Introduction
Pulmonary arterial hypertension (PAH) is
characterized by vascular remodeling and a progressive increase in
pulmonary vascular resistance, which ultimately leads to right
ventricular failure and death (1).
The abnormal proliferation of pulmonary artery smooth muscle cells
(PASMCs) is an important feature of PAH that contributes to
vascular remodeling and leads to vascular occlusion (2). It is therefore important to clarify
the specific molecular mechanisms and signaling pathways that lead
to the proliferation of PASMCs. Numerous studies have demonstrated
that the pathological proliferation of PASMCs is associated with
mitogen-activated protein kinase (MAPK) and AKT signaling pathways
(3,4).
The platelet-derived growth factor (PDGF) signaling
system consists of four ligands, PDGF-A, PDGF-B, PDGF-C and PDGF-D,
and two types of receptor, PDGF α-receptor (PDGFR-α) and β-receptor
(PDGFR-β) (5). PDGF-BB induces the
proliferation of vascular smooth muscle cells (VSMCs) and has been
proposed to function in the development of atherosclerosis, lung
fibrosis, PAH and chronic thromboembolic pulmonary hypertension
(6–8). Furthermore, the levels of PDGF in the
blood and lung tissues of patients with PAH are increased, further
suggesting that PDGF plays a critical role in the development of
pulmonary vascular remodeling and the increase in pulmonary
arterial pressure (9).
Rhodiola is a widely used medicinal plant that is
grown at high altitudes and has a long history of use by Tibetans
to enhance the resistance of the body to fatigue. Studies have
shown that Rhodiola has various pharmacological properties and
exerts anti-inflammatory, anti-anoxia, anti-oxidation, anti-aging,
anti-cancer and liver-protective effects (10–14).
Salidroside (2-(4-hydroxyphenyl)ethyl β-D-glucopyranosidee) is one
of the major bioactive components extracted from Rhodiola. In
lipopolysaccharide (LPS)-induced mastitis, salidroside has been
shown to inhibit the extracellular signal-regulated kinase (ERK),
p38 and c-Jun N-terminal kinase (JNK) signaling pathways to inhibit
inflammation (15). Salidroside
can also inhibit the reactive oxygen species-protein kinase
C-ERK1/2 signaling pathway, decreasing the proliferation of HT1080
human fibrosarcoma cells (16).
Furthermore, salidroside inhibits the proliferation of mesangial
cells, induced by high glucose levels, by blocking the ERK1/2
signaling pathway (17). MCF-7
human breast cancer cells can be arrested in G0/G1 phase by
salidroside (18). However, the
effects of salidroside on the proliferation of PASMCs and its
associated mechanisms remain unclear. This study aimed to determine
whether salidroside could inhibit the PDGF-BB-induced proliferation
of PASMCs, as well as to identify the molecular mechanisms that may
be responsible for the protective effects of salidroside on
PAH.
Materials and methods
Materials
Salidroside (98% purity as determined by
high-performance liquid chromatography analysis) was ordered from
Shanghai Medical Technology Development Co., Ltd. (Shanghai,
China). Recombinant human PDGF-BB was ordered from PeproTech (Rock
Hill, NJ, USA). Cell counting kit-8 (CCK-8) was obtained from
Dojindo Molecular Technologies Inc. (Kumamoto, Japan). A cell
proliferation ELISA, BrdU (colorimetric) kit was purchased from
Roche (Roche Diagnostics, Mannheim, Germany). TRIzol®
was purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). The antibodies used to recognize the total and phosphorylated
forms of ERK1/2, p38, JNK, AKT, glycogen synthase kinase 3 β
(GSK3β) and GAPDH were ordered from Cell Signaling Technology Inc.
(Danvers, MA, USA). Sprague Dawley rats (150–200 g) were ordered
from the Wuhan University Center for Animal Experiment (Wuhan,
China). All of the animal experiments were approved by the
Institutional Animal Care and Use Committee at Renmin Hospital,
Wuhan University (Wuhan, China). For the in vitro study,
salidroside was dissolved in double-distilled water.
Collagenase I (0.2%) digestion of PASMCs
and cell culture
Sprague Dawley rats (150–200 g) received
intraperitoneal anesthesia with 50 mg/kg 1% sodium pentobarbital.
The pleura of each rat was then rapidly sectioned, and the heart
and lung were removed and placed in a petri dish filled with
phosphate-buffered saline to clean the residual blood in a
ultra-clean platform. To separate the pulmonary artery, the outer
fibrous arterial connective tissue was stripped under a microscope
using tweezers and rinsed several times in Dulbecco’s modified
Eagle’s medium/F12 (DMEM/F12) containing 1%
penicillin-streptomycin. The artery was subsequently cut into 1-mm
pieces and placed in a centrifuge tube pre-filled with 0.2%
collagenase I, and the tube was then placed in a CO2
incubator to help remove digestive juices. Once every 20 to 30 min,
the mixture was observed and gently agitated. The total duration of
the digestion was 2–4 h. Following arterial fragment digestion, the
cells were centrifuged at 195 × g for 5 min, and the supernatant
was discarded. The pellet was rinsed with DMEM/F12 containing 20%
fetal bovine serum and placed in a 37°C, 5% CO2
incubator for culturing. Four to five days later, the cells were
passaged and grown in DMEM/F12 containing 10% fetal bovine serum.
