Introduction
Epilepsy, a disorder of recurrent seizures, is a
common and often deleterious neurological condition. It has
considerable impact on the patients’ quality of life and greatly
increases the risk of injury, socioeconomic disadvantage, and even
mortality (1). It can also
interfere with memory, cognitive function and education
opportunities, and it may cause endocrine dysfunction (2). Despite the development and
availability of >22 anti-epileptic drugs (AEDs), most of which
have been identified based on large-scale, randomized, double-blind
clinical trails, it is estimated that 25–40% of patients diagnosed
with epilepsy are resistant to drug therapy and continue to have
seizures (3,4). Thus, exploring the molecular
mechanisms underlying epilepsy may allow to identify novel
treatment methods.
The roles of the inflammatory system in the
occurrence of seizure are currently heavily investigated. Louboutin
et al (5) showed that the
C-C chemokine receptor type 5 is involved in neuronal injury caused
by kainic acid (KA) in animal models. Our previous study reported
that Toll-like receptors (TLRs) contribute to the development of
epilepsy (6). The majority of TLRs
recruit the MyD88-IRAK-TRAF6 pathway, culminating in the activation
of nuclear factor-κB, which drives the transcription of genes
encoding pro-inflammatory factors, such as interleukin (IL)-6,
IL-12, and tumor necrosis factor-α (7). Earlier studies by Whitmore et
al (8) and Gilchrist et
al (9) demonstrated that the
activating transcription factor 3 (ATF3) is induced by TLR
signaling in primary mouse macrophages and human dendritic cells.
ATF3 modulated the transcription of IL-6, IL-12b, and IL-12p40,
which highlights its key regulatory roles in TLR signaling. Recent
studies have further reported that TLR gene expression
closely interacts with that of p53 (10,11).
The hypothesis that ATF3 can modulate the activity of p53 was based
on evidence supporting the interaction between these two proteins
(12).
A previous study in an epileptic rat model suggested
that aberrant mossy fiber sprouting (MFS) may contribute to
spontaneous seizures (13). A
recent study by our group showed that TLRs modulate neurite
outgrowth in the hippocampus of pentylenetetrazole (PTZ)-kindled
rats (6). In addition, other
studies found that overexpression of ATF3 plays a crucial role in
promoting neurite outgrowth in the peripheral nervous system, both
in vitro and in vivo (14–16).
The tumor protein p53 has been also shown to promote neurite
growth: overexpression of a dominant negative form of p53 in
primary cortical neurons led to growth cone collapse and a decrease
in neurite outgrowth (17,18). In this context, we hypothesized
that ATF3 and p53 may be involved in MFS during epileptogenesis. To
verify this hypothesis, we established a kindling model of epilepsy
via intraperitoneal injection of PTZ in rats, and analyzed the
expression level of the ATF3 and p53 proteins.
Materials and methods
Animals and drug treatment
Rats were treated following the Guidelines for the
Care and Use of Laboratory Animals, published by the National
Institutes of Health (NIH; Bethesda, MD, USA). All protocols were
approved by the Animal Ethics Committee of the Central South
University in China. A total of 180 adult male Sprague-Dawley rats
(6–8 weeks of age, 180–220 g) were purchased from the Animal
Experimental Center of the Central South University (Changsha,
China). They were housed in quiet rooms with a 12–12 h light-dark
cycle (light from 07:00 a.m. to 19:00 p.m.) and a 22–24°C
temperature, and were given standard laboratory food and tap water
ad libitum. The rats were randomly divided into the control
and PTZ groups, each containing 5 subgroups of 18 rats each. There
was no statistically significant difference in weight and age
between rats of the two groups. Rats of the PTZ group received an
intraperitoneal injection of 30 mg/kg PTZ (Sigma-Aldrich, St.
Louis, MO, USA) every day until they were kindled or sacrificed,
while those of the control group were injected with an equal dose
of normal saline. The rat behavior was recorded by a video camera
(Sony Corp., Tokyo, Japan). Following PTZ injection, the rats were
monitored for a minimum of 2 h to assess the severity and duration
of the seizures. Rats were considered kindled when seizure attacks
(Racine’s scale score ≥3) occurred after each PTZ injection for 5
consecutive days. At 3 days and 1, 2, 4 and 6 weeks after the first
injection, the rats were sacrificed and perfused.
Immunohistochemistry
At different time-points, the rats were deeply
anesthetized with 10% chloral hydrate and perfused intracardially
with 300 ml of normal saline and 400 ml of 4% paraformaldehyde in
0.1 M phosphate buffer at 4°C. The brains were removed and placed
in 4% paraformaldehyde overnight, then transferred into 0.1 M
phosphate buffer containing 20–30% sucrose. Subsequently, serial,
20-μm-thick sections were performed for immunohistochemistry and
immunofluorescence analysis. The sections were subjected to
conventional rewarming and heat-induced antigen retrieval in 10 mM
sodium citrate buffer (0.01 mol/l; Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China) at boiling temperature for 24 min; cool
sodium citrate buffer was added every 6 min. Peroxidase and lipids
were eliminated by the admixture of 1% hydrogen and methanol at 4°C
for 30 min. After rinsing in 0.01 M phosphate-buffered saline
(PBS), the sections were blocked using 5% goat serum at room
temperature for 2 h and incubated overnight at 4°C with the rabbit
anti-rat monoclonal antibodies anti-ATF3 and −p53 (1:50; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). After rinsing in 0.01 M
PBS, the sections were incubated with a biotinylated goat
anti-rabbit secondary antibody (Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at room temperature for 60
min. To visualize peroxidase labeling, the sections were stained
with diaminobenzidine (Boster Biological Technology Ltd., Wuhan,
China), dehydrated and mounted. The sections were observed under a
fluorescence microscope (Leica DM5000B microscope; Leica Camera
Co., Solms, Germany). Images were processed with a Leica DM5000 B
image analysis system (Leica Microsystems, Glattbrugg,
Switzerland).
