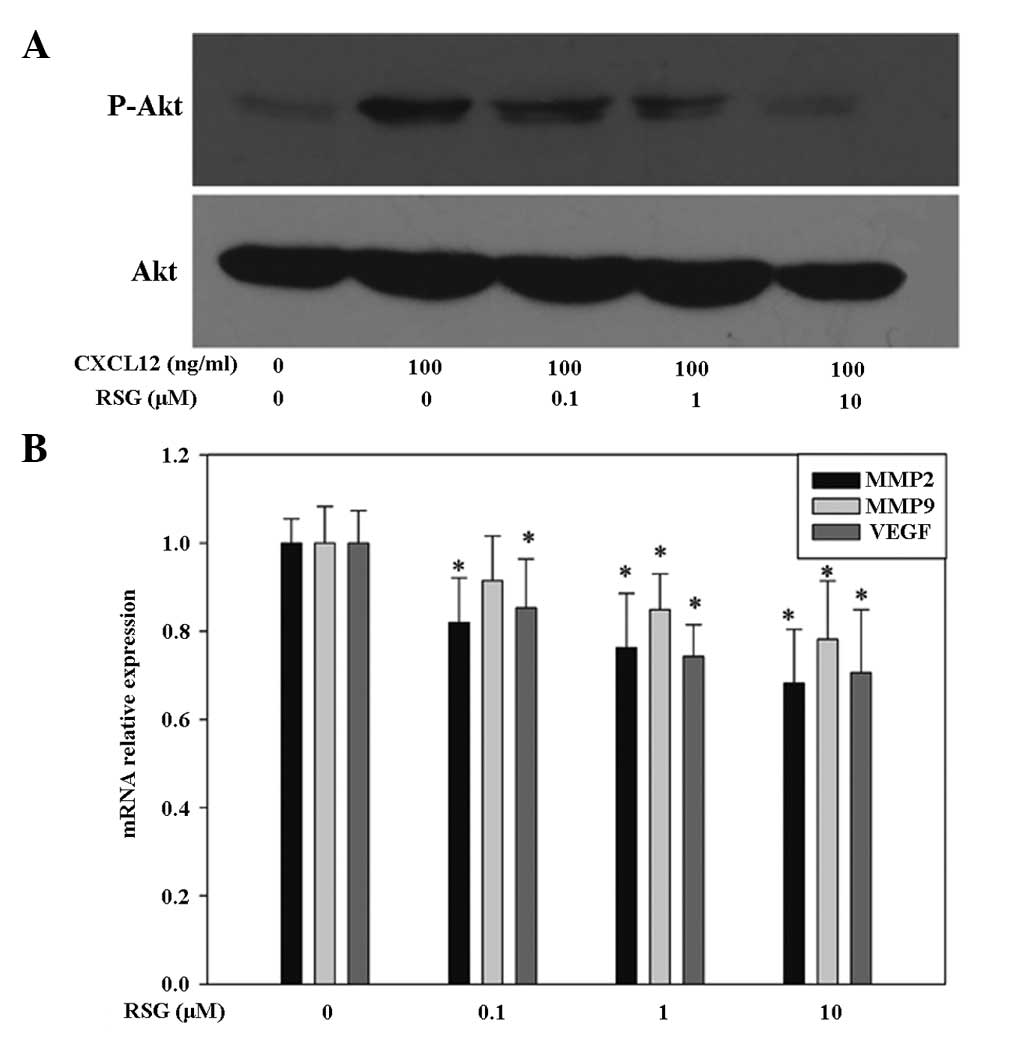
receptors, was examined. Upon rapid stimulation (30 min) of PC-3
cells with 100 ng/ml CXCL12, an increase in Akt phosphorylation at
Ser473 was observed, resulting in the activation of the enzyme.
Furthermore, it was observed that concomitant addition of 10 μM RSG
for 30 min downregulated the CXCL12-induced phosphorylation of Akt
in a dose-dependent manner in PC-3 cells (Fig. 4A). This finding suggests that the
inhibition of migration and invasion associated with RSG treatment
in PC-3 cells may partly occur through the suppression of PI3K
pathways.
In addition to the PI3K/Akt pathway, other signaling
pathways downstream of the activated CXCR4/CXCL12 axis were
investigated. PC-3 cells were treated with various concentrations
of RSG for 12 h and the expression of MMP-2, MMP-9 and vascular
endothelial growth factor (VEGF) mRNA were then analyzed using
qPCR, respectively. As demonstrated in Fig. 4, RSG significantly downregulated
MMP-2, MMP-9 and VEGF mRNA levels in a dose-dependent manner.
Discussion
Activation of PPARγ by TZDs and other ligands has
been shown to inhibit proliferation and invasion, as well as induce
apoptosis and cell cycle arrest in prostate and other cancer cells
through PPARγ-dependent and independent pathways (5–9).
These findings suggest that activation of PPARγ may demonstrate
anticarcinogenic potential. In the present study, it was observed
that RSG was capable of downregulating CXCL12-induced migration,
invasion and PI3K/Akt activation in the PC-3 PCa cell line, through
inhibition of the CXCL12/CXCR4 axis. This suggests that PPARγ may
possess antimetastatic potential.