The purity of the PASMC cultures was assessed by the
immunocytochemical localization of α-smooth muscle actin.
The cells used in this study were taken between
passages four and 10. The PASMCs were grown to 70–80% confluency
and then subjected to serum starvation for 24 h before being used
for the experiments. Cells were pretreated with different
concentrations of salidroside for 1 h prior to stimulation with
PDGF-BB (20 ng/ml).
Measurement of cell proliferation and DNA
synthesis
Cell proliferation was determined by CCK-8 assay
according to the manufacturer’s instructions (Dojindo Molecular
Technologies Inc.). PASMCs (5×103/well) were grown to
70–80% confluency in 96-well plates and their growth was arrested
by serum starvation for 24 h. Cells were subsequently preincubated
with various concentrations of salidroside for 1 h and then treated
with PDGF-BB (20 ng/ml) for 12, 24 and 48 h in the presence of
salidroside, prior to being loaded with CCK-8 solution for the
final 3 h. Cell proliferation was determined by measuring the
optical density at 450 nm. BrdU labeling mixture was added to each
well and incubated for 2 h. DNA synthesis was then measured by
assessing the incorporation of BrdU using a cell proliferation
ELISA kit.
Evaluation of cell viability
The toxicity of salidroside on PASMCs was determined
by the trypan blue exclusion test. After 12, 24 and 48 h of
incubation with salidroside at concentrations between 12.5 and 100
μM, the PASMCs were removed from culture and the cells that
excluded 0.4% trypan blue were counted using an automated cell
counter (Invitrogen Life Technologies).
Cell cycle progression assays
Cell cycle progression was determined using a cell
cycle and apoptosis analysis kit (Beyotime Institute of
Biotechnology, Haimen, China), in accordance with the
manufacturer’s instructions, and fluorescence-activated cell
sorting. Upon reaching 70–80% confluency in the six-well plates,
the PASMCs were subjected to serum starvation for 24 h. The cells
were then preincubated with salidroside (100 μM) for 1 h and
subsequently treated with PDGF-BB (20 ng/ml) for 24 h prior to
analysis.
Quantitative polymerase chain reaction
(qPCR)
Following serum starvation for 24 h and
preincubation with 100 μM salidroside for 1 h, the cells
were treated with PDGF-BB for 24 h in the absence or presence of
salidroside. Total RNA was extracted from PASMCs using TRIZol and
reverse transcribed into DNA using oligo (dT) primers with the
LightCycler 480 SYBR Green 1 Master mix and the LightCycler 480
qPCR system (both Roche Diagnostics). Target gene mRNA expression
was normalized to the internal control GAPDH and was expressed
relative to the control group. The primer sequences used were as
follows: Cyclin D1, forward 5′-GAGACCATCCCCCTGACGGC-3′ and reverse
5′-TCTTCCTCCTCCTCGGCGGC-3′; Cyclin E, forward
5′-GTCCTGGCTGAATGTATACATGC-3′ and reverse
5′-CCCTATTTTGTTCAGACAACATGGC-3′; CDK2, forward
5′-GCTTTCTGCCATTCTCATCG-3′ and reverse 5′-GTCCCCAGAGTCCGAAAGAT-3′;
CDK4, forward 5′-ATGTTGTCCGGCTGATGG-3′ and reverse
5′-CACCAGGGTTACCTTGATCTCC-3′; P27, forward
5′-TGCAACCGACGATTCTTCTACTCAA-3′ and reverse
5′-CAAGCAGTGATGTATCTGATAAACAAGGA-3′; GAPDH, forward
5′-ATTCCATGGCACCGTCAAGG-3′ and reverse
5′-AATTCGTTGTCATACCAGGA-3′.
Western blotting
Confluent, serum-starved PASMCs were treated with
salidroside (100 μM) for 1 h following exposure to 20 ng/ml
PDGF-BB for the indicated time. The cells were lysed in
radioimmunoprecipitation assay buffer containing a protease and
phosphatase inhibitor cocktail; the cells were then scraped into
1.5-ml centrifuge tubes. The cell suspension was centrifuged at
3,362 × g for 30 min at 4°C, and the protein concentration was
assessed by the bicinchoninic acid assay. A total of 20 μg protein
extract was used for SDS-PAGE, blotted onto Immobilon-FL transfer
membranes (Millipore, Billerica, MA, USA) and probed with the
relevant antibodies. The protein expression was quantified using
the Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln,
NE, USA) and protein expression levels were normalized to the GAPDH
internal control in the total cell lysate.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Differences among groups were tested by one-way analysis
of variance or unpaired two-tailed-tests. P<0.05 was considered
to indicate a statistically significant difference.