Immunofluorescence
Rewarming and antigen recovery of brain tissue
sections were performed as described above. Each section was
permeabilized with 1% Triton X-100 in Tris-buffered saline with
Tween-20. After blocking with 10% goat serum (Zhongshan Golden
Bridge Biotechnology Co., Ltd.) at room temperature for 2 h, the
samples were incubated at 4°C overnight with anti-ATF3 or −p53 at
1:12.5 dilution. After rinsing in 0.01 M PBS, the sections were
incubated in the dark for 1 h at room temperature with Alexa Fluor
555-conjugated goat anti-rabbit IgG (1:1,000; Invitrogen, Carlsbad,
CA, USA). The fluorescence intensity was measured on a Leica DM5000
B system.
Western blotting
The entire hippocampi, including both CA3 and
dentate gyrus (DG) areas, were used for western blot analysis. Rats
in the control and the PTZ groups were deeply anesthetized with
chloral hydrate (350 mg/kg), and cervical dislocation was performed
at different time-points. Tissues were snap-frozen in liquid
nitrogen, and protein samples were extracted directly from the
hippocampi by homogenization in admixture of 1 mM phenylmethyl
sulfonyfluoride (PMSF) and RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Following heating at 100°C for 10
min in 5X SDS-PAGE loading buffer, (Beijing Cowin Biotech Co.,
Ltd., Beijing, China), equal amounts of denatured protein were
separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis, and the protein bands were electrotransferred onto
polyvinylidene fluoride (PVDF) membranes (Pall Corp., Port
Washington, NY, USA) and stained with the appropriate antibody
(anti-ATF3, 1:1,000; anti-p53, 1:1,500). Immunostaining with the
3-phosphate dehydrogenase (GAPDH) antibody (1:2,000; Sigma-Aldrich)
was used to normalize the expression data. The immunoreactive bands
were visualized by enhanced chemiluminescence using Image Lab™
software with the gel imaging analysis system (Bio-Rad, Hercules,
CA, USA).
Timm staining
At different time-points, the rats were deeply
anesthetized with 10% chloral hydrate (Laboratory of The Second
Xiangya Hospital, Central South University, Changsha, China) and
perfused intracardially with 300 ml of normal saline, followed by
addition of 200 ml of 0.1 M phosphate buffer (pH, 7.2–7.6;
Sinopharm Chemical Reagent Co., Ltd.) containing 0.4% sodium
sulfide (Shanghai Aibi Chemistry Preparation Co., Ltd., Shanghai,
China) and 400 ml of 4% paraformaldehyde (Tianjin Chemical Reagent
Co., Ltd., Tianjin, China), at 4°C. The brains were removed, fixed
in 4% paraformaldehyde for 24 h, transferred to 0.1 M phosphate
buffer containing 30% sucrose (Sinopharm Chemical Reagent Co.,
Ltd.), and cut into 30-μm coronal sections. The sections were
stained in the dark for 90 min in a solution containing 60 ml of
50% arabic gum (Sinopharm Chemical Reagent Co., Ltd.), 10 ml of 2 M
citrate buffer (Sinopharm Chemical Reagent Co., Ltd.), 30 ml of 0.5
M hydroquinone (Shanghai Aibi Chemistry Preparation Co., Ltd.) and
0.5 ml of 17% silver nitrate (Sinopharm Chemical Reagent Co.,
Ltd.). The glass slides were washed in de-ionized water and
counterstained by Nissl solution (Beyotime Institute of
Biotechnology, China) for 5 min. Subsequently, the glass slides
were dehydrated with gradient ethanol between 50 and 100%. They
were made transparent by xylene and mounted with permount mounting
medium (Sinopharm Chemical Reagent Co., Ltd.). Mossy fiber
sprouting was evaluated by rating the distribution of supragranular
Timm granules (TG) at a standard location in the dorsal and the
ventral hippocampus. Timm scoring scale ranged between 0 and 5
according to the following criteria: 0, no TG in the supragranular
region; 1, sparse TG in the supragranular regions in a patchy
distribution; 2, several TG in a continuous distribution; 3,
prominent TG on a continuous distribution with occasional patches
of confluent TG; 4, prominent TG forming a confluent dense laminar
band and 5, a confluent dense laminar band of TG that further
extends into the inner molecular layer.
Statistical analysis
Statistical analysis was performed with the GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA), and
the data were expressed as the mean ± standard deviation (SD).
Differences among multiple groups were assessed by a one-way
analysis of variance (ANOVA), and differences between 2 groups were
evaluated using the independent samples t-test. Differences with
p<0.05 were considered significant.
Result
Behavior of PTZ-treated rats
With the exception of 5 rats in the PTZ group that
died as a result of status epilepticus or generalized clonic-tonic
seizures at 1 or 2 weeks, the remaining rats in this group
developed seizure activity of stage 3, 4 or 5 after continuous PTZ
injection for 18–22 days. The PTZ-induced seizure activity
generally occurred 5–10 min after the PTZ injection, and had a
duration of 5–30 min. Spontaneous recurrent seizures of grade 2–3
were detected in kindled rats as early as 23 days after the first
injection. No epileptiform activity was observed in the control
groups.
