Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive
disease of unknown etiology associated with a high rate of
mortality. The histology of IPF demonstrates the features of usual
interstitial pneumonia with a patchy distribution of fibrosis
adjacent to fibroblastic foci (FF) (1). FF are composed of migrating and
proliferating fibroblasts and myofibroblasts accounting for
extracellular matrix (ECM) deposition. This excessive and
uncontrolled deposition of ECM compromises normal lung function and
structure (2,3). Myofibroblasts are a key factor in
organ fibrosis and are characterised by the expression of α-smooth
muscle actin (α-SMA) (4). The
pathogenesis of IPF is unknown and the role of inflammation remains
controversial as anti-inflammatory treatment does not produce
significant beneficial effects against the progression of the
disease (5). Eventually, fibrosis
self-maintains and progresses by an unknown process. Several
studies have indicated that IPF is the result of injury to the
alveolar epithelium that leads to a cascade of dysregulated
epithelial-fibroblast crosstalk and abnormal wound healing
(6,7). In this model, through the release of
growth factors and cytokines, epithelial injury leads to the
excessive proliferation of fibroblasts, transformation of
fibroblasts to myofibroblasts and ECM deposition culminating in
parenchymal destruction (6,8).
Previous studies indicated that the Wnt signalling pathway is
active in pulmonary interstitial fibrosis and other fibrous
proliferative diseases (9,10). Königshoff et al found that
the mRNA expression and immunohistochemical reactivity of Wnts 1
and 3a increase in adjacent pulmonary epithelium in IPF patients
(11). However, the signalling
pathways involved are not completely understood. The current study
investigated Wnt1 stimulation of human embryonic lung fibroblasts,
the transition of human embryonic pulmonary fibroblasts (HEPF) to
myofibroblasts, as well as the synthesis, function and
proliferation of the ECM. Furthermore, bronchoalveolar lavage fluid
(BALF) obtained from fibrosis mouse model cultured HEPF cells were
used to observe if this fluid was able to induce fibrosis
transition to microfibrosis, as well as its association with the
Wnt/β-catenin signalling pathway. Furthermore, after the lentivirus
containing β-catenin shRNA knocked-out the β-catenin gene, HEPF
cells were cultured with BALF to observe whether HEPF microfibrosis
transformation was affected. The present study investigated Wnt
signalling in lung fibre formation to determine the possible
mechanism of this formation and to provide a theoretical basis for
IPF-targeted therapy.
Materials and methods
Cell culture
HEPF cells were provided by the Institute of
Biochemistry and Cell Biology (Shanghai Institute of Biological
Science, Chinese Academy of Sciences, Shanghai, China) and were
cultured with Dulbecco’s modified Eagle’s medium (DMEM; Sunshine
Biotechnology Co., Ltd., Nanjing, Jiangsu, China) supplemented with
10% foetal bovine serum (FBS; Invitrogen Life Technologies,
Carlsbad, CA, USA) at 37°C in a 5% CO2 and 95% air
incubator.
Animal treatments
We used 6- to 8-week old (18±2 g) SPF female C57BL/6
mice (Laboratory Animal Center of Jiangsu University, Zhenjiang,
Jiangsu, China). The mice had free access to water and rodent
laboratory chow. A group of mice were injected intratracheally with
5 mg/kg of bleomycin (BLM) solutions (Nippon Kayaku Co., Ltd.,
Tokyo, Japan) and the control group received the same volume of
saline, as previously described (12–14).
On day 7 post-modelling, mice were sacrificed by cervical
dislocation and bronchoalveolar lavage (BAL) was performed.
Following excision of the trachea, a plastic cannula was inserted
into the trachea and 1.0 ml of saline solution was gently injected
using a syringe and then withdrawn. This procedure was repeated
three times. Then BALF was centrifuged at 716 × g for 5 min. The
supernatants were preserved at −70°C and the lung tissues were
harvested. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Jiangsu University
(Zhenjiang, Jiangsu, China). This study was approved by the
Laboratory Animal Management Committee of Jiangsu University.
H&E staining
On day 7 post-modelling, mice were sacrificed. The
left lung was rinsed in PBS, fixed in 4% paraformaldehyde (Sunshine
Biotechnology Co., Ltd.) for 24 h, embedded in paraffin and cut
into 5 μm sections. The slides were stained with H&E for cell
alignment to evaluate the degree of inflammation.
Methylthiazoltetrazolium (MTT) assay
The day prior to the experiment, HEPF cells were
diluted to 1×104 cells/ml and seeded in 96-well culture
plates, with 100 μl in each well. After HEPF cells were cultured to
60–70% confluence, HEPF cells were placed in a serum-free DMEM
medium 24 h prior to treatment. Following that, the cells were
grouped randomly. The blank group consisted of serum-free DMEM in
wells without cells and the experimental groups were exposed to
DMEM plus 10% FBS containing various concentrations of Wnt1 (0, 5,
10, 20, 40 and 80 μg/l) (Peprotech, Rocky Hill, NJ, USA) for 48 h.
Following the experimental periods, 20 μl of MTT (5 mg/ml; Amresco
Inc., Solon, OH, USA) was added to each well and the plates were
incubated for another 4 h at 37°C in a humidified 5% CO2
atmosphere. The supernatant was then discarded and 150 μl of
dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA) was added to
each well. The optical density (OD) was measured with a microplate
reader (SpectraMax 340; Molecular Devices Corp., Sunnyvale, CA,
USA) at a wavelength of 570 nm. The proliferation rate was
calculated using the equation: Proliferation rate (%) =
Ab570 treated/Ab570 control × 100%.