Results
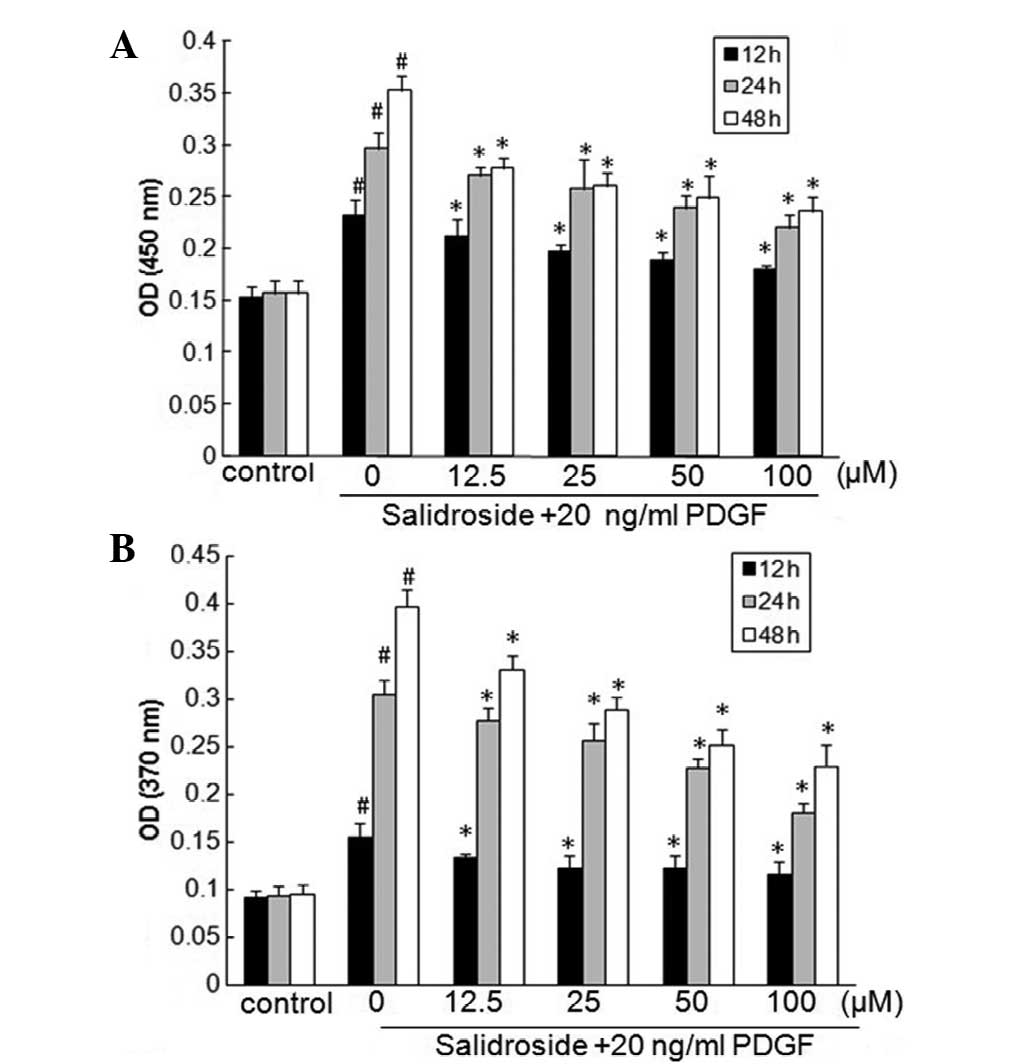
Salidroside inhibits the proliferation
and DNA synthesis of PASMCs induced by PDGF-BB
The abnormal proliferation of PASMCs contributes to
vascular lesion formation (2).
Salidroside is a proven antitumor compound that leads to the
suppression of cancer cell growth (18); however, whether salidroside
suppresses the growth of PASMC is currently unknown. To determine
the effect of salidroside on PASMC proliferation, the effect of
different doses of salidroside (12.5–100 μM) on
proliferation in 12, 24 and 48 h was investigated using the CCK-8
cell proliferation assay. Compared with the control, PDGF-BB
induced a time-dependent proliferation of PASMCs that was blocked
in a concentration-dependent manner by treatment with salidroside
for different lengths of time. The greatest level of suppression of
proliferation was induced by salidroside at a concentration of 100
μM (Fig. 1A). The inhibitory
effects of salidroside on DNA synthesis were next investigated by
measuring the incorporation of BrdU. Treatment with PDGF-BB
increased DNA synthesis in PASMCs in a time-dependent manner, and
salidroside significantly suppressed the increase in DNA synthesis
in a dose- and time-dependent manner (Fig. 1B).
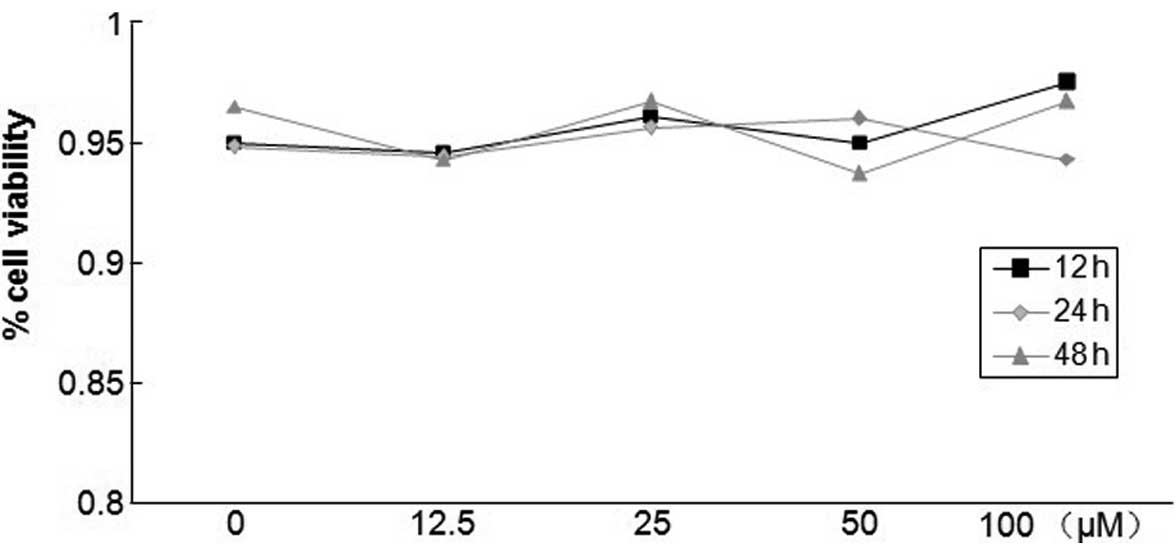
Different concentrations of salidroside
have no effect on PASMC survival
The toxicity of salidroside on PASMCs was determined
by the trypan blue exclusion test in the absence or presence of
salidroside. As shown in Fig. 2,
salidroside concentrations between 12.5 and 100 μM did not
induce significant levels of cell necrosis in PASMCs after 12, 24
or 48 h compared with untreated cells. Regardless of whether cells
were treated with salidroside, cell viability was maintained at
~95%, suggesting that salidroside was not cytotoxic at the tested
concentrations.