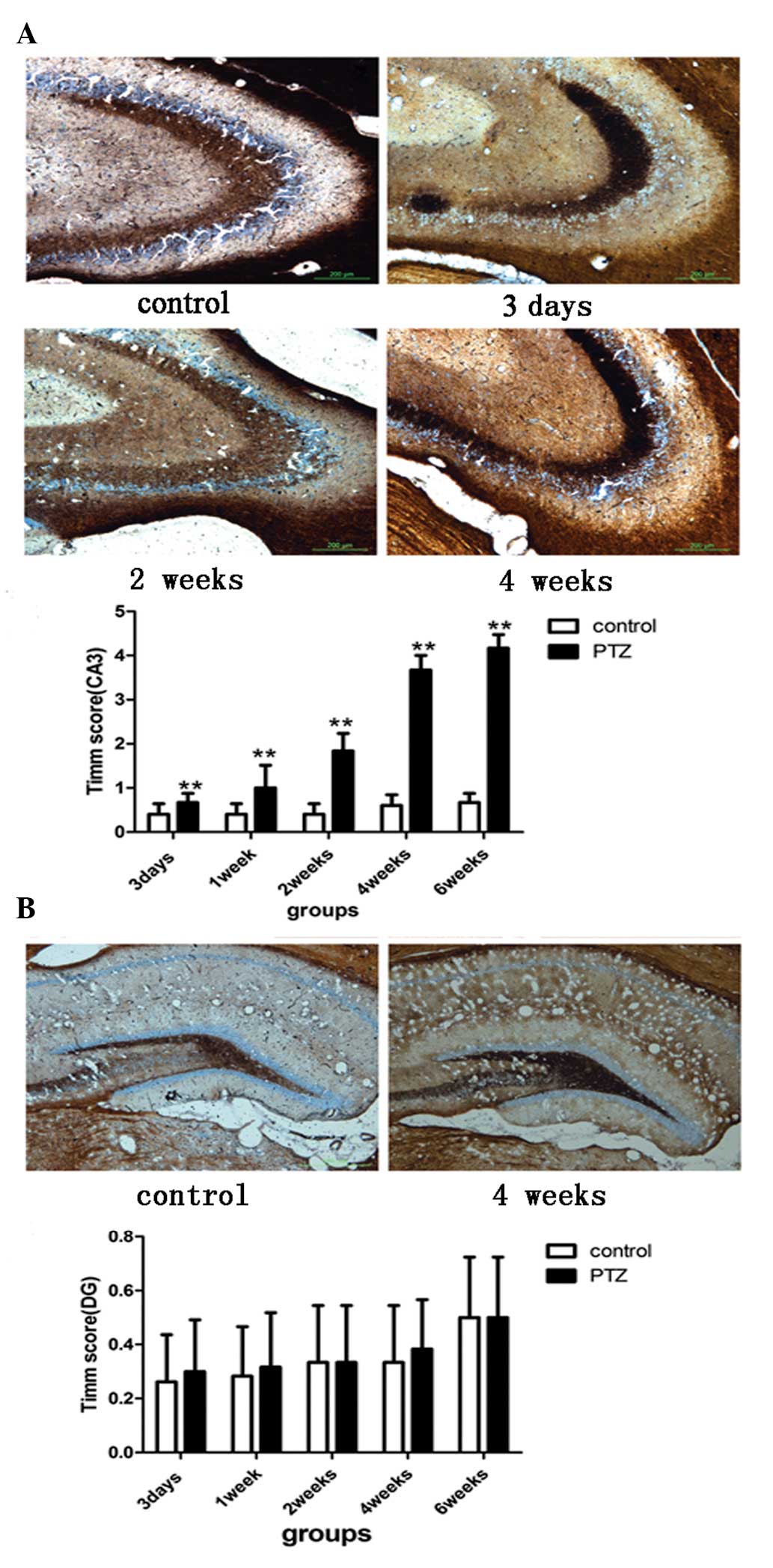
The severity of MFS in the CA3 region is
associated with the evolution of seizure behavior
The Timm scores in the CA3 area of the PTZ group
were significantly different from those of the control group in all
time-points (p<0.05; Fig. 1A),
and were prominently increased at 2, 4 and 6 weeks after the first
injection. The degree of MFS in the CA3 area of the PTZ group,
indicated by the corresponding Timm scores, was consistent with the
grade of seizures. At 3 days and 1 week post-PTZ treatment, most of
the rats in the PTZ group did not show epileptic seizures. At 2
weeks, the pathology of most of the rats did not change, with only
few rats showing head myoclonus (n=3) or forelimb clonus (n=5). At
4 and 6 weeks, most of the rats in the PTZ group were kindled, and
MFS reached its highest degree as compared to other time-points. In
the DG area, the Timm scores ranged between 0 and 1 for all rats.
There was no significant difference in MFS between the control and
the PTZ groups in this area (p>0.05; Fig. 1B).