In addition to PPARγ-dependent effects, TZDs have
been reported to induce cellular effects independent of PPARγ
activation (15). Therefore,
although the potency correlation in the present study was
indicative of PPARγ involvement, a PPARγ-selective antagonist was
used to confirm PPARγ involvement. The PPARγ antagonist, GW9662,
reduced the effect of RSG on CXCR4 expression. Therefore, these
findings suggest that TZD-induced CXCR4 downregulation occurs in a
PPARγ-dependent manner. This result was in accordance with that of
Richard and Blay, who demonstrated that PPARγ agonists inhibited
CXCR4 expression in a PPARγ-dependent manner, which was also time-
and dose-dependent (16).
Furthermore, pre-clinical studies have shown that the parent
compound TZD is capable of reducing metastasis of HT-29 cells
implanted in the rectums of mice (17). However, in a Phase II study of
patients with advanced metastatic colorectal cancer that had not
responded to chemotherapy, troglitazone failed to produce an
objective tumor response (18).
Whether treatment with a more potent and selective PPARγ agonist,
such as RSG, is also associated with a lack of tumor response is
yet to be elucidated. The present data suggest that such an agent
may be capable of reducing CXCR4 expression to a greater extent,
and may therefore be anticipated to demonstrate greater potential
for reducing metastasis.
Metastasis is the spread of a disease from one organ
or tissue to another non-adjacent organ or tissue, and is regulated
by numerous signaling pathways in cancer cells and the
microenvironment. The CXCR4/CXCL12 axis has a role in cancer cell
metastasis and proliferation, the importance of which may vary
between different types of cancer cells, due to differences in
expression. For example, overexpression of CXCR4 in PCa cells has
been shown to accelerate prostate tumor metastasis and
vascularization, as well as tumor growth in vivo (19,20).
Furthermore, CXCL12 stimulates chemotaxis of metastatic PCa cells
expressing high levels of CXCR4, and accelerates their migration
(20). Conversely, blockade of
CXCR4/CXCL12 interaction in prostate cancer cells via CXCR4
knockdown has been found to significantly inhibit bone metastasis
in vivo (21).
Another important signaling pathway in PCa cells is
the PI3K/Akt pathway (22,23). Akt is a serine-threonine kinase
whose phosphorylation is associated with mitogenic signals. In
addition to its role in survival, Akt has been found to participate
in numerous intracellular signaling pathways, including the
integration of proliferation and differentiation signals, such as
migration and angiogenesis. Previous studies have demonstrated that
the PI3K/Akt pathway may also have a role in CXCL12/CXCR4-mediated
PCa cell migration and angiogenesis (24,25).
In this study, it was observed that concomitant addition of RSG
downregulated CXCL12-induced phosphorylation of Akt in a
dose-dependent manner in PC-3 cells, indicating that the
inhibition of migration and invasion by RSG may partly occur
through suppression of PI3K pathways. In accordance with the
present study, it has previously been reported that PPARγ agonists
may be capable of decreasing Akt phosphorylation (26,27).
RSG was also observed to demonstrate a similar affect on insulin
growth factor 1-induced phosphorylation of Akt and extracellular
signal-regulated kinases in adrenocortical cancer cells (28).
In conclusion, the present data indicate that RSG is
capable of inhibiting CXCL12-induced invasion and migration of PC-3
cells in vitro, through inhibition of CXCR4 expression and
Akt phosphorylation. This study may provide preliminary evidence
for further research into the mechanisms underlying the inhibition
of metastasis by TZDs. In the present study, RSG was used only as a
proof-of-principle to determine the efficacy of the PPARγ agonist
class of drugs to inhibit CXCR4 expression. Based on the unclear
association between RSG and risk of myocardial infarction, as well
as the apparently higher risk of long-bone fracture in females that
is associated with RSG, detailed safety, pharmacokinetic and
pharmacodynamic studies of this class of drugs are necessary prior
to clinical use in patients.
Acknowledgements
This study was supported by a grant from the Key
Laboratory of Cancer Invasion and Metastasis of the Ministry of
Education, China and the Ministry of Education of People’s Republic
of China (no. 200804871-051).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Kota BP, Huang TH and Roufogalis BD: An
overview on biological mechanisms of PPARs. Pharmacol Res.
51:85–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou J, Zhang W, Liang B, et al: PPARgamma
activation induces autophagy in breast cancer cells. Int J Biochem
Cell Biol. 41:2334–2342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim S, Lee JJ and Heo DS: PPARγ ligands
induce growth inhibition and apoptosis through p63 and p73 in human
ovarian cancer cells. Biochem Biophys Res Commun. 406:389–395.
2011.
|
|
6
|
Lyles BE, Akinyeke TO, Moss PE and Stewart
LV: Thiazolidinediones regulate expression of cell cycle proteins
in human prostate cancer cells via PPARgamma-dependent and
PPARgamma-independent pathways. Cell Cycle. 8:268–277. 2009.