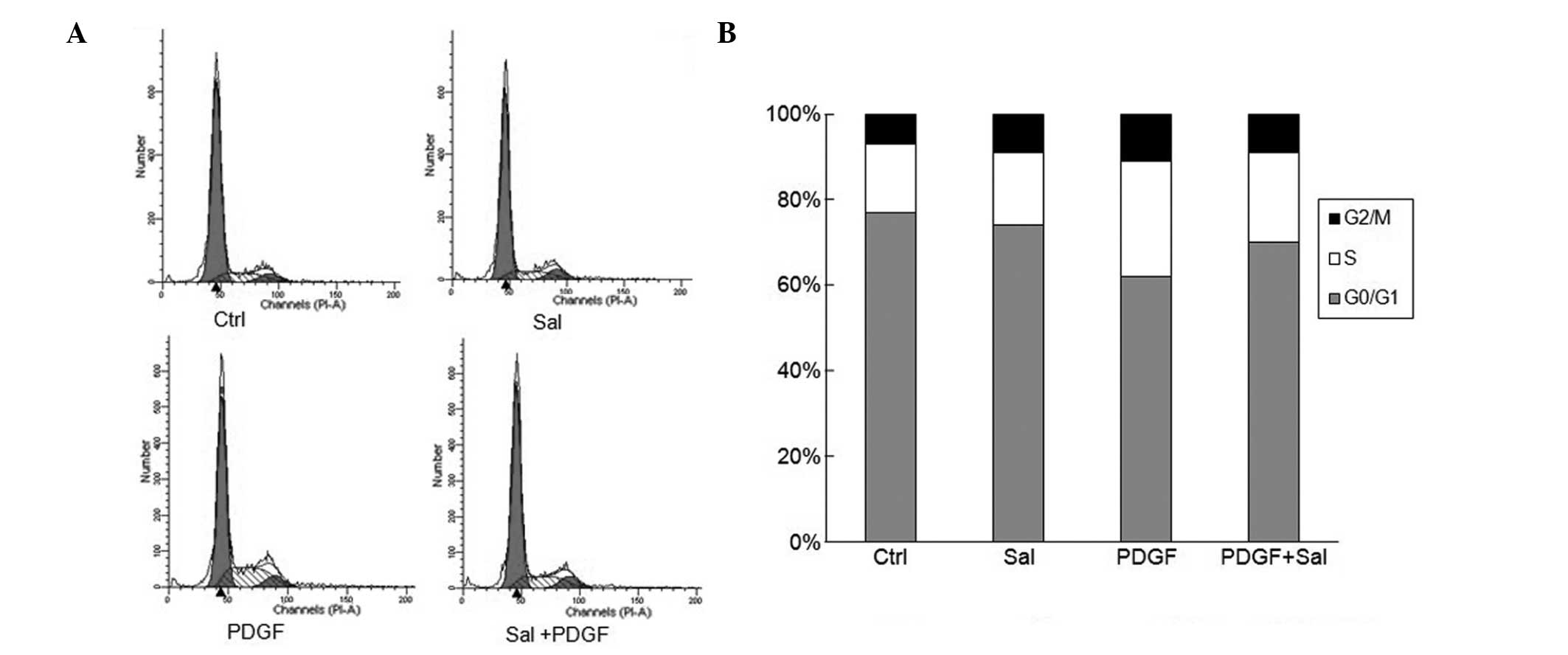
Salidroside blocks PDGF-BB-induced cell
cycle progression through G0/G1- to S-phase cell cycle arrest
The effect of salidroside on cell cycle progression
was analyzed using flow cytometric analysis. PDGF-BB treatment
alone significantly increased the percentage of cells in S phase
whilst decreasing the G0/G1 populations (Fig. 3). By contrast, salidroside-treated
cells showed a significant suppression of cell cycle progression.
Salidroside at a dose of 100 μM reduced the percentage of
cells in S phase and increased the G0/G1 populations among the
PDGF-BB-stimulated cells. This suggests that salidroside affects
the G0/G1- to S-phase transition rather than being involved in the
S or G2/M phases (Fig. 3).