Immunofluorescence
HEPF cells were seeded in a 24-well plate and slides
at a density of 1×103 cells per well, following being
synchronously cultured in DMEM serum-free medium for 24 h and then
in 10% FBS containing Wnt1 at a concentration of 20 μg/l for 48 h.
Following the experimental treatment, HEPF cells were fixed with 4%
paraformaldehyde for 15 min. The cells were permeabilised for 8 min
with 0.5% Triton X-100 (BD Biosciences) in PBS, then inhibited in
PBS supplemented with 1% BSA (BD Biosciences, Shanghai, China) for
1 h and incubated overnight at 4°C with 1:100 rabbit anti-β-catenin
antibody (Cell Signaling Technology, Inc., Danvers, MA, USA). The
cells were washed for 20 min, incubated with secondary antibody
(1:100 goat anti-rabbit; labelled with cy3; Boster Biological
Technology, Wuhan, Hubei, China) for 1 h at 37°C and then stained
with Hoechst (10 μg/ml in PBS) for 20 min. The cells were finally
mounted on glass slides and observed using fluorescence microscopy
(BX-51; Olympus, Tokyo, Japan).
Western blot analysis
Total proteins were extracted after the cells were
collected, then separated using SDS-PAGE (Sunshine Biotechnology
Co., Ltd.) and transferred onto polyvinylidene difluoride
membranes. The membranes were inhibited with skimmed milk powder
and then probed for proteins of interest. These membranes were
probed using mouse anti-human antibody against α-SMA, vimentin and
β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
rabbit anti-human collagen I (Santa Cruz Biotechnology, Inc.) and
β-catenin. The primary antibodies were used in the following
concentrations: anti-β-actin, 1:1,000; anti-SMA, 1:200 and
anti-collagen I, 1:1,000. The horseradish peroxidase-goat
anti-rabbit and anti-mouse secondary antibodies (Santa Cruz
Biotechnology, Inc.) concentrations were 1:5,000. ECL detection
reagents were used for visualisation (Amersham Biosciences,
Piscataway, NJ, USA). The band densities for each phenotype marker
were quantified using Lane 1D software (Beijing Sage Creation
Science And Technology Co., Ltd., Beijing, China) following
scanning with a GS-710 calibrated imaging densitometer ChampChemi
basic (Bio-Rad, Hercules, CA, USA). The results were expressed as a
ratio of band density to total β-actin.
Real-time polymerase chain reaction
(RT-PCR)
RT-PCR was used to determine the mRNA expression of
α-SMA, vimentin, collagen I and β-catenin. Total RNA was isolated
using TRIzol reagent (Invitrogen Life Technologies) and cDNAs were
generated using a PrimeScript RT reagent kit (Takara, Dalian,
China) at 37°C for 15 min and then at 85°C for 5 sec. The specific
primers for the PCR reaction were as follows: α-SMA, forward
5′-TCAAATACCCCATTGAACACGG-3′ and reverse 5′-GGTGCTCTTCAGGTGCTACA-3′
with a product size of 178 bp; vimentin, forward
5′-TGCGTGAAATGGAAGAGAACT-3′ and reverse 5′-TCAGGTTCAGGGAGGAAAAGT-3′
with a product size of 240 bp; collagen I, forward
5′-TCTGACTGGAAGAGTGGAGAGTAC-3′ and reverse
5′-ATCCATCGGTCATGCTCTCG-3′ with a product size of 202 bp;
β-catenin, forward 5′-GCTACTCAAGCTGATTTGATGGA-3′ and reverse
5′-GGTAGTGGCACCAGAATGGATT-3′ with a product size of 120 bp; GAPDH,
forward 5′-GGATTTGGTCGTATTGGG-3′ and reverse
5′-GGAAGATGGTGATGGGATT-3′ with a product size of 205 bp.
Quantitative PCR was performed using the Mx3000P qPCR System
(Stratagene, La Jolla, CA, USA) with SYBR Premix Ex Taq (Takara). A
total of 1 μl of reverse transcription reaction mixture was
utilised for a quantitative PCR in a total volume of 20 μl. The PCR
cycles were 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec
and 58°C for 30 sec. Data were analysed according to the
comparative Ct method and then normalised to GAPDH expression
levels within each sample. The relative expression levels of target
genes, following normalisation to an endogenous sequence, were
given by 2−ΔΔCt.
Statistical analysis
Statistical analysis was performed using a
statistical software package (SPSS for Windows version 16.0; SPSS,
Chicago, IL, USA). Data are presented as the mean ± standard
deviation (SD). Statistical comparisons between the groups were
performed using a two-tailed unpaired t-test or a one-way analysis
of variance (AVOVA) followed by a SNK-q test for studies with more
than two groups. The Pearson’s correlation analysis was also
adopted. P<0.05 was considered to indicate a statistically
significant difference.
Results
Proliferation of HEPF cells
An MTT assay revealed that the OD of HEPF cells in
the experimental groups were higher than that of the control group
following 48 h of treatment. When the concentration exceeded 20
μg/l, the OD significantly increased (P<0.05; Fig. 1A). The present study indicated that
Wnt1 was able to induce the proliferation of HEPF cells and that
this proliferation was concentration dependent.