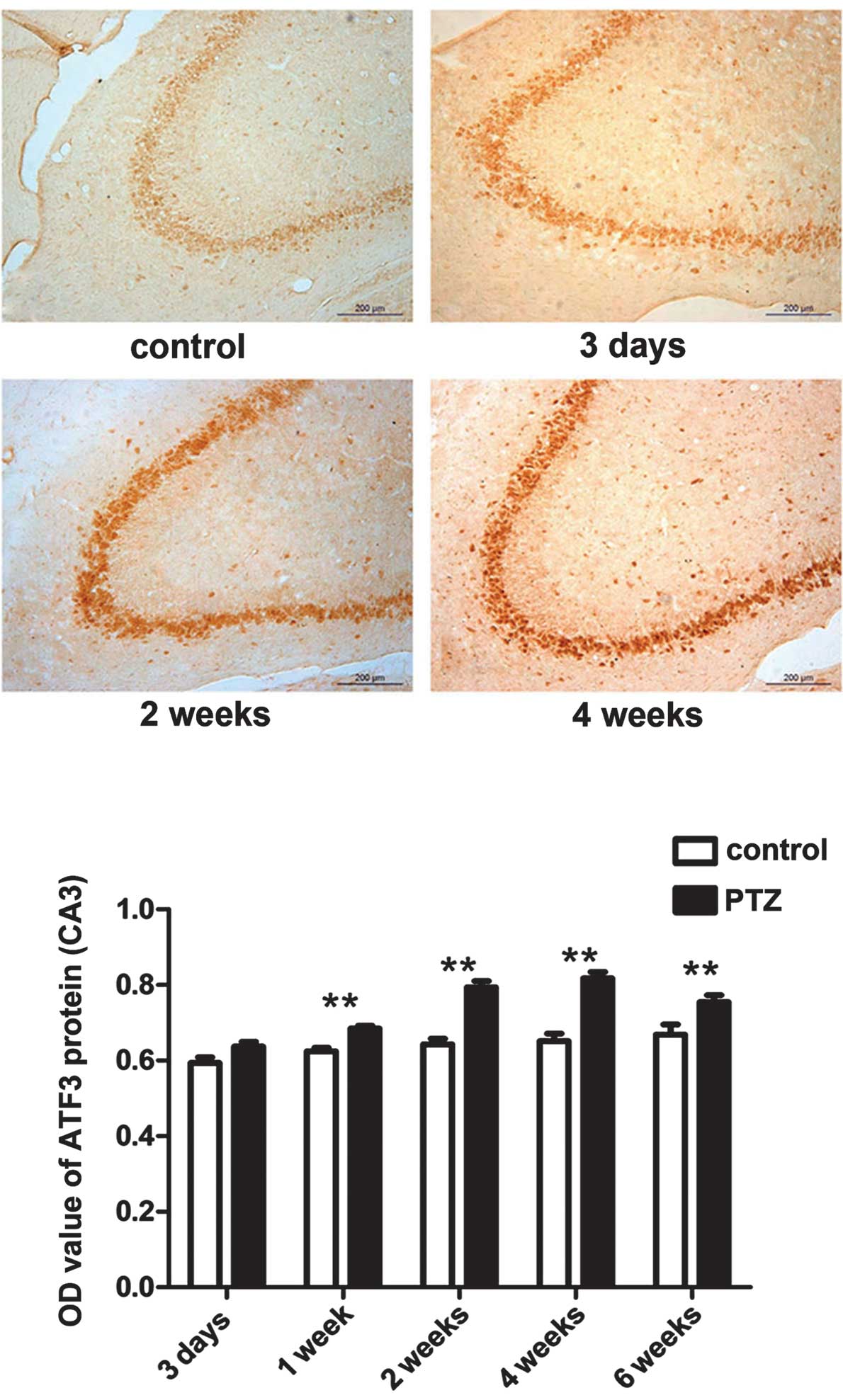
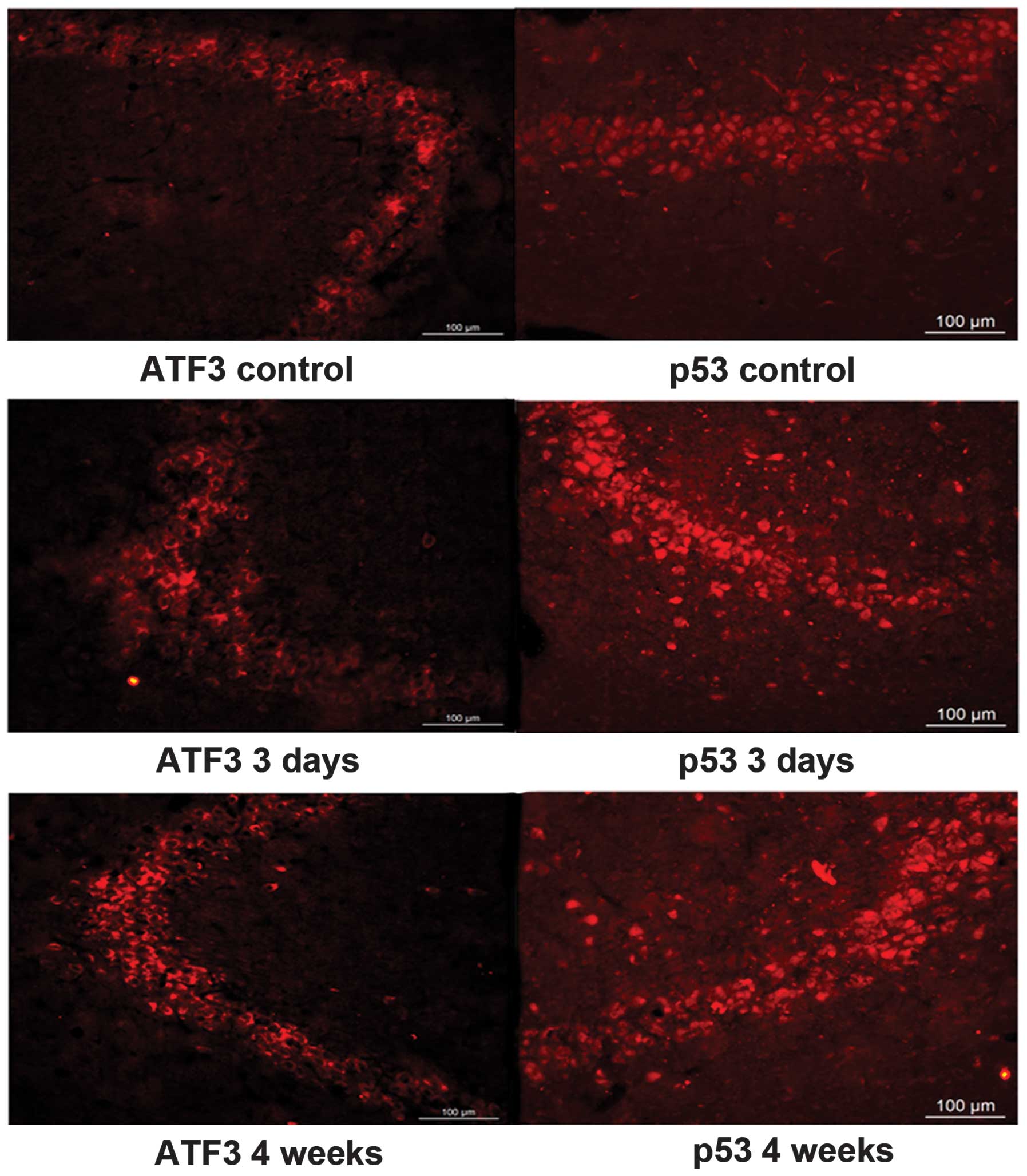
The expression of ATF3 and p53 in the CA3
areas is significantly increased during progression of PTZ-induced
kindling
The expression of the ATF3 and p53 proteins was
mainly observed in the pyramidal cells in the CA3 region and in
hilar neurons in the DG within the hippocampus. Compared to the
control group, expression of ATF3 and p53 in the CA3 area of the
PTZ group was significantly increased (P<0.05).
Immunohistochemistry analysis showed that the expression of ATF3 in
the PTZ group gradually increased from 3 days to 4 weeks, peaked at
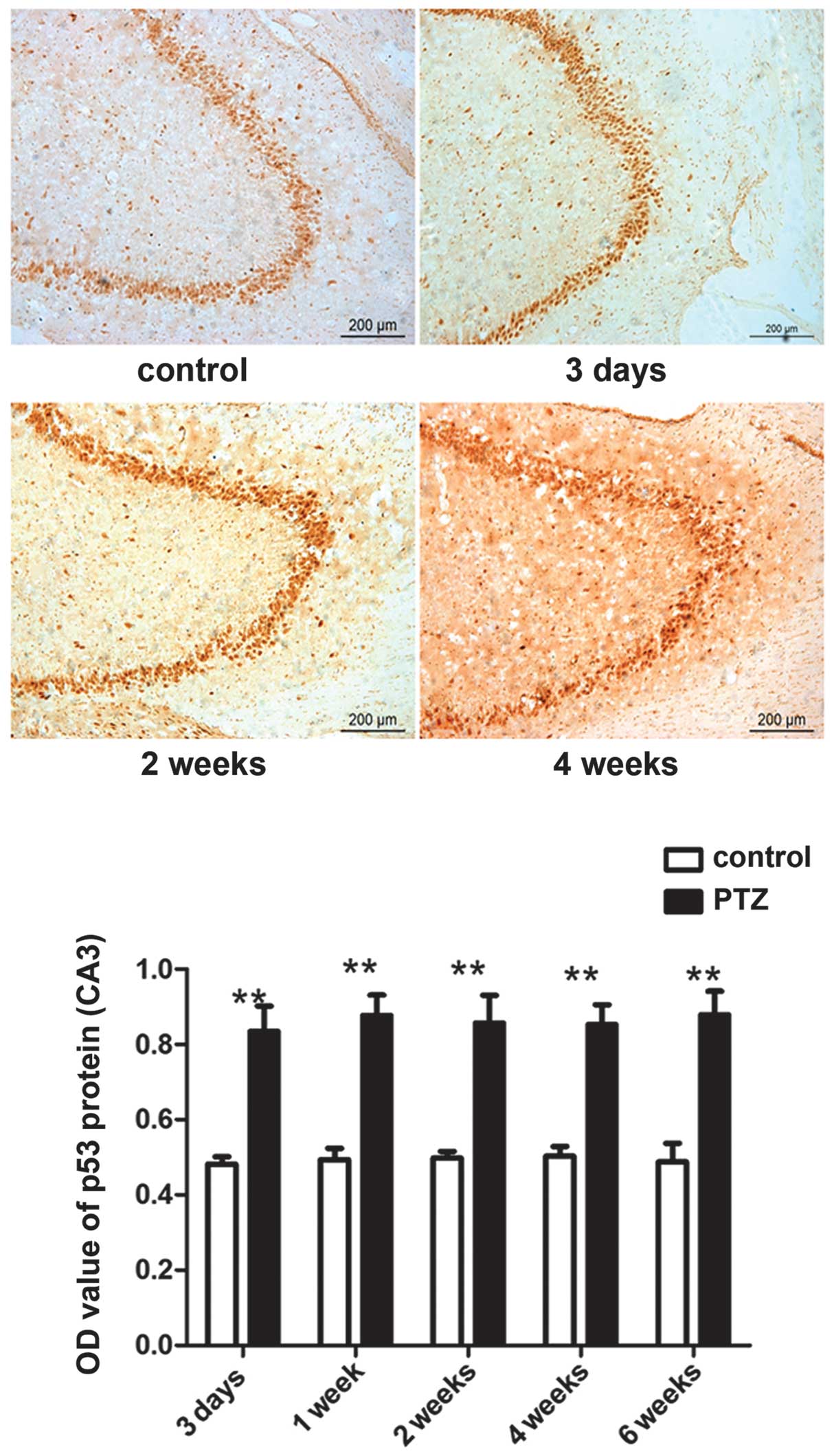
4 weeks, and slightly decreased at 6 weeks (Figs. 2 and 4). The expression of p53 was also higher