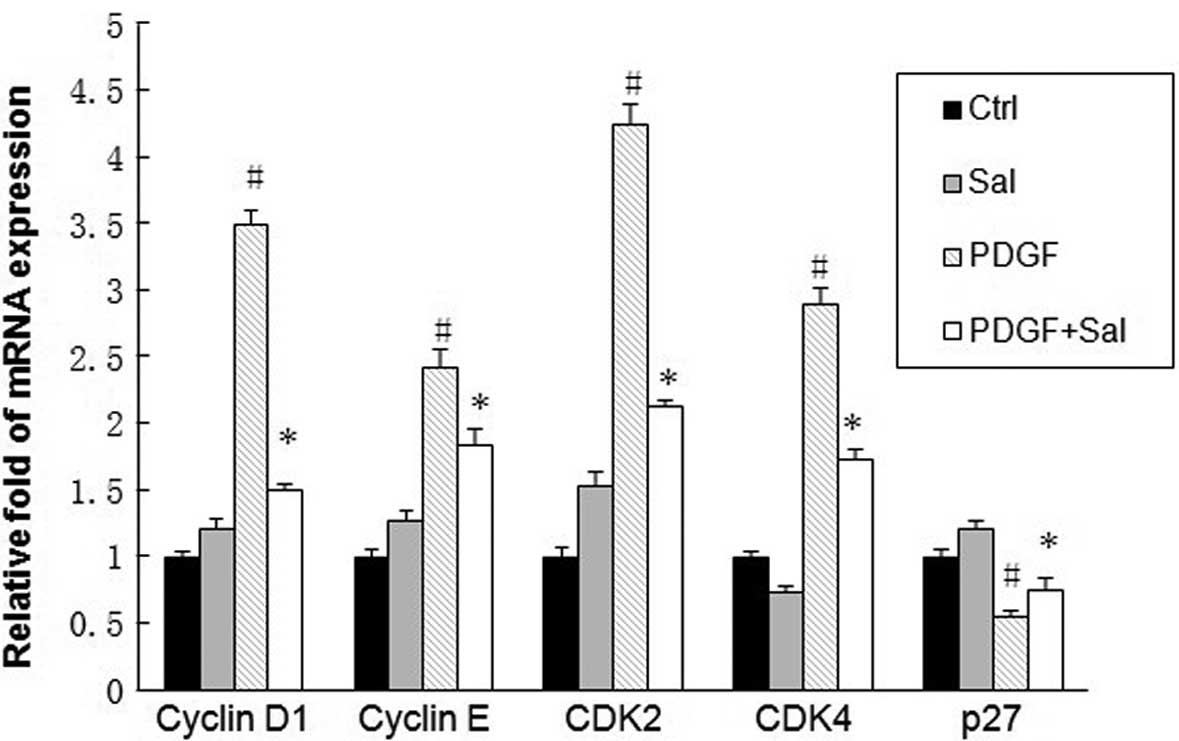
Salidroside downregulates the mRNA
expression of cyclin D1, cyclin E, cyclin-dependent kinase 2 (CDK2)
and CDK4, and upregulates p27 mRNA expression
To explore the potential mechanisms by which
salidroside influences the cell cycle of PASMCs, the mRNA levels of
cell cycle regulatory genes, including cyclins, the CDKs and cell
cycle inhibitory genes, were examined. PDGF induction significantly
increased the mRNA levels of cyclin D1, cyclin E, CDK2 and CDK4.
Conversely, pretreatment with salidroside significantly suppressed
the PDGF-induced upregulation of the studied genes (Fig. 4). The cyclin-CDK complexes formed
in cell cycle progression are regulated by CDK inhibitors, such as
p27, which leads to cell cycle arrest at the G1 and G1/S boundary
(23). Pretreatment with
salidroside upregulated the expression of p27.
Molecular mechanisms involved in the
salidroside-induced inhibition of the proliferation of PASMCs
To explore the molecular mechanisms by which
salidroside inhibits the proliferation of PASMCs, the effects of
salidroside on MAPK and AKT/GSK3β signaling were examined.
Significant activation of ERK1/2, p38, JNK and AKT/GSK3β was
observed 5, 10 and 15 min after PDGF treatment, without affecting
the total levels of these molecules (assessed by comparison with
internal controls using western blotting) (Fig. 5). Salidroside significantly reduced
the phosphorylation of AKT/GSK3β, but did not exhibit any
inhibitory effects on the phosphorylation of ERK1/2, p38 and JNK
(Fig. 5).