View Article : Google Scholar
|
|
7
|
Shen D, Deng C and Zhang M: Peroxisome
proliferator-activated receptor gamma agonists inhibit the
proliferation and invasion of human colon cancer cells. Postgrad
Med J. 83:414–419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jan HJ, Lee CC, Lin YM, Lai JH, Wei HW and
Lee HM: Rosiglitazone reduces cell invasiveness by inducing MKP-1
in human U87MG glioma cells. Cancer Lett. 277:141–148. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita M, Yagami T, Fujio M, et al:
Cytotoxicity of troglitazone through PPARγ-independent pathway and
p38 MAPK pathway in renal cell carcinoma. Cancer Lett. 312:219–227.
2011.
|
|
10
|
Liu H, Chen A, Guo F and Yuan L: A
short-hairpin RNA targeting osteopontin downregulates MMP-2 and
MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett.
295:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang K, Chen D, Jiao X, et al: Slug
enhances invasion ability of pancreatic cancer cells through
upregulation of matrix metalloproteinase-9 and actin cytoskeleton
remodeling. Lab Invest. 91:426–438. 2011. View Article : Google Scholar
|
|
12
|
Shanmugam MK, Manu KA, Ong TH, et al:
Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to
suppression of metastasis in transgenic adenocarcinoma of mouse
prostate model. Int J Cancer. 129:1552–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita D, Shimizu M and Osumi T:
Mechanism for the action of PPARs. Nihon Rinsho. 63:536–537.
2005.(In Japanese).
|
|
14
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han S and Roman J: Rosiglitazone
suppresses human lung carcinoma cell growth through
PPARgamma-dependent and PPARgamma-independent signal pathways. Mol
Cancer Ther. 5:430–437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Richard CL and Blay J: Thiazolidinedione
drugs down-regulate CXCR4 expression on human colorectal cancer
cells in a peroxisome proliferator activated receptor
gamma-dependent manner. Int J Oncol. 30:1215–1222. 2007.
|
|
17
|
Yoshizumi T, Ohta T, Ninomiya I, et al:
Thiazolidinedione, a peroxisome proliferator-activated
receptor-gamma ligand, inhibits growth and metastasis of HT-29
human colon cancer cells through differentiation-promoting effects.
Int J Oncol. 25:631–639. 2004.
|
|
18
|
Kulke MH, Demetri GD, Sharpless NE, et al:
A phase II study of troglitazone, an activator of the PPARgamma
receptor, in patients with chemotherapy-resistant metastatic
colorectal cancer. Cancer J. 8:395–399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Darash-Yahana M, Pikarsky E, Abramovitch
R, et al: Role of high expression levels of CXCR4 in tumor growth,
vascularization, and metastasis. FASEB J. 18:1240–1242.
2004.PubMed/NCBI
|
|
20
|
Arya M, Patel HR, McGurk C, et al: The
importance of the CXCL12-CXCR4 chemokine ligand-receptor
interaction in prostate cancer metastasis. J Exp Ther Oncol.
4:291–303. 2004.PubMed/NCBI
|
|
21
|
Xing Y, Liu M, Du Y, et al: Tumor
cell-specific blockade of CXCR4/SDF-1 interactions in prostate
cancer cells by hTERT promoter induced CXCR4 knockdown: A possible
metastasis preventing and minimizing approach. Cancer Biol Ther.
7:1839–1848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kreisberg JI, Malik SN, Prihoda TJ, et al:
Phosphorylation of Akt (Ser473) is an excellent predictor of poor
clinical outcome in prostate cancer. Cancer Res. 64:5232–5236.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ayala G, Thompson T, Yang G, et al: High
levels of phosphorylated form of Akt-1 in prostate cancer and
non-neoplastic prostate tissues are strong predictors of
biochemical recurrence. Clin Cancer Res. 10:6572–6578. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Wang J, Sun Y, Song W, Nor JE,
Wang CY and Taichman RS: Diverse signaling pathways through the
SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to
altered patterns of cytokine secretion and angiogenesis. Cell
Signal. 17:1578–1592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinni SR, Sivalogan S, Dong Z, et al:
CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in
prostate cancer cells: the role of bone microenvironment-associated
CXCL12. Prostate. 66:32–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goetze S, Eilers F, Bungenstock A, et al:
PPAR activators inhibit endothelial cell migration by targeting
Akt. Biochem Biophys Res Commun. 293:1431–1437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen WC, Lin MS and Bai X: Induction of
apoptosis in colorectal cancer cells by peroxisome
proliferators-activated receptor gamma activation up-regulating
PTEN and inhibiting PI3K activity. Chin Med J (Engl).
118:1477–1481. 2005.PubMed/NCBI
|
|
28
|
Cantini G, Lombardi A, Piscitelli E, et
al: Rosiglitazone inhibits adrenocortical cancer cell proliferation
by interfering with the IGF-IR intracellular signaling. PPAR Res.
2008:9040412008. View Article : Google Scholar : PubMed/NCBI
|