in the PTZ group compared to the control group, but no significant
difference (P>0.05) was observed between 3 days and 6 weeks
following PTZ treatment (Figs. 3
and 4). No obvious differences in
the expression of the two proteins were observed in the DG area
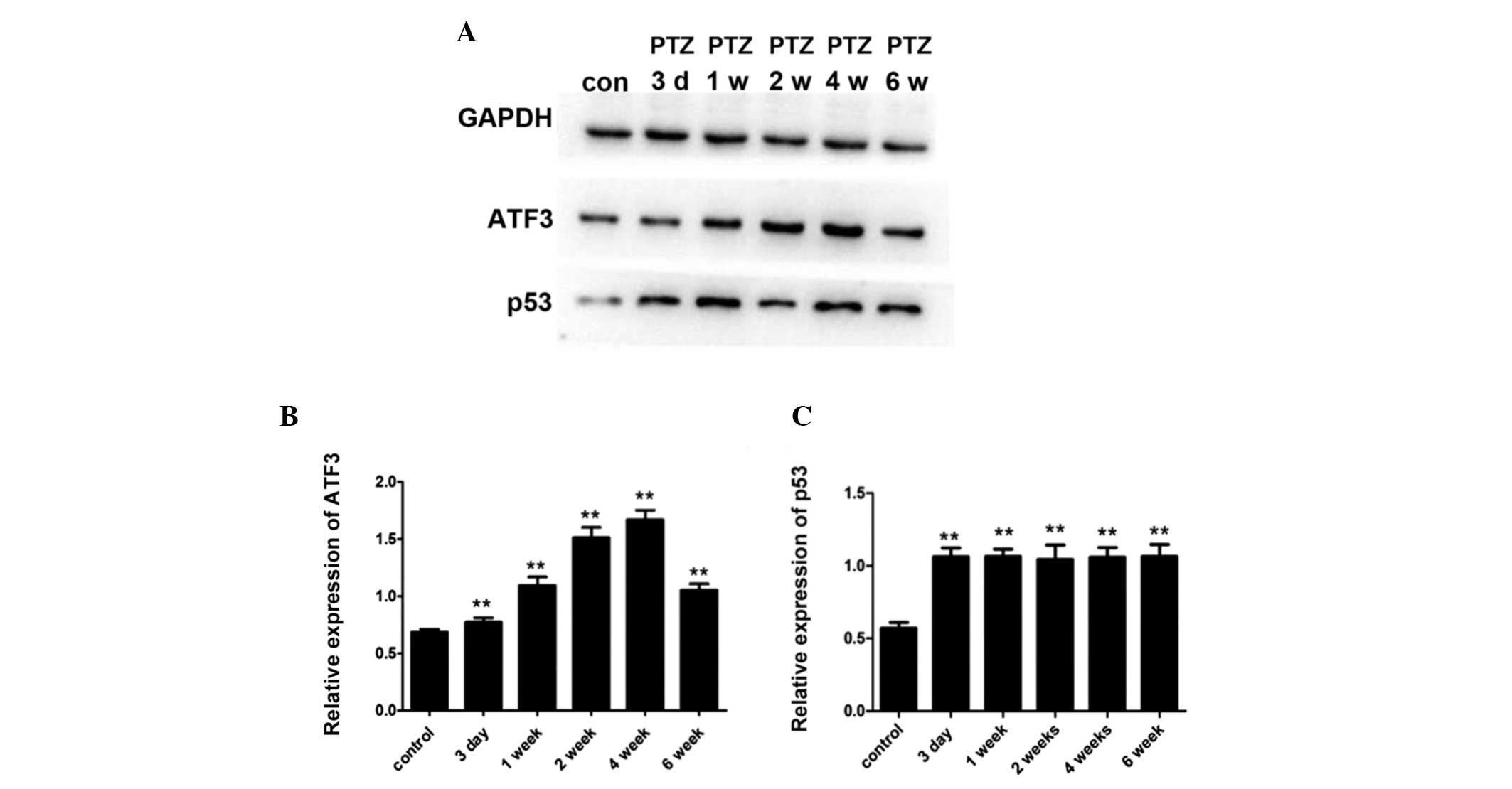
between the PTZ and the control group (data not shown). Figs. 4 and 5 show western blot and immunofluorescence
analysis results, confirming the above-described patterns of ATF3
and p53 expression. ATF3 mainly accumulated in the cytoplasm of the
neurons in the CA3 area of the hippocampus, while p53 mostly
localized in the cell nuclei in the CA3 area of the
hippocampus.
Discussion
Increasing evidence has highlighted the roles of
immunity and inflammatory processes in epilepsy (19–23).
Our previous study demonstrated that TLR signaling contributes to
the occurrence of epilepsy, and that suppressors of cytokine
signaling may act as negative regulators of TLR signaling (6). In the current study, we studied the
dynamic changes in the expression of the TLR downstream effectors
ATF3 and p53 in the hippocampus of a PTZ-induced kindling rat
model.
The expression of both ATF3 and p53 proteins was
increased in the PTZ compared to the control group, indicating that
these proteins may participate in the occurrence of epilepsy. ATF3
is encoded by an early-response gene, the expression of which is
induced in cells exposed to a variety of stress stimuli (24,25).