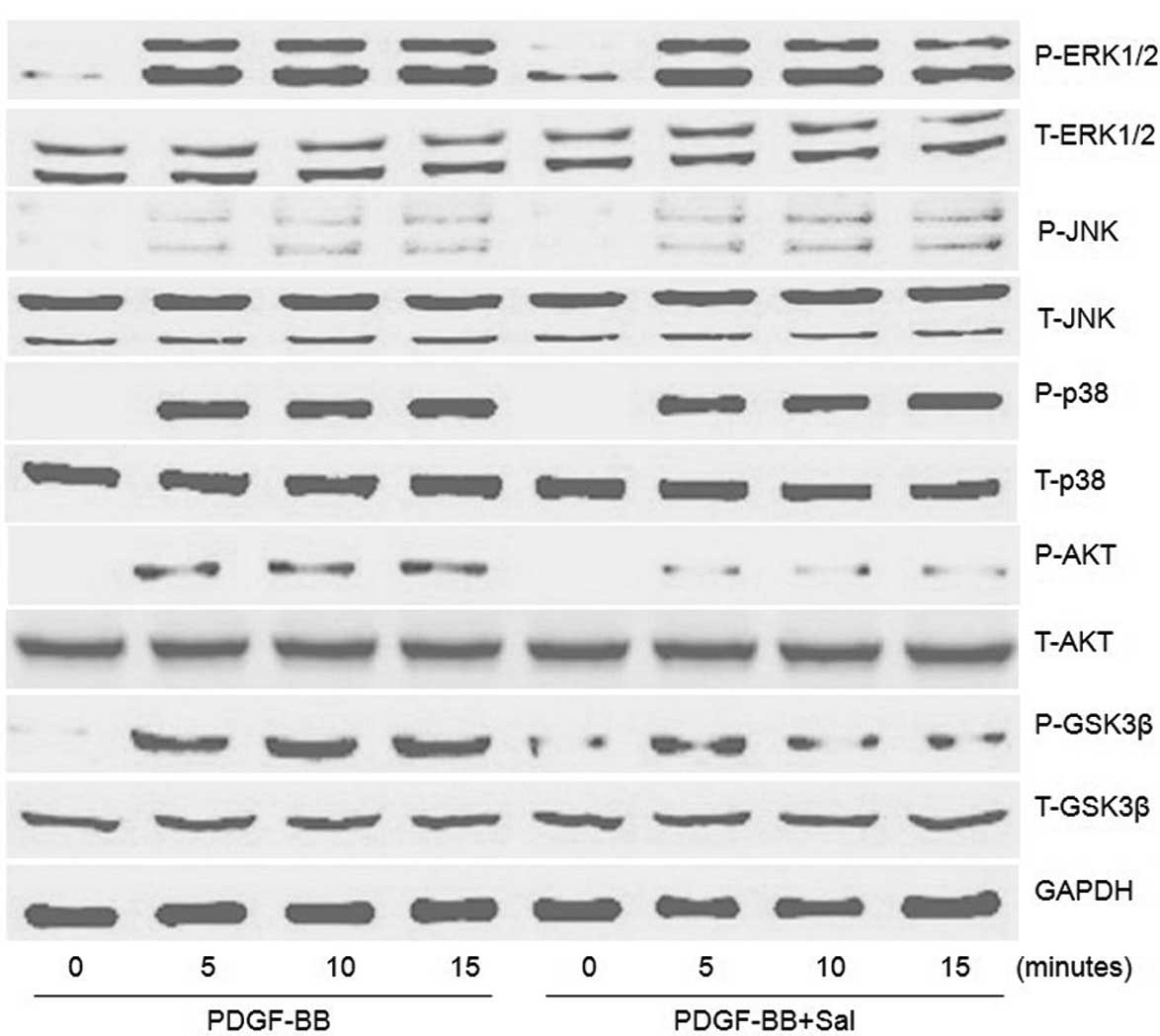 | Figure 5Effect of salidroside on the
activation of signaling pathways in PDGF-BB-stimulated PASMCs.
PASMCs were pretreated with salidroside (100 μM) for 1 h prior to
20 ng/ml PDGF-BB treatment. The protein levels of P-ERK1/2, ERK1/2,
P-JNK, JNK, P-p38, p38, P-AKT, AKT, P-GSK3β and GSK3β induced by
PDGF-BB at 5, 10 and 15 min were determined by western blot
analysis. One representative image out of three independently
performed experiments is shown. PDGF-BB, platelet-derived growth
factor-BB; PASMCs, pulmonary artery smooth muscle cells; Sal,
salidroside; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; GSK3β, glycogen synthase kinase 3 β; P-,
phosphorylated. |
Discussion
The present study demonstrated that salidroside
inhibits PDGF-induced PASMC proliferation and DNA synthesis in a
dose- and time-dependent manner without cell cytotoxicity. It also
showed that salidroside inhibits the cell cycle at G0/G1 to S phase
through inhibition of the mRNA expression of cyclin D1, cyclin E,
CDK2 and CDK4, as well as through an increase in the mRNA
expression of p27, in PDGF-BB-stimulated PASMCs. These effects of
salidroside on the proliferation of PASMCs were associated with
inhibition of the AKT/GSK3β pathway. These results suggest that
salidroside may be a novel therapy for preventing pulmonary
vascular remodeling diseases.
Abnormal proliferation of PASMCs leads to medial
vascular lumen narrowing and vascular remodeling, which are
critical to the development of PAH (19,20).