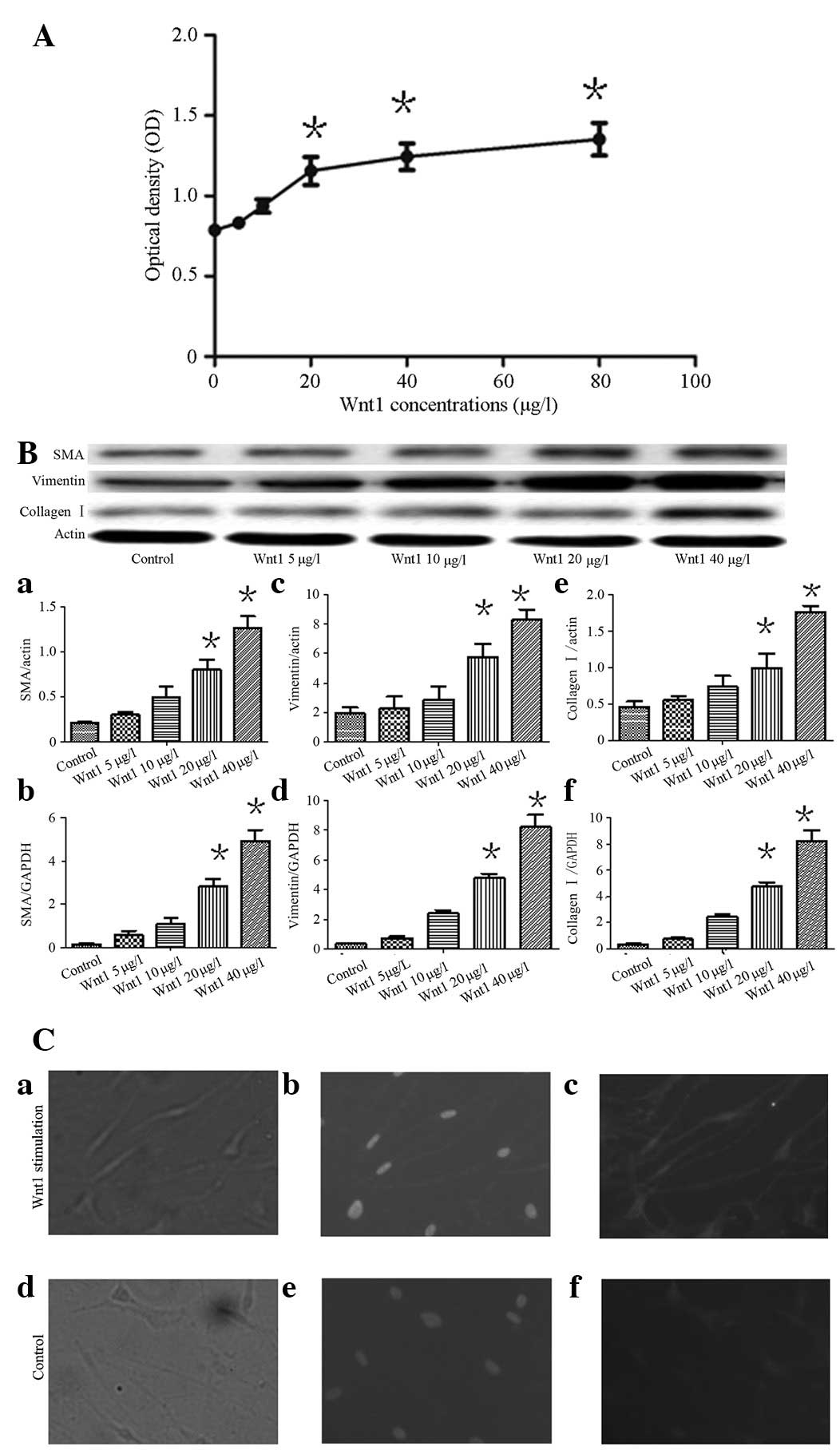 | Figure 1(A) Effects on cell proliferation
stimulated by Wnt1, detected by MTT assay. Wnt1 was able to induce
HEPF cell proliferation in a concentration-dependent manner. When
the concentration was >20 μg/l, Wnt1 was able to markedly
increase proliferation compared with the control group
(*P<0.05). (B) Effects of Wnt1 on the expression of
α-SMA, vimentin and collagen I in HEPF cells. (Ba) Western blot
analysis demonstrating SMA protein expression. (Bb) qRT-PCR
demonstrated SMA mRNA expression. (Bc) Vimentin protein is shown.
(Bd) Vimentin mRNA was expressed. (Be) Expression of collagen I
protein. (Bf) qRT-PCR for collagen I mRNA. From left: medium
treated as the control group, various concentrations of Wnt1 5, 10,
20 and 40 μg/l. Each bar represents the mean ± SD;
*P<0.05, versus the control. (Ca and d) HEPF cells
were observed under a light microscope. (Cb and e) Hoechst staining
demonstrated cell nuclei under fluorescence microscopy. (Cc) HEPF
cells stimulated with Wnt1 demonstrated β-catenin protein
expression mostly localized in the nucleus. (Cf) In the controls,
the β-catenin protein was expressed in the membrane (magnification,
×200). HEPF, human embryonic pulmonary fibroblast; α-SMA, α-smooth
muscle actin; MTT, methylthiazoltetrazolium; qRT-PCR, quantitative
real-time polymerase chain reaction. |
Wnt1 stimulates SMA, vimentin and
collagen I mRNA and protein expression
Western blot analysis and qRT-PCR revealed that SMA
vimentin and collagen I mRNA and protein expression, increased in a
concentration-dependent manner following the stimulation of HEPF
cells with various concentrations of Wnt1 (0, 5, 10, 20 and 40
μg/l) for 48 h. When the concentration of Wnt1 exceeded 20 μg/l,
this expression significantly increased in the experimental group
compared with the control group (P<0.05; Fig. 1B). Following the stimulation of
HEPF cells with Wnt1, β-catenin was mostly distributed in the
nucleus as demonstrated by immunofluorescence (Fig. 1Ca–Cc). However, in the control
cells, β-catenin was associated with the cell membrane in regions
of cell-cell contact (Fig.