It was previously proposed that there is a regulatory feedback
regulation loop between p53 and ATF3 (26). In the nervous system, ATF3 appears
to contribute to the regenerative response (27,28),
and p53 was found to be involved in neurite outgrowth and nerve
regeneration (18). In our study,
the expression of ATF3 in the CA3 area of the hippocampus gradually
increased and then slightly decreased during the studied period.
The ATF3 expression profile and the degree of MFS in the CA3 area
were concordant over time, as well as their localization. Our
results indicated that ATF3 may modulate neurite outgrowth and
affect neurogenesis in the hippocampus during the process of
kindling. These results are however not in agreement with those
reported by Francis et al (29), which may be probably attributed to
the different type of convulsants used. Compared to KA used in the
study by Francis et al, PTZ is a relatively mild convulsant
that leads to a more mild cell death. Therefore, the main function
of ATF3 may differ in different experimental models. The expression
of p53 in the PTZ group was not in complete accordance with the
degree of MFS; it increased 3 days post-PTZ injection and was
maintained at similar levels after this time-point. As Sakhi et
al (30) reported, p53 acts as
a marker of irreversible neuronal injury. In our study, p53 was
upregulated at the early stage of the kindling process. This
indicates that p53-induced neuronal death may occursonly during the
early stages of epileptogenesis, and that p53 may be an important
factor in maintaining neurite outgrowth.
In addition, it has been reported that p53 is
involved in ATF3-mediated injury response, potentially via
ATF3-mediated regulation of the p53 stability, by interference upon
p53 ubiquitination (31). In a
study by Yan et al (32),
ATF3 was found to directly bind to p53 and repress the
p53-dependent transactivation of the type IV collagenase gene
(MMP-2) promoter. The expression patterns of the ATF3 and
p53 proteins were highly similar during the kindling process in our
experiments. We hypothesize that p53 may be one of the perpetuating
factors in the development of MFS, since its expression increased
before manifestation of MFS, and was maintained at similar levels
afterwards. Following the injection of PTZ, p53 expression was
maintained at high levels, probably in order to promote the
development of MFS. Considering the consistency in ATF3 expression
and the changes in the degree of MFS, ATF3 may be more important in
promoting the development of MFS. Whether there is an interaction
between ATF3 and p53 needs to be further investigated.
In summary, our results demonstrated an increase in
the levels of ATF3 and p53 in the PTZ groups compared to the
control groups in the rat hippocampus. This study indicated that
ATF3 may play a role in aberrant MFS in a p53-dependent manner
during the early stage of epileptogenesis. We did not knockout or
overexpress the rat ATF3 and p53 genes or proteins in this study;
these experiments may provide additional evidence to explain our
results and to elucidate the functions of the two proteins. Further
studies are needed to elucidate their roles in the neurons, both in
rat models and epileptic patients. The results reported herein
provide important perspectives for future studies on ATF3 and p53,
aiming to identify more effective treatments for epilepsy.
Acknowledgements
This study was supported by the 2011FJ4271 grant
from the Natural Science foundation of Hunan Province. We thank
Zhao-hui Luo for technical assistance.
References
|
1
|
Pitkänen A and Sutula TP: Is epilepsy a
progressive disorder? Prospects for new therapeutic approaches in
temporal-lobe epilepsy. Lancet Neurol. 1:173–181. 2002.PubMed/NCBI
|
|
2
|
England MJ, Liverman CT, Schultz AM and
Strawbridge LM: Epilepsy across the spectrum: promoting health and
understanding A summary of the Institute of Medicine report.
Epilepsy Behav. 25:266–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pitkänen A: Therapeutic approaches to
epileptogenesis-hope on the horizon. Epilepsia. 51(Suppl 3): 2–17.
2010.PubMed/NCBI
|
|
4
|
Perucca E, French J and Bialer M:
Development of new antiepileptic drugs: challenges, incentives, and
recent advances. Lancet Neurol. 6:793–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louboutin JP and Strayer DS: Relationship
between the chemokine receptor CCR5 and microglia in neurological
disorders: consequences of targeting CCR5 on neuroinflammation,
neuronal death and regeneration in a model of epilepsy. CNS Neurol
Disord Drug Targets. 12:815–829. 2013. View Article : Google Scholar
|
|
6
|
Song MY, Tian FF, Liu H, Wang YZ, Dang J,
Huang WJ and Ding DX: Expression of SOCSs and TLRs in the
hippocampus of a pentylenetetrazole kindling model. Clin Lab.
60:233–240. 2014.PubMed/NCBI
|
|
7
|
Thompson MR, Xu D and Williams BR:
Activating transcription factor 3 contributes to Toll-like
receptor-mediated macrophage survival via repression of Bax and
Bak. J Interferon Cytokine Res. 33:682–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitmore MM, Iparraguirre A, Kubelka L,
Weninger W, Hai T and Williams BR: Negative regulation of
TLR-signaling pathways by activating transcription factor-3. J
Immunol. 179:3622–3630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilchrist M, Thorsson V, Li B, Rust AG,
Korb M, Roach JC, Kennedy K, Hai T, Bolouri H and Aderem A: Systems
biology approaches identify ATF3 as a negative regulator of
Toll-like receptor 4. Nature. 441:173–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fávaro WJ, Nunes OS, Seiva FR, Nunes IS,
Woolhiser LK, Durán N and Lenaerts AJ: Effects of P-MAPA
immunomodulator on Toll-like receptors and p53: potential
therapeutic strategies for infectious diseases and cancer. Infect
Agent Cancer. 7:142012.PubMed/NCBI
|
|
11
|
Menendez D, Shatz M, Azzam K, Garantziotis
S, Fessler MB and Resnick MA: The Toll-like receptor gene family is
integrated into human DNA damage and p53 networks. PLoS Genet.