In the present study, it was demonstrated that salidroside
inhibited PDGF-induced PASMC proliferation and DNA synthesis in a
dose- and time-dependent manner without cell cytotoxicity. Cell
proliferation is tightly regulated by the cell cycle, and
salidroside has been demonstrated to cause cell cycle arrest in
cancer cell lines (16,18). For this reason, the effects of
salidroside on the cell cycle in PDGF-BB-stimulated PASMCs were
examined. Flow cytometric analysis results demonstrated that 100
μM salidroside treatment for 24 h led to a significant
increase in the number of cells in the G0/G1 phase and a reduction
in the number of cells in the S phase, without any significant
effect on the number of cells in the G2/M phase. Taken together,
these results indicate that salidroside targets a signaling
transduction event evoked in the G0/G1-S boundary. Key factors of
cell cycle regulation include the CDKs, which may be activated in a
specific cell cycle phase by phosphorylation of the corresponding
substrates, allowing for progression through the cell cycle. The
CDKs are also dependent on cyclins, whose expression levels are
associated with different cell cycle phases. Therefore, the degree
of CDK activation is different for each stage of the cell cycle and
plays a critical role in cell cycle regulation. The activity of
CDKs may be inhibited by cell cycle inhibitory proteins (CKIs).
CDK2 and CDK4 are known to form complexes with cyclin E and cyclin
D1, which are essential for the mediation of cell cycle progression
from the G0/G1 to S phases (21,22).
Another regulator controlling cell cycle progression is the CKI
p27, which forms heterotrimeric complexes with cyclins and CDKs to
inhibit their activity, such as cyclin D-CDK4 and cyclin E-CDK2
(23).
In this study, the expression of cell cycle
regulatory genes in response to PDGF-BB in PASMCs was investigated.
Salidroside reduced the PDGF-BB-induced mRNA expression of cyclin
D1, cyclin E, CDK2 and CDK4. Consistent with these changes, the
mRNA expression of p27 was increased by salidroside. These
observations suggest that the antiproliferative activity of
salidroside has a multifaceted effect on numerous target molecules
critically involved in growth inhibition.
MAPK families, including ERKs, p38 and JNK, as well
as the AKT pathway, play an important role in the regulation of
cell proliferation. A previous study by our group demonstrated that
PDGF-BB can stimulate the activation of ERK, JNK and p38, as well
as the AKT/GSK3β pathway in VSMCs (24). In the present study, it was found
that PDGF-BB can significantly stimulate the phosphorylation of
ERK1/2, JNK, p38 and AKT/GSK3β in PASMCs. The PI3K/AKT signaling
pathway is implicated in multiple cellular processes, including
proliferation, differentiation, apoptosis and migration. AKT has
numerous downstream targets, and GSK3β is one of its critical
downstream molecules. Data from this study showed that salidroside
inhibited the phosphorylation of AKT/GSK3β during PDGF-BB
induction. However, this result contrasts those obtained by other
studies, in which salidroside was reported to stimulate the AKT
pathway (25,26). Whether these differences are
associated with different types of cells and different salidroside
concentrations used in the studies remains to be elucidated. Cyclin
D1 is regulated by GSK3β, which can be inactivated by
phosphorylation (27,28). Activation of GSK3β has been found
to regulate cyclin D1 export from the nucleus to the cytoplasm for
proteolysis and thus decreases the expression of cyclin D1
(29). Furthermore, inhibition of
the phosphorylation of the AKT/GSK3β signaling pathway has been
shown to decrease the expression of cyclin D1 in cultured VSMCs
(30). GSK3β inhibition has also
been shown to decrease expression of the CKI p27 (31). Based on these results, it is
possible that salidroside suppresses the proliferation of PASMCs
through the AKT/GSK3β signaling pathway. The effect of salidroside
on the activation of MAPKs, including ERK1/2, p38 and JNK, which
are other important factors implicated in the PASMC proliferation
induced by PDGF-BB, was also examined. Unlike its effect on the
AKT/GSK3β pathway, salidroside failed to affect the PDGF-stimulated
activation of ERK1/2, p38 and JNK. However, a previous study
indicated that salidroside can inhibit the ERK, p38 and JNK
signaling pathways in LPS-induced mastitis (15). It is possible that salidroside has
different effects on the MAPK signaling pathways in different types
of cells. These results indicate that the PDGF-BB-mediated
activation of ERK, p38 and JNK may not be involved in the
inhibitory effect of salidroside on the proliferation of
PASMCs.
In conclusion, this study, to the best of our
knowledge, demonstrated for the first time that salidroside
inhibits the proliferation of PASMCs induced by PDGF-BB. Notably,
this process appears to be associated with an inhibition of cyclin
D1 expression and an increase in p27 accumulation through blockade
of the AKT/GSK3β signaling pathway. The results of this study
suggest that the use of salidroside may be feasible for therapies
to treat pulmonary vascular remodeling diseases.
References
|
1
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakao S, Tatsumi K and Voelkel NF:
Reversible or irreversible remodeling in pulmonary arterial
hypertension. Am J Respir Cell Mol Biol. 43:629–634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agbani EO, Coats P, Mills A and Wadsworth
RM: Peroxynitrite stimulates pulmonary artery endothelial and
smooth muscle cell proliferation: involvement of ERK and PKC. Pulm
Pharmacol Ther. 24:100–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo C, Yi B, Bai L, et al: Suppression of
Akt1 phosphorylation by adenoviral transfer of the PTEN gene
inhibits hypoxia-induced proliferation of rat pulmonary arterial
smooth muscle cells. Biochem Biophys Res Commun. 397:486–492. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giese NA, Marijianowski MM, McCook O, et
al: The role of alpha and beta platelet-derived growth factor
receptor in the vascular response to injury in nonhuman primates.