1Cd–Cf).
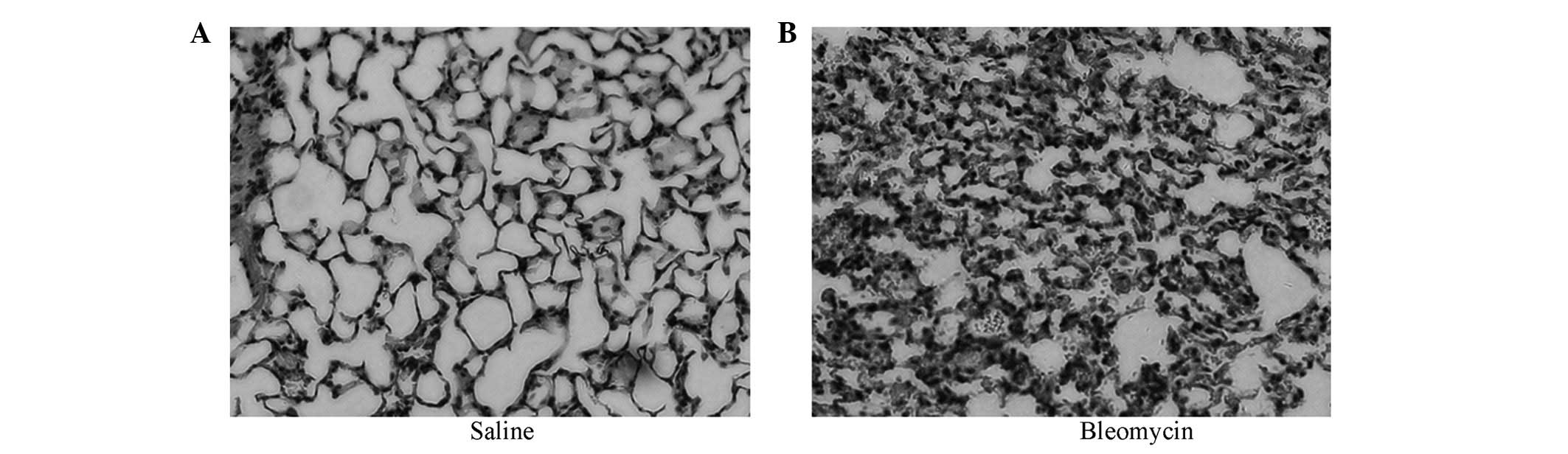
Histopathological changes
At present, the most widely used experimental model
of lung fibrosis is the BLM-induced model. In the present study,
BALF from BLM-induced models of pulmonary fibrosis on day 7 were
obtained along with simultaneous mouse lung biopsy. In the sections
stained with H&E, we observed massive inflammatory cells and
erythrocytes in the septum and alveoli, which were accompanied by
fibroblast proliferation (Fig.
2B), unlike in the control group (Fig. 2A), which was given the same volume
of saline. These results demonstrated that BLM-induced models of
pulmonary fibrosis were constructed successfully.
BALF induces α-SMA, vimentin and collagen
I expression
To determine whether lung alveolar epithelial cell
injury induces fibroblast activation and ECM deposition, we used
BALF obtained from BLM-induced models of pulmonary fibrosis on day
7 to culture HEPF cells. Using western blot analysis and real-time
RT-PCR, HEPF cells exposed to BALF overexpressed α-SMA, vimentin
and collagen I compared with the control (P<0.05), as shown in
Fig. 3. Whether these changes were
associated with the Wnt signalling pathway was evaluated. β-catenin
mRNA and protein was simultaneously detected. The increase in α-SMA
mRNA and protein was associated with β-catenin levels (r=0.829,
0.842). The increase in vimentin mRNA and protein was associated
with β-catenin levels (r=0.867, 0.837) and the increase in collagen
I mRNA and protein was associated with β-catenin levels (r=0.817,
0.881). Furthermore, we knocked down the β-catenin gene in the HEPF
cells by infecting them with the lentivirus (sh β-catenin).
Following 96 h, unlike the untreated cells (Fig. 4Ad), the cells treated with
lentiviruses expressing GFP (Fig.
4Ab) indicated a successful infection. Western blot analysis
demonstrated that the β-catenin levels in HEPF siRNA-infected cells
were significantly lower than in cells infected with pLL-shNC
(control siRNA; Fig. 4Ba and 4Bb).
The qRT-PCR demonstrated the same result (Fig. 4Bc). The cells were cultured using
BALF. Notably, as shown in Fig.
4C, compared with the shNC-infected cells, siRNA improved the
expression of the α-SMA, vimentin and collagen I of sh
β-catenin-infected HEPF cells, which were induced by BALF (Fig. 4C).