7:e10013602011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan C, Lu D, Hai T and Boyd DD: Activating
transcription factor 3, a stress sensor, activates p53 by blocking
its ubiquitination. EMBO J. 24:2425–2435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epsztein J, Represa A, Jorquera I, Ben-Ari
Y and Crepel V: Recurrent mossy fibers establish aberrant kainate
receptor-operated synapses on granule cells from epileptic rats. J
Neurosci. 25:8229–8239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peddie CJ and Keast JR: Pelvic nerve
injury causes a rapid decrease in expression of choline
acetyltransferase and upregulation of c-Jun and ATF-3 in a distinct
population of sacral preganglionic neurons. Front Neurosci.
5:62011. View Article : Google Scholar
|
|
15
|
Shokouhi BN, Wong BZ, Siddiqui S,
Lieberman AR, Campbell G, Tohyama K and Anderson PN: Microglial
responses around intrinsic CNS neurons are correlated with axonal
regeneration. BMC Neurosci. 11:132010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deshpande LS, Lou JK, Mian A, Blair RE,
Sombati S and DeLorenzo RJ: In vitro status epilepticus but not
spontaneous recurrent seizures cause cell death in cultured
hippocampal neurons. Epilepsy Res. 75:171–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tedeschi A, Nguyen T, Puttagunta R, Gaub P
and Di Giovanni S: A p53-CBP/p300 transcription module is required
for GAP-43 expression, axon outgrowth, and regeneration. Cell Death
Differ. 16:543–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Giovanni S, Knights CD, Rao M, Yakovlev
A, Beers J, Catania J, Avantaggiati ML and Faden AI: The tumor
suppressor protein p53 is required for neurite outgrowth and axon
regeneration. EMBO J. 25:4084–4096. 2006.PubMed/NCBI
|
|
19
|
Auvin S, Shin D, Mazarati A, Nakagawa J,
Miyamoto J and Sankar R: Inflammation exacerbates seizure-induced
injury in the immature brain. Epilepsia. 48(Suppl 5): 27–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aalbers MW, Rijkers K, Majoie HJ, Dings
JT, Schijns OE, Schipper S, De Baets MH, Kessels A, Vles JS and
Hoogland G: The influence of neuropathology on brain inflammation
in human and experimental temporal lobe epilepsy. J Neuroimmunol.
Mar 29–2014.(Epub ahead of print). View Article : Google Scholar
|
|
21
|
Diamond ML, Ritter AC, Failla MD, Boles
JA, Conley YP, Kochanek PM and Wagner AK: IL-1beta associations
with posttraumatic epilepsy development: A genetics and biomarker
cohort study. Epilepsia. Apr 22–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
22
|
Simões PS, Visniauskas B, Perosa SR, et
al: Expression and activity of thimet oligopeptidase (TOP) are
modified in the hippocampus of subjects with temporal lobe epilepsy
(TLE). Epilepsia. Apr 4–2014.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Avanzini G, Depaulis A, Tassinari A and de
Curtis M: Do seizures and epileptic activity worsen epilepsy and
deteriorate cognitive function? Epilepsia. 54(Suppl 8): 14–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamanaka H, Obata K, Fukuoka T, Dai Y,
Kobayashi K, Tokunaga A and Noguchi K: Induction of plasminogen
activator inhibitor-1 and -2 in dorsal root ganglion neurons after
peripheral nerve injury. Neuroscience. 132:183–191. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuzuki K, Kondo E, Fukuoka T, Yi D,
Tsujino H, Sakagami M and Noguchi K: Differential regulation of
P2X(3) mRNA expression by peripheral nerve injury in intact and
injured neurons in the rat sensory ganglia. Pain. 91:351–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Gao C, Kawauchi J, Hashimoto Y,
Tsuchida N and Kitajima S: Transcriptional activation of the human
stress-inducible transcriptional repressor ATF3 gene promoter by
p53. Biochem Biophys Res Commun. 297:1302–1310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindå H, Sköld MK and Ochsmann T:
Activating transcription factor 3, a useful marker for regenerative
response after nerve root injury. Front Neurol. 2:302011.PubMed/NCBI
|
|
28
|
Yano K, Kawasaki K, Hattori T, Tawara S,
Toshima Y, Ikegaki I, Sasaki Y, Satoh S, Asano T and Seto M:
Demonstration of elevation and localization of Rho-kinase activity
in the brain of a rat model of cerebral infarction. Eur J
Pharmacol. 594:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Francis JS, Dragunow M and During MJ: Over
expression of ATF-3 protects rat hippocampal neurons from in vivo
injection of kainic acid. Brain Res Mol Brain Res. 124:199–203.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakhi S, Bruce A, Sun N, Tocco G, Baudry M
and Schreiber SS: p53 induction is associated with neuronal damage
in the central nervous system. Proc Natl Acad Sci USA.
91:7525–7529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buganim Y, Kalo E, Brosh R, Besserglick H,
Nachmany I, Rais Y, Stambolsky P, Tang X, Milyavsky M, Shats I,
Kalis M, Goldfinger N and Rotter V: Mutant p53 protects cells from
12-O-tetradecanoylphorbol-13-acetate-induced death by attenuating
activating transcription factor 3 induction. Cancer Res.
66:10750–10759. 2006. View Article : Google Scholar
|
|
32
|
Yan C, Wang H and Boyd DD: ATF3 represses
72-kDa type IV collagenase (MMP-2) expression by antagonizing
p53-dependent trans-activation of the collagenase promoter. J Biol
Chem. 277:10804–10812. 2002. View Article : Google Scholar : PubMed/NCBI
|