Arterioscler Thromb Vasc Biol. 19:900–909. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perros F, Montani D, Dorfmüller P, et al:
Platelet-derived growth factor expression and function in
idiopathic pulmonary arterial hypertension. Am J Respir Crit Care
Med. 178:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa A, Nakamura K, Matsubara H, et al:
Prednisolone inhibits proliferation of cultured pulmonary artery
smooth muscle cells of patients with idiopathic pulmonary arterial
hypertension. Circulation. 112:1806–1812. 2005. View Article : Google Scholar
|
|
8
|
Sanchez O, Marcos E, Perros F, et al: Role
of endothelium-derived CC chemokine ligand 2 in idiopathic
pulmonary arterial hypertension. Am J Respir Crit Care Med.
176:1041–1047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berg JT, Breen EC, Fu Z, et al: Alveolar
hypoxia increases gene expression of extracellular matrix proteins
and platelet-derived growth factor-B in lung parenchyma. Am J
Respir Crit Care Med. 158:1920–1928. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz Lanza AM, Abad Martínez MJ, Fernández
Matellano L, et al: Lignan and phenylpropanoid glycosides from
Phillyrea latifolia and their in vitro anti-inflammatory
activity. Planta Med. 67:219–223. 2001.
|
|
11
|
De Sanctis R, De Bellis R, Scesa C, et al:
In vitro protective effect of Rhodiola rosea extract against
hypochlorous acid-induced oxidative damage in human erythrocytes.
Biofactors. 20:147–159. 2004.
|
|
12
|
Mattioli L and Perfumi M: Rhodiola
rosea L. extract reduces stress- and CRF-induced anorexia in
rats. J Psychopharmacol. 21:742–750. 2007. View Article : Google Scholar
|
|
13
|
Ming DS, Hillhouse BJ, Guns ES, et al:
Bioactive compounds from Rhodiola rosea (Crassulaceae).
Phytother Res. 19:740–743. 2005. View
Article : Google Scholar
|
|
14
|
Kanupriya, Prasad D, Sai Ram M, et al:
Cytoprotective and antioxidant activity of Rhodiola
imbricata against tert-butyl hydroperoxide induced oxidative
injury in U-937 human macrophages. Mol Cell Biochem. 275:1–6.
2005.PubMed/NCBI
|
|
15
|
Li D, Fu Y, Zhang W, et al: Salidroside
attenuates inflammatory responses by suppressing nuclear factor-κB
and mitogen activated protein kinases activation in
lipopolysaccharide-induced mastitis in mice. Inflamm Res. 62:9–15.
2013.PubMed/NCBI
|
|
16
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine. 19:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin D, Yao W, Chen S, et al: Salidroside,
the main active compound of Rhodiola plants, inhibits high
glucose-induced mesangial cell proliferation. Planta Med.
75:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Zhang X, Qiu S, et al: Salidroside
induces cell-cycle arrest and apoptosis in human breast cancer
cells. Biochem Biophys Res Commun. 398:62–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pietra GG, Capron F, Stewart S, et al:
Pathologic assessment of vasculopathies in pulmonary hypertension.
Am Coll Cardiol. 43(12 Suppl S): 25S–32S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rabinovitch M: The mouse through the
looking glass: a new door into the pathophysiology of pulmonary
hypertension. Circ Res. 94:1001–1004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jirawatnotai S, Aziyu A, Osmundson EC, et
al: Cdk4 is indispensable for postnatal proliferation of the
anterior pituitary. J Biol Chem. 279:51100–51106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martín A, Odajima J, Hunt SL, et al: Cdk2
is dispensable for cell cycle inhibition and tumor suppression
mediated by p27(Kip1) and p21(Cip1). Cancer Cell. 7:591–598.
2005.PubMed/NCBI
|
|
23
|
Abukhdeir AM and Park BH: P21 and p27:
roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan H, Chen C, Zhu L, et al:
Indole-3-carbinol blocks platelet-derived growth factor-stimulated
vascular smooth muscle cell function and reduces neointima
formation in vivo. J Nutr Biochem. 24:62–69. 2013. View Article : Google Scholar
|
|
25
|
Zhang L, Ding W, Sun H, et al: Salidroside
protects PC12 cells from MPP+-induced apoptosis via
activation of the PI3K/Akt pathway. Food Chem Toxicol.
50:2591–2597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Shi YP, Wu D, et al: Salidroside
protects against hydrogen peroxide-induced injury in cardiac H9c2
cells via PI3K-Akt dependent pathway. DNA Cell Biol. 30:809–819.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu XJ, Han QB, Wen ZS, et al: Gambogenic
acid induces G1 arrest via GSK3β-dependent cyclin D1 degradation
and triggers autophagy in lung cancer cells. Cancer Lett.
322:185–194. 2012.PubMed/NCBI
|
|
29
|
Malumbres M and Barbacid M: To cycle or
not to cycle: a critical decision in cancer. Nat Rev Cancer.
1:222–231. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin M, Tian S, Huang X, et al: Role and
mechanism of tissue plasminogen activator in venous wall fibrosis
remodeling after deep venous thrombosis via the glycogen synthase
kinase-3 beta signaling pathway. J Surg Res. 184:1182–1195. 2013.
View Article : Google Scholar
|
|
31
|
Tseng AS, Engel FB and Keating MT: The
GSK-3 inhibitor BIO promotes proliferation in mammalian
cardiomyocytes. Chem Biol. 13:957–963. 2006. View Article : Google Scholar : PubMed/NCBI
|