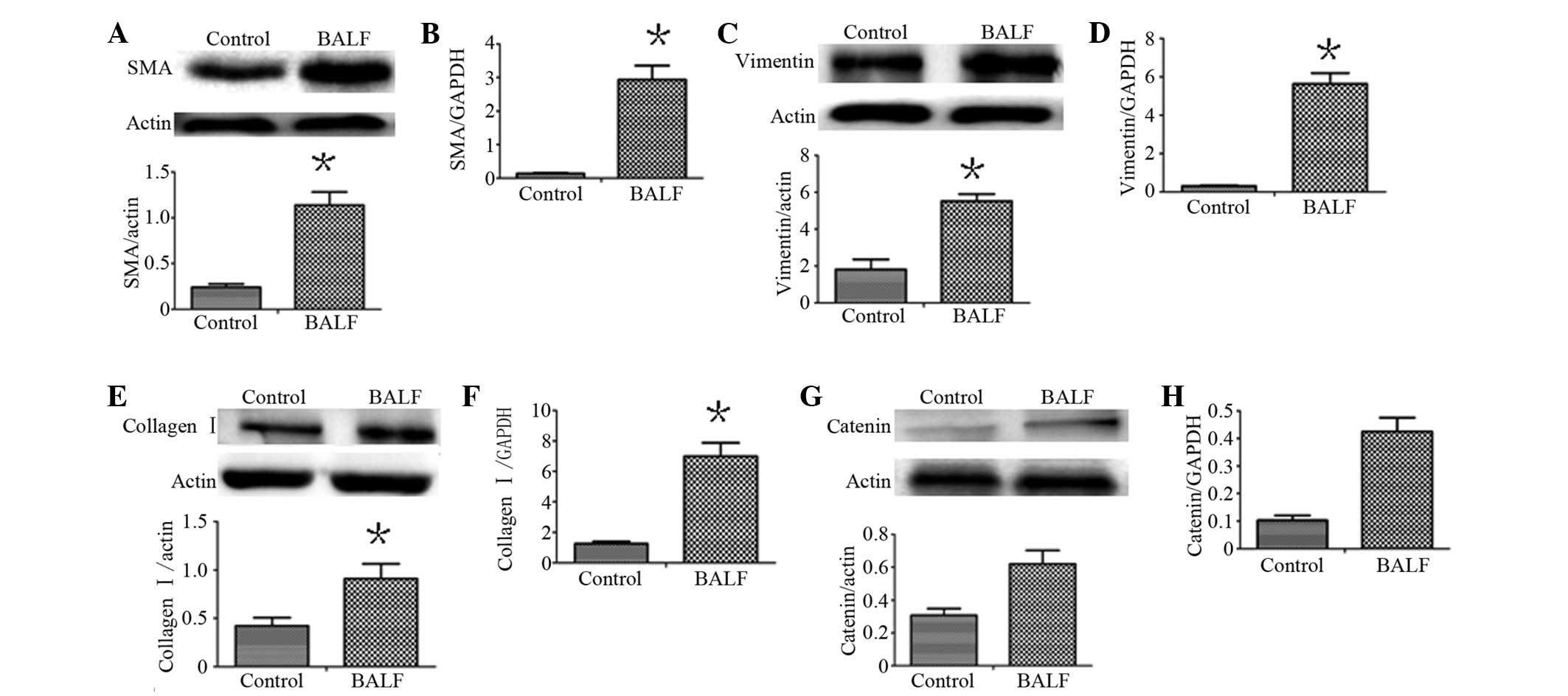 | Figure 3Expression of α-SMA, vimentin and
collagen I in HEPF cells cultured with BALF. (A, C and E) Western
blot analysis demonstrating SMA, vimentin and collagen I protein
expression; (B, D and F) qRT-PCR demonstrated SMA, vimentin and
collagen I mRNA expression; (G and H) Western blot analysis and
qRT-PCR demonstrated the expression of the β-catenin protein and
mRNA. Expression of α-SMA, vimentin and collagen I associated with
the expression of β-catenin. Each bar represents the mean ± SD;
*P<0.05, compared with the control. HEPF, human
embryonic pulmonary fibroblast; α-SMA, α-smooth muscle actin; BALF,
bronchoalveolar lavage fluid; qRT-PCR, quantitative real-time
polymerase chain reaction; SD, standard deviation. |
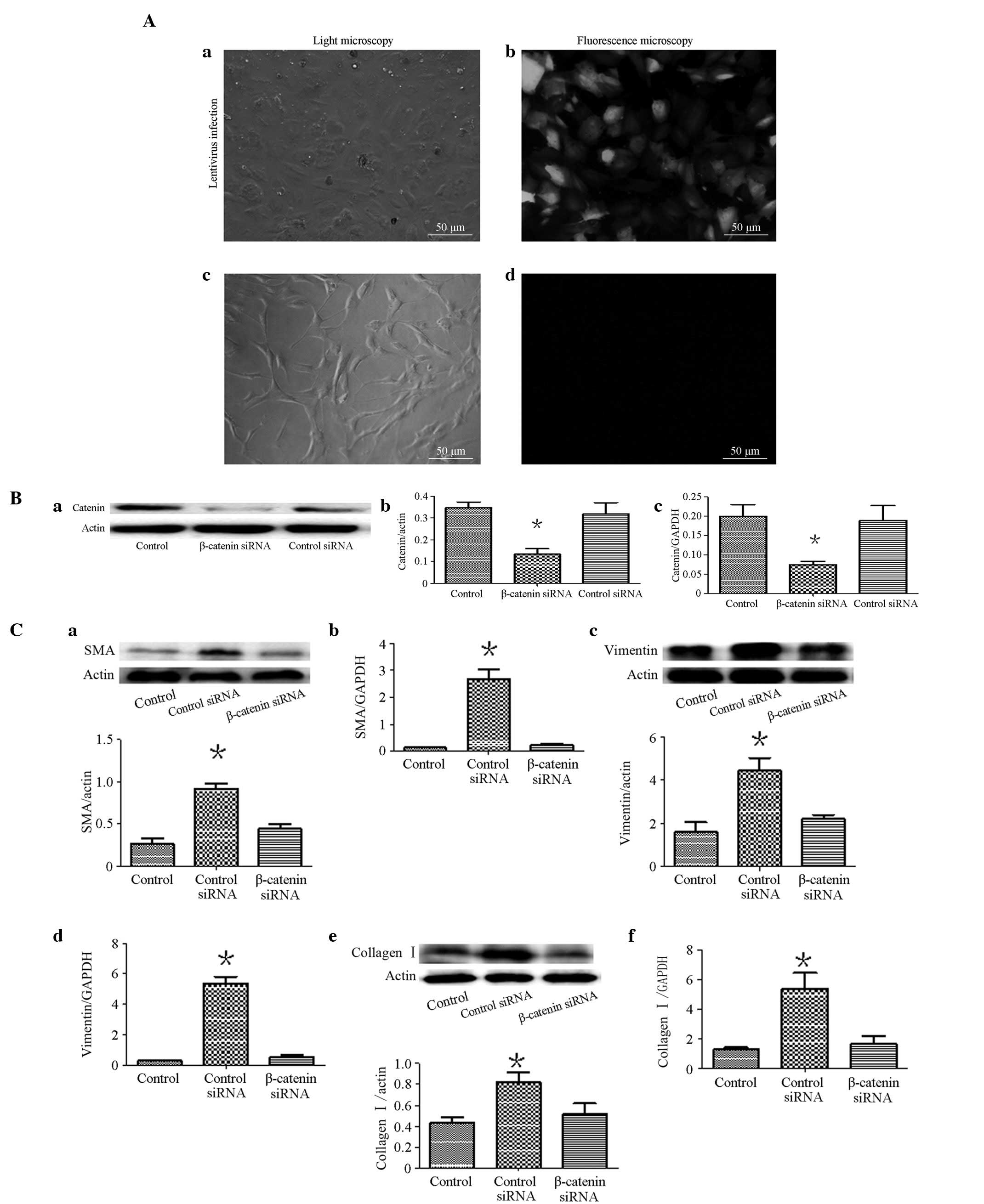 | Figure 4(A) HEPF cells observation results.
(Aa and Ac) HEPF cells observed under light microscope. (Ab) GFP
fluorescence (right panel) of HEPF cells observed under
fluorescence microscopy (magnification, ×200) 96 h after infection
with the lentivirus containing pLL-sh catenin or shNC. (Ad) GFP
fluorescence cannot be observed when the cells were not infected
with the lentivirus. (B) Expression of β-catenin in HEPF cells
infected with the lentivirus (sh β-catenin). Western blot analysis
and qRT-PCR assessment that β-catenin siRNA suppresses β-catenin
protein (Ba and Bb) and (Bc) mRNA expression in HEPF cells. Each
bar represents the mean ± SD; *P<0.05 compared with
the control. (C) Effects of β-catenin siRNA attenuated HEPF cell
differentiation into myofibroblasts. (Ca, Cc and Ce) Western blot
analysis demonstratd α-SMA, vimentin and collagen I protein
expression; (Cb, Cd and Cf) qRT-PCR demonstrated α-SMA, vimentin
and collagen I mRNA expression. From left: medium-treated HEPF
cells, β-catenin siRNA-treated BALF-treated cells and control
siRNA-treated BALF-treated cells. Each bar represents the mean ±
SD, *P<0.05. HEPF, human embryonic pulmonary
fibroblast; α-SMA, α-smooth muscle actin; BALF, bronchoalveolar
lavage fluid; qRT-PCR, quantitative real-time polymerase chain
reaction; SD, standard deviation. |
Discussion
IPF is characterised by fibroblast/myofibroblast
activation, ECM deposition and alveolar epithelial type II cell
dysfunction, which all lead to parenchymal destruction. However,
the origins of these characteristics remain to be elucidated.
Recently, a new hypothesis has been proposed based on the evidence
that injury is key to the irreversible process of fibrosis and
tissue remodelling. However, the molecular pathways linking injury
with the development of fibrosis are poorly understood. Previous
studies demonstrated that the Wnt signalling pathway is abnormally
activated in lung fibrosis (10,15–17).
Classical Wnt signalling is initiated by extracellular ligands
known as Wnts, including Wnt1 and Wnt3. In the absence of ligands,
β-catenin is phosphorylated on a serine residue by glycogen
synthase kinase-3β (GSK-3β) in a complex that includes adenomatous
polyposis coli and axin. Phosphorylation targets β-catenin for
ubiquitin-mediated degradation. Following ligand binding and
complexing with LRP, the inhibition of GSK-3β results in a
stabilised β-catenin, which leads to β-catenin accumulation in the
cytoplasm. β-catenin is then translocated into the nucleus and acts
as a co-transcriptional activator of T-cell factor and lymphoid
enhancer factor that regulate downstream target gene expression
(18).
The present study used different concentrations of
Wnt1 to intervene in cell proliferation in a dose-dependent manner.
When the concentration of Wnt1 exceeded 20 μg/l, cell proliferation
was more apparent. Furthermore, we used Wnt1 to intervene with HEPF
cells. The results demonstrated that the mRNA and protein
expression of α-SMA, vimentin and collagen I increased with
corresponding Wnt1 in a dose-dependent manner. α-SMA in progressive
lesions of IPF reflected the transition of fibroblasts to
myofibroblasts (4,19,20).
Vimentin was a key molecule involved in the post-transcriptional
regulation of collagen expression (21). The results demonstrated that Wnt1
activated myofibroblasts, as well as synthesised and deposited a
collagen-rich ECM. The present study demonstrated that Wnt1 is able
to activate β-catenin-mediated signalling.
The model of BLM-induced lung injury has been
extensively used to investigate potential pathways in the
pathogenesis of pulmonary fibrosis (22,23).
Our previous studies in BLM-induced pulmonary fibrosis in mice
demonstrated that alveolar epithelial injury is severe and
β-catenin expression increases on day 7.
On day 7 of BALF in a BLM mouse model, it was
demonstrated that increases in the mRNA and protein expression of
α-SMA vimentin and collagen I were positively correlated with
β-catenin expression. HEPF cells were infected with a lentivirus
containing β-catenin shRNA, which knocked down the β-catenin gene
and then HEPF cells were cultured with the pulmonary lavage fluid.
The present study revealed that the mRNA and protein expression of
α-SMA, vimentin and collagen I did not significantly increase. The
expression of the Wnt ligands and β-catenin was not measured in the
pulmonary lavage fluid from the mouse in the BLM model by ELISA. A
possible mechanism may be that in response to injury induced by BLM
to the lung epithelium, lung repair mechanisms are initiated
immediately. These mechanisms trigger an acute inflammatory
response that results in the release of a wide variety of
cytokines, in immune cell recruitment and activation of the Wnt
signalling pathway. The Wnt family proteins are released by the
injured epithelial cells and endothelial cells (11) to the surrounding tissues and shed
into the BALF. When BALF was used to culture HEPF cells, it caused
Wnt/β-catenin signalling activation, even in the absence of the
initial injury factors. TGF-β is assumed to be a major mediator of
fibrosis progression (24). A
number of studies demonstrated that Wnt/β-catenin signalling is
able to upregulate the expression of TGF-β (25,26)
and TGF-β1 can promote β-catenin signalling (27–31).
Crosstalk exists between the Wnt/β-catenin pathway and TGF-β
signalling. In BLM-induced mice, TGF-β protein levels in BALF were
significantly increased (32–34).
We assumed that the BALF obtained from BLM-induced pulmonary
fibrosis in a mouse model contained TGF-β that activated the Wnt
signalling pathway through crosstalk with the Wnt/β-catenin
pathway.
BALF from the pulmonary fibrosis mouse model
contains interleukin (IL)-1α, TNF-α, IL-6 (32) and connective tissue growth factor
(35). Previous studies
demonstrated that the proinflammatory cytokine IL-1β is one of the
most upregulated genes in primary murine alveolar epithelial type
II cells following Wnt3a treatment (36). Injury to the epithelium initiates
the Wnt/β-catenin signalling pathway to induce proinflammatory
cytokine IL release. Otherwise, Wnt signalling may be triggered by
proinflammatory cytokines, including IL-1α, TNF-α and IL-6 to
induce a profibrotic cascade. This profibrotic cascade may result
in fibroblast expansion and progressive fibrosis reminiscent of an
abnormal wound healing, which requires further investigation.
In summary, the results from the present study
demonstrated that sustained activation of Wnt1/β-catenin signalling
increases the number of myofibroblasts in pulmonary fibrosis and
promotes fibroblasts to change into myofibroblasts upon tissue
injury or inflammation. Furthermore, these results also
demonstrated that the activation of a biological repair response
and persistence at the injury site is a key factor in the formation
of pulmonary fibrosis. The Wnt1/β-catenin signalling pathway is
important in the formation of fibrotic disease in lung injury and
may provide opportunities for treatment and intervention in
IPF.
Acknowledgements
This study was supported by grants from the
Scientific Research Project of Ministry of Public Health (no.
wkj2006-2-026) and funds from Shanghai Science and Technology
Development (no. 10ZR1422600).
References
|
1
|
Chilosi M, Zamò A, Doglioni C, et al:
Migratory marker expression in fibroblast foci of idiopathic
pulmonary fibrosis. Respir Res. 7:952006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gross TJ and Hunninghake GW: Idiopathic
pulmonary fibrosis. N Engl J Med. 345:517–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katzenstein AL, Zisman DA, Litzky LA,
Nguyen BT and Kotloff RM: Usual interstitial pneumonia: histologic
study of biopsy and explant specimens. Am J Surg Pahol.
26:1567–1577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scotton CJ and Chambers RC: Molecular
targets in pulmonary fibrosis: the myofibroblast in focus. Chest.
132:1311–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meltzer EB and Noble PW: Idiopathic
pulmonary fibrosis. Orphanet J Rare Dis. 3:82008. View Article : Google Scholar
|
|
6
|
Selman M, King TE and Pardo A: Idiopathic
pulmonary fibrosis: prevailing and evolving hypotheses about its
pathogenesis and implications for therapy. Ann Intern Med.
134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horowitz JC and Thannickal VJ:
Epithelial-mesenchymal interactions in pulmonary fibrosis. Semin
Respir Crit Care Med. 27:600–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selman M and Pardo A: Idiopathic pulmonary
fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir
Res. 3:32002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chilosi M, Poletti V, Zamò A, et al:
Aberrant Wnt/beta-catenin pathway activation in idiopathic
pulmonary fibrosis. Am J Pathol. 162:1495–1502. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Xiao L, Sun L and Liu F:
Wnt/beta-catenin signaling: a promising new target for fibrosis
diseases. Physiol Res. 61:337–346. 2012.PubMed/NCBI
|
|
11
|
Königshoff M, Balsara N, Pfaff EM, et al:
Functional Wnt signaling is increased in idiopathic pulmonary
fibrosis. PLoS One. 3:e21422008.PubMed/NCBI
|
|
12
|
Lawson WE, Polosukhin VV, Stathopoulos GT,
et al: Increased and prolonged pulmonary fibrosis in surfactant
protein C-deficient mice following intratracheal bleomycin. Am J
Pathol. 167:1267–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang D, Liang J, Campanella GS, et al:
Inhibition of pulmonary fibrosis in mice by CXCL10 requires
glycosaminoglycan binding and syndecan-4. J Clin Invest.
120:2049–2057. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang D, Liang J, Hodge J, et al:
Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J
Clin Invest. 114:291–299. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meuten T, Hickey A, Franklin K, et al:
WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis. Respir
Res. 13:622012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, McLean S, Carter DE and Leask A:
The gene expression profile induced by Wnt 3a in NIH 3T3
fibroblasts. J Cell Commun Signal. 1:175–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Velden JL, Guala AS, Leggett SE,
Sluimer J, Badura EC and Janssen-Heininger YM: Induction of a
mesenchymal expression program in lung epithelial cells by wingless
protein (Wnt)/β-catenin requires the presence of c-Jun N-terminal
kinase-1 (JNK1). Am J Respir Cell Mol Biol. 47:306–314.
2012.PubMed/NCBI
|
|
18
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and β-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004.
|
|
19
|
Broekelmann TJ, Limper AH, Colby TV and
McDonald JA: Transforming growth factor beta 1 is present at sites
of extracellular matrix gene expression in human pulmonary
fibrosis. Proc Natl Acad Sci USA. 88:6642–6646. 1991. View Article : Google Scholar
|
|
20
|
Salazar KD, Lankford SM and Brody AR:
Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that
mediate proliferation and procollagen expression by lung
fibroblasts. Am J Physiol Lung Cell Mol Physiol. 297:L1002–L1011.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Challa AA and Stefanovic B: A novel role
of vimentin filaments: binding and stabilization of collagen mRNAs.
Mol Cell Biol. 31:3773–3789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moeller A, Ask K, Warburton D, Gauldie J
and Kolb M: The bleomycin animal model: a useful tool to
investigate treatment options for idiopathic pulmonary fibrosis?
Int J Biochem Cell Biol. 40:362–382. 2008. View Article : Google Scholar
|
|
23
|
Moore BB and Hogaboam CM: Murine models of
pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
294:L152–L160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011.
|
|
25
|
Carre AL, James AW, MacLeod L, et al:
Interaction of wingless protein (Wnt), transforming growth
factor-beta1, and hyaluronan production in fetal and postnatal
fibroblasts. Plast Reconstr Surg. 125:74–88. 2010. View Article : Google Scholar
|
|
26
|
Cheon SS, Wei Q, Gurung A, et al:
Beta-catenin regulates wound size and mediates the effect of
TGF-beta in cutaneous healing. FASEB J. 20:692–701. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato M: Upregulation of the
Wnt/beta-catenin pathway induced by transforming growth factor-beta
in hypertrophic scars and keloids. Acta Derm Venereol. 86:300–307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satterwhite DJ and Neufeld KL: TGF-beta
targets the Wnt pathway components, APC and beta-catenin, as Mv1Lu
cells undergo cell cycle arrest. Cell Cycle. 3:1069–1073. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheon SS, Nadesan P, Poon R and Alman BA:
Growth factors regulate beta-catenin-mediated TCF-dependent
transcriptional activation in fibroblasts during the proliferative
phase of wound healing. Exp Cell Res. 293:267–274. 2004. View Article : Google Scholar
|
|
30
|
Ulsamer A, Wei Y, Kim KK, et al: Axin
pathway activity regulates in vivo pY654-β-catenin accumulation and
pulmonary fibrosis. J Biol Chem. 287:5164–5172. 2012.PubMed/NCBI
|
|
31
|
Zhou B, Liu Y, Kahn M, et al: Interactions
between β-catenin and transforming growth factor-β signaling
pathways mediate epithelial-mesenchymal transition and are
dependent on the transcriptional co-activator cAMP-response
element-binding protein (CREB)-binding protein (CBP). J Biol Chem.
287:7026–7038. 2012.
|
|
32
|
Gurujeyalakshmi G, Wang Y and Giri SN:
Taurine and niacin block lung injury and fibrosis by
down-regulating bleomycin-induced activation of transcription
nuclear factor-kappaB in mice. J Pharmacol Exp Ther. 293:82–90.
2000.PubMed/NCBI
|
|
33
|
Izumo T, Kondo M and Nagai A: Effects of a
leukotriene B4 receptor antagonist on bleomycin-induced pulmonary
fibrosis. Eur Respir J. 34:1444–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robb WB, Condron C, Moriarty M, Walsh TN
and Bouchier-Hayes DJ: Taurine attenuates radiation-induced lung
fibrosis in C57/Bl6 fibrosis prone mice. Ir J Med Sci. 179:99–105.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang C, Huang H, Liu J, Wang Y, Lu Z and
Xu Z: Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced
pulmonary fibrosis in mice. Int J Mol Sci. 13:8293–8307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aumiller V, Balsara N, Wilhelm J, Günther
A and Königshoff M: Wntβ-catenin signaling induces IL-1β expression
by alveolar epithelial cells in pulmonary fibrosis. Am J Respir
Cell Mol Biol. 49:96–104. 2013.
|